Scratching the Itch: Updated Perspectives on the Schistosomes Responsible for Swimmer’s Itch around the World
Abstract
1. Introduction
2. A Growing Appreciation for the Full Extent of Schistosome Diversity
3. How Much More Diversity Is out There?
4. The Many Paths to Swimmer’s Itch, a Complex Array of Zoonotic Players
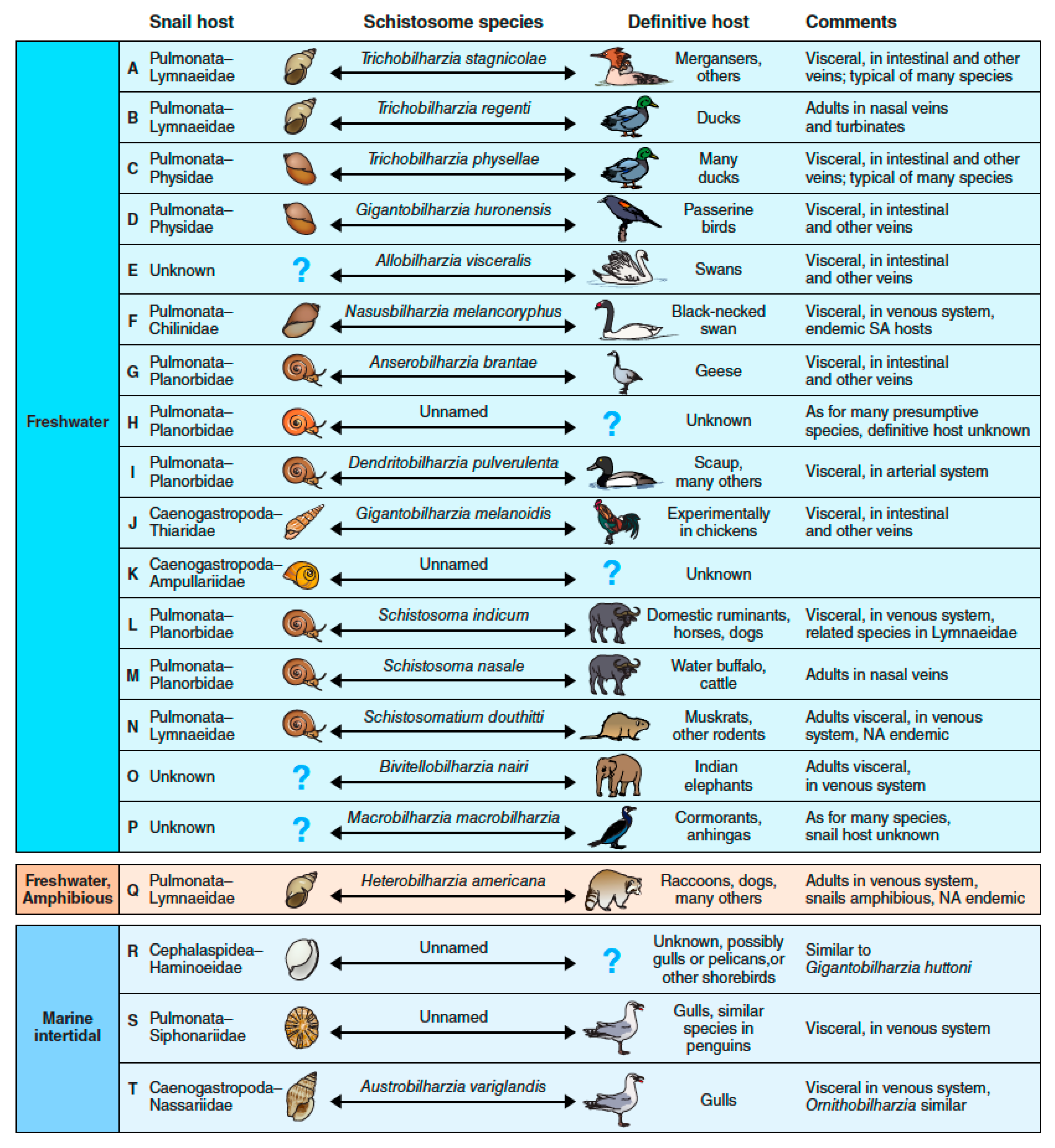
5. Swimmer’s Itch—A One Health Perspective
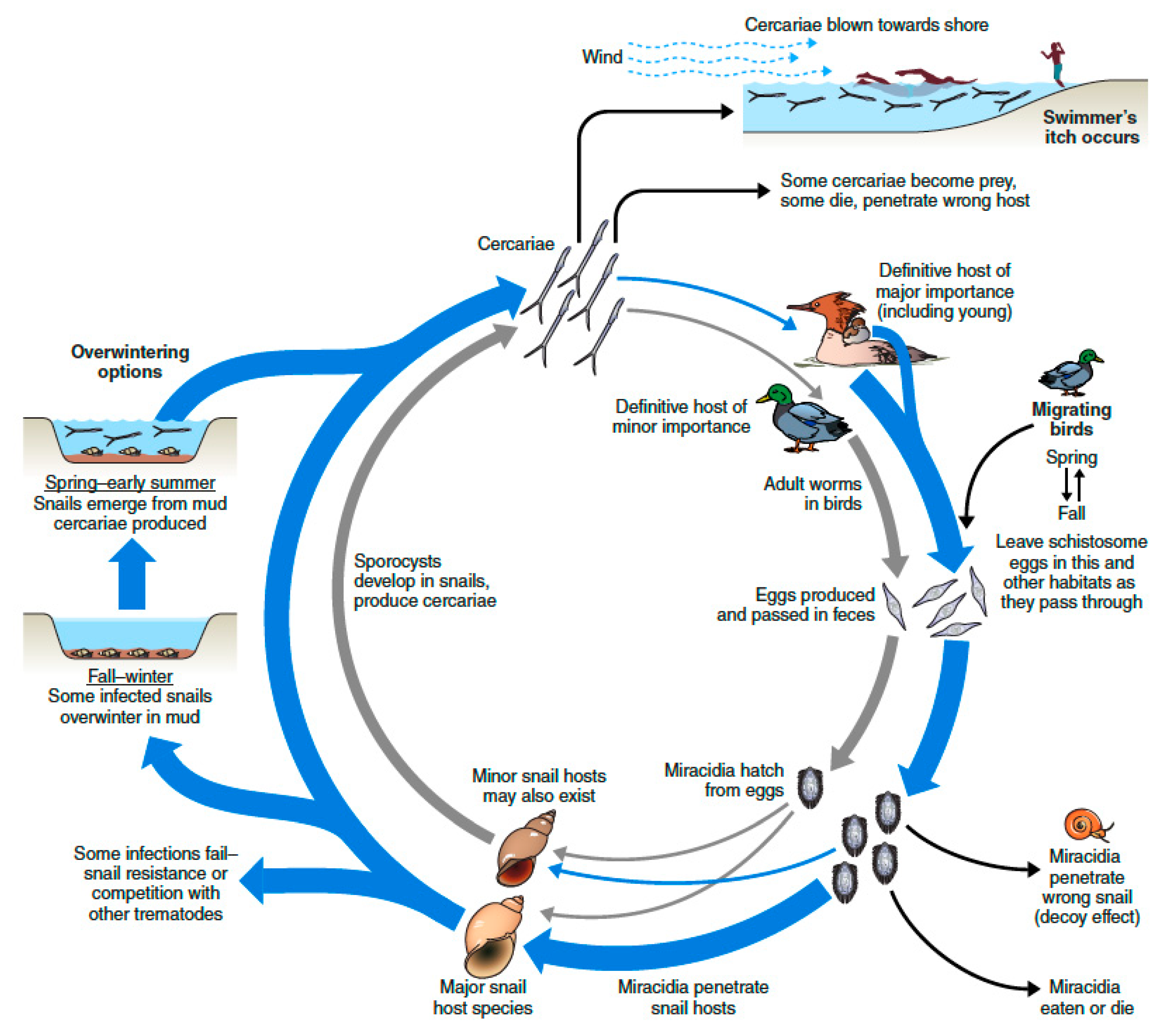
6. Monitoring Itch-Causing Parasites in Natural Habitats—Some Pros and Cons of Different Methods
7. Controlling, or Should We Say Managing, Swimmer’s Itch—The Need for Effective Yet Specific and Environmentally Acceptable Solutions
8. Outlook and Priorities for Future Work
9. Increased Funding Opportunities for Swimmer’s Itch Research?
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishnamurthy, D.; Katsikis, G.; Bhargava, A.; Prakash, M. Schistosoma mansoni Cercariae Swim Efficiently by Exploiting an Elastohydrodynamic Coupling. Nat. Phys. 2017, 13, 266–271. [Google Scholar] [CrossRef]
- Zhu, B.; Luo, F.; Shen, Y.; Yang, W.; Sun, C.; Wang, J.; Li, J.; Mo, X.; Xu, B.; Zhang, X.; et al. Schistosoma japonicum Cathepsin B2 (SjCB2) Facilitates Parasite Invasion through the Skin. PLoS Negl. Trop. Dis. 2020, 14, e0008810. [Google Scholar] [CrossRef] [PubMed]
- Vondráček, O.; Mikeš, L.; Talacko, P.; Leontovyč, R.; Bulantová, J.; Horák, P. Differential Proteomic Analysis of Laser-Microdissected Penetration Glands of Avian Schistosome Cercariae with a Focus on Proteins Involved in Host Invasion. Int. J. Parasitol. 2022, 52, S0020751922000170. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human Schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Brant, S.V.; Loker, E.S. Discovery-Based Studies of Schistosome Diversity Stimulate New Hypotheses about Parasite Biology. Trends Parasitol. 2013, 29, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Brant, S.V.; Loker, E.S. Can Specialized Pathogens Colonize Distantly Related Hosts? Schistosome Evolution as a Case Study. PLOS Pathog. 2005, 1, e38. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, M.C.C.; Hoogerwerf, M.-A.; Koopman, J.P.R.; Janse, J.J.; Kos-van Oosterhoud, J.; Feijt, C.; Jochems, S.P.; de Dood, C.J.; van Schuijlenburg, R.; Ozir-Fazalalikhan, A.; et al. A Controlled Human Schistosoma mansoni Infection Model to Advance Novel Drugs, Vaccines and Diagnostics. Nat. Med. 2020, 26, 326–332. [Google Scholar] [CrossRef]
- Barlow, C.H. Is There Dermatitis in Egyptian Schistosomiasis? Am. J. Hyg. 1936, 24, 587–599. [Google Scholar] [CrossRef]
- Olivier, L. The Penetration Of Dermatitis-Producing Schistosome Cercariae. Am. J. Hyg. 1949, 49, 134–139. [Google Scholar]
- Olivier, L. Schistosome Dermatitis, a Sensitization Phenomenon. Am. J. Hyg. 1949, 49, 290–302. [Google Scholar]
- Kolářová, L. Schistosomes Causing Cercarial Dermatitis: A Mini-Review of Current Trends in Systematics and of Host Specificity and Pathogenicity. Folia Parasitol. 2007, 54, 81–87. [Google Scholar] [CrossRef]
- Lashaki, E.K.; Gholami, S.; Fakhar, M.; Karamian, M.; Daryani, A. Association between Human Cercarial Dermatitis (HCD) and the Occurrence of Trichibilarizia in Duck and Snail in Main Wetlands from Mazandaran Province, Northern Iran. Parasite Epidemiol. Control 2021, 13, e00211. [Google Scholar] [CrossRef] [PubMed]
- Chamot, E.; Toscani, L.; Rougemont, A. Public Health Importance and Risk Factors for Cercarial Dermatitis Associated with Swimming in Lake Leman at Geneva, Switzerland. Epidemiol. Infect. 1998, 120, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, L.M.; Rainey, J.J.; Reimink, R.L.; Blankespoor, H.D. Prospective Study of Swimmer’s Itch Incidence and Severity. J. Parasitol. 2004, 90, 697–704. [Google Scholar] [CrossRef]
- Verbrugge, L.M.; Rainey, J.J.; Reimink, R.L.; Blankespoor, H.D. Swimmer’s Itch: Incidence and Risk Factors. Am. J. Public Health 2004, 94, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Gordy, M.A.; Cobb, T.P.; Hanington, P.C. Swimmer’s Itch in Canada: A Look at the Past and a Survey of the Present to Plan for the Future. Environ. Health 2018, 17, 73. [Google Scholar] [CrossRef]
- Soldánová, M.; Selbach, C.; Kalbe, M.; Kostadinova, A.; Sures, B. Swimmer’s Itch: Etiology, Impact, and Risk Factors in Europe. Trends Parasitol. 2013, 29, 65–74. [Google Scholar] [CrossRef]
- Marszewska, A.; Cichy, A.; Bulantová, J.; Horák, P.; Żbikowska, E. Potamopyrgus antipodarum as a Potential Defender against Swimmer’s Itch in European Recreational Water Bodies—Experimental Study. PeerJ 2018, 6, e5045. [Google Scholar] [CrossRef]
- Loker, E.S.; Dolginow, S.Z.; Pape, S.; Topper, C.D.; Alda, P.; Pointier, J.P.; Ebbs, E.T.; Sanchez, M.C.; Verocai, G.G.; DeJong, R.J.; et al. An Outbreak of Canine Schistosomiasis in Utah: Acquisition of a New Snail Host (Galba Humilis) by Heterobilharzia americana, a Pathogenic Parasite on the Move. One Health 2021, 13, 100280. [Google Scholar] [CrossRef]
- Rudko, S.P.; McPhail, B.A.; Reimink, R.L.; Froelich, K.; Turnbull, A.; Hanington, P.C. Non-Resident Definitive Host Presence Is Sufficient to Sustain Avian Schistosome Populations. Int. J. Parasitol. 2022, 52, 305–315. [Google Scholar] [CrossRef]
- Harris, N.C.; Livieri, T.M.; Dunn, R.R. Ectoparasites in Black-Footed Ferrets (Mustela nigripes) from the Largest Reintroduced Population of the Conata Basin, South Dakota, USA. J. Wildl. Dis. 2014, 50, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.; Viozzi, G.; Casalins, L.; Loker, E.S.; Brant, S.V. A New Schistosome (Digenea: Schistosomatidae) from the Nasal Tissue of South America Black-Necked Swans, Cygnus melancoryphus (Anatidae) and the Endemic Pulmonate Snail Chilina gibbosa. Zootaxa 2021, 4948, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Devkota, R.; Brant, S.V.; Thapa, A.; Loker, E.S. Sharing Schistosomes: The Elephant Schistosome Bivitellobilharzia nairi Also Infects the Greater One-Horned Rhinoceros (Rhinoceros unicornis) in Chitwan National Park, Nepal. J. Helminthol. 2014, 88, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Costa, I.; Cutmore, S.C.; Miller, T.L.; Nolan, M.J. Molecular Approaches to Trematode Systematics: ‘Best Practice’ and Implications for Future Study. Syst. Parasitol. 2016, 93, 295–306. [Google Scholar] [CrossRef]
- Brant, S.V.; Morgan, J.A.T.; Mkoji, G.M.; Snyder, S.D.; Rajapakse, R.P.V.J.; Loker, E.S. An Approach to Revealing Blood Fluke Life Cycles, Taxonomy, and Diversity: Provision of Key Reference Data Including DNA Sequence from Single Life Cycle Stages. J. Parasitol. 2006, 92, 77–88. [Google Scholar] [CrossRef]
- Devkota, R.; Brant, S.V.; Loker, E.S. The Schistosoma indicum Species Group in Nepal: Presence of a New Lineage of Schistosome and Use of the Indoplanorbis exustus Species Complex of Snail Hosts. Int. J. Parasitol. 2015, 45, 857–870. [Google Scholar] [CrossRef]
- McPhail, B.A.; Rudko, S.P.; Turnbull, A.; Gordy, M.A.; Reimink, R.L.; Clyde, D.; Froelich, K.; Brant, S.V.; Hanington, P.C. Evidence of a Putative Novel Species of Avian Schistosome Infecting Planorbella trivolvis. J. Parasitol. 2021, 107, 89–97. [Google Scholar] [CrossRef]
- Lorenti, E.; Brant, S.V.; Gilardoni, C.; Diaz, J.I.; Cremonte, F. Two New Genera and Species of Avian Schistosomes from Argentina with Proposed Recommendations and Discussion of the Polyphyletic Genus Gigantobilharzia (Trematoda, Schistosomatidae). Parasitology 2022, 149, 675–694. [Google Scholar] [CrossRef]
- Brant, S.V.; Loker, E.S.; Casalins, L.; Flores, V. Phylogenetic Placement of a Schistosome from an Unusual Marine Snail Host, the False Limpet (Siphonaria lessoni) and Gulls (Larus dominicanus) from Argentina with a Brief Review of Marine Schistosomes from Snails. J. Parasitol. 2017, 103, 75–82. [Google Scholar] [CrossRef]
- Pennance, T. Genetic Diversity and Evolution within the Genus Bulinus and Species-Level Interactions with the Transmission of Schistosoma haematobium Group Parasites. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2020. [Google Scholar]
- Moles, J.; Giribet, G. A Polyvalent and Universal Tool for Genomic Studies in Gastropod Molluscs (Heterobranchia). Mol. Phylogenet. Evol. 2021, 155, 106996. [Google Scholar] [CrossRef]
- Gauffre-Autelin, P.; von Rintelen, T.; Stelbrink, B.; Albrecht, C. Recent Range Expansion of an Intermediate Host for Animal Schistosome Parasites in the Indo-Australian Archipelago: Phylogeography of the Freshwater Gastropod Indoplanorbis exustus in South and Southeast Asia. Parasit. Vectors 2017, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; DeJong, R.J.; Jung, Y.; Khallaayoune, K.; Kock, S.; Mkoji, G.M.; Loker, E.S. A Phylogeny of Planorbid Snails, with Implications for the Evolution of Schistosoma Parasites. Mol. Phylogenet. Evol. 2002, 25, 477–488. [Google Scholar] [CrossRef]
- Aksenova, O.V.; Bolotov, I.N.; Gofarov, M.Y.; Kondakov, A.V.; Vinarski, M.V.; Bespalaya, Y.V.; Kolosova, Y.S.; Palatov, D.M.; Sokolova, S.E.; Spitsyn, V.M.; et al. Species Richness, Molecular Taxonomy and Biogeography of the Radicine Pond Snails (Gastropoda: Lymnaeidae) in the Old World. Sci. Rep. 2018, 8, 11199. [Google Scholar] [CrossRef] [PubMed]
- Alda, P.; Lounnas, M.; Vázquez, A.A.; Ayaqui, R.; Calvopiña, M.; Celi-Erazo, M.; Dillon, R.T.; González Ramírez, L.C.; Loker, E.S.; Muzzio-Aroca, J.; et al. Systematics and Geographical Distribution of Galba Species, a Group of Cryptic and Worldwide Freshwater Snails. Mol. Phylogenet. Evol. 2021, 157, 107035. [Google Scholar] [CrossRef]
- Cunha, T.J.; Giribet, G. A Congruent Topology for Deep Gastropod Relationships. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182776. [Google Scholar] [CrossRef]
- Jørgensen, A.; Madsen, H.; Nalugwa, A.; Nyakaana, S.; Rollinson, D.; Stothard, J.R.; Kristensen, T.K. A Molecular Phylogenetic Analysis of Bulinus (Gastropoda: Planorbidae) with Conserved Nuclear Genes. Zool. Scr. 2011, 40, 126–136. [Google Scholar] [CrossRef]
- Saijuntha, W.; Tantrawatpan, C.; Agatsuma, T.; Rajapakse, R.P.V.J.; Karunathilake, K.J.K.; Pilap, W.; Tawong, W.; Petney, T.N.; Andrews, R.H. Phylogeographic Genetic Variation of Indoplanorbis exustus (Deshayes, 1834) (Gastropoda: Planorbidae) in South and Southeast Asia. One Health 2021, 12, 100211. [Google Scholar] [CrossRef]
- Zapata, F.; Wilson, N.G.; Howison, M.; Andrade, S.C.S.; Jörger, K.M.; Schrödl, M.; Goetz, F.E.; Giribet, G.; Dunn, C.W. Phylogenomic Analyses of Deep Gastropod Relationships Reject Orthogastropoda. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141739. [Google Scholar] [CrossRef]
- Laidemitt, M.R.; Brant, S.V.; Mutuku, M.W.; Mkoji, G.M.; Loker, E.S. The Diverse Echinostomes from East Africa: With a Focus on Species That Use Biomphalaria and Bulinus as Intermediate Hosts. Acta Trop. 2019, 193, 38–49. [Google Scholar] [CrossRef]
- Thompson, C.W.; Phelps, K.L.; Allard, M.W.; Cook, J.A.; Dunnum, J.L.; Ferguson, A.W.; Gelang, M.; Khan, F.A.A.; Paul, D.L.; Reeder, D.M.; et al. Preserve a Voucher Specimen! The Critical Need for Integrating Natural History Collections in Infectious Disease Studies. mBio 2021, 12, e02698-20. [Google Scholar] [CrossRef]
- Snyder, S.D.; Loker, E.S. Evolutionary relationships amont the Schistosomatidae (Platyhelminthes: Digenea) and an Asian origin for Schistosoma. J. Parasitol. 2000, 86, 283–288. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Olson, P.D.; Østergaard, P.; Rollinson, D.; Johnston, D.A.; Attwood, S.W.; Southgate, V.R.; Horak, P.; Snyder, S.D.; Le, T.H.; et al. The Phylogeny of the Schistosomatidae Based on Three Genes with Emphasis on the Interrelationships of Schistosoma Weinland, 1858. Parasitology 2003, 126, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Lawton, S.P.; Hirai, H.; Ironside, J.E.; Johnston, D.A.; Rollinson, D. Genomes and Geography: Genomic Insights into the Evolution and Phylogeography of the Genus Schistosoma. Parasit. Vectors 2011, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Norman, B.F.; Borrett, H.E.; Attwood, S.W.; Mondal, M.M.H.; Walker, A.J.; Webster, J.P.; Rajapakse, R.P.V.J.; Lawton, S.P. Divergence across Mitochondrial Genomes of Sympatric Members of the Schistosoma indicum Group and Clues into the Evolution of Schistosoma spindale. Sci. Rep. 2020, 10, 2480. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Beacham, E.; Macaranas, J. Molecular Phylogenetics of Caenogastropoda (Gastropoda: Mollusca). Mol. Phylogenet. Evol. 2007, 42, 717–737. [Google Scholar] [CrossRef]
- Varney, R.M.; Brenzinger, B.; Malaquias, M.A.E.; Meyer, C.P.; Schrödl, M.; Kocot, K.M. Assessment of Mitochondrial Genomes for Heterobranch Gastropod Phylogenetics. BMC Ecol. Evol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Cribb, T.H.; Chick, R.C.; O’Connor, W.; O’Connor, S.; Johnson, D.; Sewell, K.B.; Cutmore, S.C. Evidence That Blood Flukes (Trematoda: Aporocotylidae) of Chondrichthyans Infect Bivalves as Intermediate Hosts: Indications of an Ancient Diversification of the Schistosomatoidea. Int. J. Parasitol. 2017, 47, 885–891. [Google Scholar] [CrossRef]
- De Buron, I.; Colon, B.; Siegel, S.; Oberstaller, J.; Rivero, A.; Kyle, D. First Evidence of Polychaete Intermediate Hosts for Neospirorchis spp. Marine Turtle Blood Flukes (Trematoda: Spirorchiidae). Int. J. Parasitol. 2018, 48, 1097–1106. [Google Scholar] [CrossRef]
- Nützel, A.; Erwin, D.H.; Mapes, R.H. Identity and Phylogeny of the Late Paleozoic Subulitoidea (Gastropoda). J. Paleontol. 2000, 74, 575–598. [Google Scholar] [CrossRef]
- Shaffer, H.B.; McCartney-Melstad, E.; Near, T.J.; Mount, G.G.; Spinks, P.Q. Phylogenomic Analyses of 539 Highly Informative Loci Dates a Fully Resolved Time Tree for the Major Clades of Living Turtles (Testudines). Mol. Phylogenet. Evol. 2017, 115, 7–15. [Google Scholar] [CrossRef]
- Crellen, T.; Allan, F.; David, S.; Durrant, C.; Huckvale, T.; Holroyd, N.; Emery, A.M.; Rollinson, D.; Aanensen, D.M.; Berriman, M.; et al. Whole Genome Resequencing of the Human Parasite Schistosoma mansoni Reveals Population History and Effects of Selection. Sci. Rep. 2016, 6, 20954. [Google Scholar] [CrossRef] [PubMed]
- DeJong, R.J.; Morgan, J.A.T.; Paraense, W.L.; Pointier, J.-P.; Amarista, M.; Ayeh-Kumi, P.F.K.; Babiker, A.; Barbosa, C.S.; Bremond, P.; Pedro Canese, A.; et al. Evolutionary Relationships and Biogeography of Biomphalaria (Gastropoda: Planorbidae) with Implications Regarding Its Role as Host of the Human Bloodfluke, Schistosoma mansoni. Mol. Biol. Evol. 2001, 18, 2225–2239. [Google Scholar] [CrossRef] [PubMed]
- Hutson, K.S.; Vaughan, D.B.; Blair, D. First Record of a ‘Fish’ Blood Fluke (Digenea: Aporocotylidae) from a Marine Mammal: Cardicola dhangali n. sp. Int. J. Parasitol. Parasites Wildl. 2019, 10, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Coady, N.R.; Muzzall, P.M.; Burton, T.M.; Snider, R.J.; Saxton, J.; Sergeant, M.; Sommers, A. Ubiquitous Variability in the Prevalence of Trichobilharzia stagnicolae (Schistosomatidae) Infecting Stagnicola emarginata in Three Northern Michigan Lakes. J. Parasitol. 2006, 92, 10–15. [Google Scholar] [CrossRef]
- Rudko, S.P.; Turnbull, A.; Reimink, R.L.; Froelich, K.; Hanington, P.C. Species-Specific QPCR Assays Allow for High-Resolution Population Assessment of Four Species Avian Schistosome That Cause Swimmer’s Itch in Recreational Lakes. Int. J. Parasitol. Parasites Wildl. 2019, 9, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Leighton, B.J.; Zervos, S.; Webster, J.M. Ecological Factors in Schistosome Transmission, and an Environmentally Benign Method for Controlling Snails in a Recreational Lake with a Record of Schistosome Dermatitis. Parasitol. Int. 2000, 49, 9–17. [Google Scholar] [CrossRef]
- Loken, B.R.; Spencer, C.N.; Granath, W.O. Prevalence and Transmission of Cercariae Causing Schistosome Dermatitis in Flathead Lake, Montana. J. Parasitol. 1995, 81, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Cort, W.W.; McMullen, D.B.; Brackett, S. Ecological Studies on the Cercariae in Stagnicola emarginata angulata (Sowerby) in the Douglas Lake Region, Michigan. J. Parasitol. 1937, 23, 504–532. [Google Scholar] [CrossRef]
- Cort, W.W.; Olivier, L. The Development of the Sporocysts of a Schistosome, Cercaria Stagnicolae Talbot, 1936. J. Parasitol. 1943, 29, 164–176. [Google Scholar] [CrossRef]
- McMullen, D.B.; Brackett, S. The Distribution and Control of Schistosome Dermatitis in Wisconsin and Michigan. Am. J. Trop. Med. Hyg. 1941, 21, 725–729. [Google Scholar] [CrossRef]
- Caron, Y.; Cabaraux, A.; Marechal, F.; Losson, B. Swimmer’s Itch in Belgium: First Recorded Outbreaks, Molecular Identification of the Parasite Species and Intermediate Hosts. Vector-Borne Zoonotic Dis. 2017, 17, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Skírnisson, K.; Aldhoun, J.A.; Kolářová, L. A Review on Swimmer’s Itch and the Occurrence of Bird Schistosomes in Iceland. J. Helminthol. 2009, 83, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jouet, D.; Ferté, H.; Depaquit, J.; Rudolfová, J.; Latour, P.; Zanella, D.; Kaltenbach, M.L.; Léger, N. Trichobilharzia spp. in Natural Conditions in Annecy Lake, France. Parasitol. Res. 2008, 103, 51. [Google Scholar] [CrossRef] [PubMed]
- Rizevsky, S.V.; Cherviakovsky, E.M.; Kurchenko, V.P. Molecular Taxonomic Identification of Schistosomatidae from Naroch Lake and Polonevichi Lake in Belarus. Biochem. Syst. Ecol. 2011, 39, 14–21. [Google Scholar] [CrossRef]
- Lawton, S.P.; Lim, R.M.; Dukes, J.P.; Cook, R.T.; Walker, A.J.; Kirk, R.S. Identification of a Major Causative Agent of Human Cercarial Dermatitis, Trichobilharzia franki (Müller and Kimmig 1994), in Southern England and Its Evolutionary Relationships with Other European Populations. Parasit. Vectors 2014, 7, 277. [Google Scholar] [CrossRef]
- Selbach, C.; Soldánová, M.; Sures, B. Estimating the Risk of Swimmer’s Itch in Surface Waters—A Case Study from Lake Baldeney, River Ruhr. Int. J. Hyg. Environ. Health 2016, 219, 693–699. [Google Scholar] [CrossRef]
- Christiansen, A.Ø.; Olsen, A.; Buchmann, K.; Kania, P.W.; Nejsum, P.; Vennervald, B.J. Molecular Diversity of Avian Schistosomes in Danish Freshwater Snails. Parasitol. Res. 2016, 115, 1027–1037. [Google Scholar] [CrossRef]
- Marszewska, A.; Cichy, A.; Heese, T.; Żbikowska, E. The Real Threat of Swimmers’ Itch in Anthropogenic Recreational Water Body of the Polish Lowland. Parasitol. Res. 2016, 115, 3049–3056. [Google Scholar] [CrossRef]
- Gulyás, K.; Soldánová, M.; Orosová, M.; Oros, M. Confirmation of the Presence of Zoonotic Trichobilharzia franki Following a Human Cercarial Dermatitis Outbreak in Recreational Water in Slovakia. Parasitol. Res. 2020, 119, 2531–2537. [Google Scholar] [CrossRef]
- Davis, N.E.; Blair, D.; Brant, S.V. Diversity of Trichobilharzia in New Zealand with a New Species and a Redescription, and Their Likely Contribution to Cercarial Dermatitis. Parasitology 2022, 149, 380–395. [Google Scholar] [CrossRef]
- Horák, P.; Kolářová, L. Bird Schistosomes: Do They Die in Mammalian Skin? Trends Parasitol. 2001, 17, 66–69. [Google Scholar] [CrossRef]
- Horák, P.; Dvořák, J.; Kolářová, L.; Trefil, L. Trichobilharzia regenti, a Pathogen of the Avian and Mammalian Central Nervous Systems. Parasitology 1999, 119, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Prüter, H.; Sitko, J.; Krone, O. Having Bird Schistosomes in Mind—the First Detection of Bilharziella polonica (Kowalewski 1895) in the Bird Neural System. Parasitol. Res. 2017, 116, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, E.T.; Loker, E.S.; Brant, S.V. Phylogeography and Genetics of the Globally Invasive Snail Physa acuta Draparnaud 1805, and Its Potential to Serve as an Intermediate Host to Larval Digenetic Trematodes. BMC Evol. Biol. 2018, 18, 103. [Google Scholar] [CrossRef]
- Ebbs, E.T.; Loker, E.S.; Davis, N.E.; Flores, V.; Veleizan, A.; Brant, S.V. Schistosomes with Wings: How Host Phylogeny and Ecology Shape the Global Distribution of Trichobilharzia querquedulae (Schistosomatidae). Int. J. Parasitol. 2016, 46, 669–677. [Google Scholar] [CrossRef]
- Helmer, N.; Blatterer, H.; Hörweg, C.; Reier, S.; Sattmann, H.; Schindelar, J.; Szucsich, N.U.; Haring, E. First Record of Trichobilharzia physellae (Talbot, 1936) in Europe, a Possible Causative Agent of Cercarial Dermatitis. Pathogens 2021, 10, 1473. [Google Scholar] [CrossRef]
- Khosravi, M.; Thieltges, D.W.; Shamseddin, J.; Georgieva, S. Schistosomes from the Persian Gulf: Phylogenetic Relationships, Host Associations and Life-Cycle Elucidation of Ornithobilharzia canaliculata (Rudolphi, 1819) Odhner, 1912. 2022. Research Square. Available online: https://doi.org/10.21203/rs.3.rs-1387572/v1 (accessed on 19 April 2022). [CrossRef]
- Ahmed, M.S.; Khalafalla, R.E.; Al-Brakati, A.; Yanai, T.; Elmahallawy, E.K. Descriptive Pathological Study of Avian Schistosomes Infection in Whooper Swans (Cygnus cygnus) in Japan. Animals 2020, 10, 2361. [Google Scholar] [CrossRef]
- Castellanos, Z.A.; Gaillard, M.C. Mollusca Gasterópoda: Chilinidae. In Fauna de Agua Dulce de la República Argentina; PROFADU (CONICET): Buenos Aires, Argentina; Volume 15, pp. 423–451.
- Flores, V.; Brant, S.V.; Loker, E.S. Avian Schistosomes from the South American Endemic Gastropod Genus Chilina (Pulmonata: Chilinidae), with a Brief Review of South American Schistosome Species. J. Parasitol. 2015, 101, 565–576. [Google Scholar] [CrossRef]
- Oyarzún-Ruiz, P.; Thomas, R.; Santodomingo, A.; Collado, G.; Muñoz, P.; Moreno, L. Morphological, Behavioral, and Molecular Characterization of Avian Schistosomes (Digenea: Schistosomatidae) in the Native Snail Chilina dombeyana (Chilinidae) from Southern Chile. Pathogens 2022, 11, 332. [Google Scholar] [CrossRef]
- Brant, S.V.; Loker, E.S. Schistosomes in the Southwest United States and Their Potential for Causing Cercarial Dermatitis or ‘Swimmer’s Itch. J. Helminthol. 2009, 83, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Brant, S.V.; Jouet, D.; Ferte, H.; Loker, E.S. Anserobilharzia gen. n. (Digenea, Schistosomatidae) and Redescription of A. brantae (Farr & Blankemeyer, 1956) comb. n. (Syn. Trichobilharzia brantae), a Parasite of Geese (Anseriformes). Zootaxa 2013, 3670, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Vusse, F.J.V. A Review of the Genus Dendritobilharzia Skrjabin and Zakharow 1920 (Trematoda: Schistosomatidae). J. Parasitol. 1980, 66, 814–822. [Google Scholar] [CrossRef]
- Leedom, W.S.; Short, R.B. Cercaria pomaceae sp. n., a Dermatitis-Producing Schistosome Cercaria from Pomacea paludosa, the Florida Apple Snail. J. Parasitol. 1981, 67, 257–261. [Google Scholar] [CrossRef]
- Schuster, R.K.; Aldhoun, J.A.; O’Donovan, D. Gigantobilharzia melanoidis n. sp. (Trematoda: Schistosomatidae) from Melanoides tuberculata (Gastropoda: Thiaridae) in the United Arab Emirates. Parasitol. Res. 2014, 113, 959–972. [Google Scholar] [CrossRef]
- Agrawal, M.C.; Gupta, S.; George, J. Cercarial Dermatitis in India. Bull. World Health Organ. 2000, 78, 278. [Google Scholar]
- Rao, V.G.; Dash, A.P.; Agrawal, M.C.; Yadav, R.S.; Anvikar, A.R.; Vohra, S.; Bhondeley, M.K.; Ukey, M.J.; Das, S.K.; Minocha, R.K.; et al. Cercarial Dermatitis in Central India: An Emerging Health Problem among Tribal Communities. Ann. Trop. Med. Parasitol. 2007, 101, 409–413. [Google Scholar] [CrossRef]
- Bont, J.D.; Aken, D.V.; Vercruysse, J.; Fransen, J.; Southgate, V.R.; Rollinson, D. The Prevalence and Pathology of Schistosoma nasale Rao, 1933 in Cattle in Sri Lanka. Parasitology 1989, 98, 197–202. [Google Scholar] [CrossRef]
- Hrádková, K.; Horák, P. Neurotropic Behaviour of Trichobilharzia regenti in Ducks and Mice. J. Helminthol. 2002, 76, 137–141. [Google Scholar] [CrossRef]
- Olivier, L. The Influence of Light on the Emergence of Schistosomatium douthitti Cercariae from Their Snail Host. J. Parasitol. 1951, 37, 201–204. [Google Scholar] [CrossRef]
- Kagan, I.G.; Short, R.B.; Nez, M.M. Maintenance of Schistosomatium douthitti (Cort, 1914) in the Laboratory (Trematoda: Schistosomatidae). J. Parasitol. 1954, 40, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Devkota, R.; Brant, S.V.; Thapa, S.; Loker, E.S. Two Avian Schistosome Cercariae from Nepal, Including a Macrobilharzia-like Species from Indoplanorbis exustus. Parasitol. Int. 2014, 63, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Malek, E.A.; Armstrong, J.C. Infection with Heterobilharzia americana in Primates. Am. J. Trop. Med. Hyg. 1967, 16, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Brant, S.V.; Cohen, A.N.; James, D.; Hui, L.; Hom, A.; Loker, E.S. Cercarial Dermatitis Transmitted by Exotic Marine Snail. Emerg. Infect. Dis. J. 2010, 16, 1357. [Google Scholar] [CrossRef]
- Barber, K.E.; Caira, J.N. Investigation of the Life Cycle and Adult Morphology of the Avian Blood Fluke Austrobilharzia variglandis (Trematoda: Schistosomatidae) from Connecticut. J. Parasitol. 1995, 81, 584–592. [Google Scholar] [CrossRef]
- Horák, P.; Mikeš, L.; Lichtenbergová, L.; Skála, V.; Soldánová, M.; Brant, S.V. Avian Schistosomes and Outbreaks of Cercarial Dermatitis. Clin. Microbiol. Rev. 2015, 28, 165–190. [Google Scholar] [CrossRef]
- Ashrafi, K.; Sharifdini, M.; Darjani, A.; Brant, S.V. Migratory Routes, Domesticated Birds and Cercarial Dermatitis: The Distribution of Trichobilharzia franki in Northern Iran. Parasite 2021, 28, 4. [Google Scholar] [CrossRef]
- Cieplok, A.; Spyra, A. The Roles of Spatial and Environmental Variables in the Appearance of a Globally Invasive Physa Acuta in Water Bodies Created Due to Human Activity. Sci. Total Environ. 2020, 744, 140928. [Google Scholar] [CrossRef]
- Früh, D.; Haase, P.; Stoll, S. Temperature Drives Asymmetric Competition between Alien and Indigenous Freshwater Snail Species, Physa Acuta and Physa Fontinalis. Aquat. Sci. 2017, 79, 187–195. [Google Scholar] [CrossRef]
- Strayer, D.L. Effects of Alien Species on Freshwater Mollusks in North America. J. N. Am. Benthol. Soc. 1999, 18, 74–98. [Google Scholar] [CrossRef]
- Riley, L.A.; Dybdahl, M.F.; Hall, R.O. Invasive Species Impact: Asymmetric Interactions between Invasive and Endemic Freshwater Snails. J. N. Am. Benthol. Soc. 2008, 27, 509–520. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Olden, J.D.; Solomon, C.T.; Vander Zanden, M.J. Interactions among Invaders: Community and Ecosystem Effects of Multiple Invasive Species in an Experimental Aquatic System. Oecologia 2009, 159, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.; Hetherington, J.; Schwarzman, L.; Vaughan, J. The Effect of Zebra Mussel Colonization on Native Snail Species of Douglas Lake in Northern Michigan. 2012. Deep Blue Documents, Research Collections, Biological Station, University of Michigan (UMBS). Available online: https://deepblue.lib.umich.edu/handle/2027.42/95922 (accessed on 19 April 2022).
- Kreps, T.A.; Baldridge, A.K.; Lodge, D.M. The Impact of an Invasive Predator (Orconectes rusticus) on Freshwater Snail Communities: Insights on Habitat-Specific Effects from a Multilake Long-Term Study. Can. J. Fish. Aquat. Sci. 2012, 69, 1164–1173. [Google Scholar] [CrossRef]
- Sckrabulis, J.P.; Flory, A.R.; Raffel, T.R. Direct Onshore Wind Predicts Daily Swimmer’s Itch (Avian Schistosome) Incidence at a Michigan Beach. Parasitology 2020, 147, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Rudko, S.P.; Reimink, R.L.; Froelich, K.; Gordy, M.A.; Blankespoor, C.L.; Hanington, P.C. Use of qPCR-Based Cercariometry to Assess Swimmer’s Itch in Recreational Lakes. EcoHealth 2018, 15, 827–839. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Dobson, A.; Lafferty, K.D.; Marcogliese, D.J.; Memmott, J.; Orlofske, S.A.; Poulin, R.; Thieltges, D.W. When Parasites Become Prey: Ecological and Epidemiological Significance of Eating Parasites. Trends Ecol. Evol. 2010, 25, 362–371. [Google Scholar] [CrossRef]
- Hobart, B.K.; Moss, W.E.; McDevitt-Galles, T.; Stewart Merrill, T.E.; Johnson, P.T.J. It’s a Worm-Eat-Worm World: Consumption of Parasite Free-Living Stages Protects Hosts and Benefits Predators. J. Anim. Ecol. 2022, 91, 35–45. [Google Scholar] [CrossRef]
- Rohr, J.R.; Civitello, D.J.; Halliday, F.W.; Hudson, P.J.; Lafferty, K.D.; Wood, C.L.; Mordecai, E.A. Towards Common Ground in the Biodiversity–Disease Debate. Nat. Ecol. Evol. 2020, 4, 24–33. [Google Scholar] [CrossRef]
- Laracuente, A.; Brown, R.A.; Jobin, W. Comparison of Four Species of Snails as Potential Decoys to Intercept Schistosome Miracidia. Am. J. Trop. Med. Hyg. 1979, 28, 99–105. [Google Scholar] [CrossRef]
- Marszewska, A.; Cichy, A.; Bulantová, J.; Horák, P.; Żbikowska, E. The Chemotactic Swimming Behavior of Bird Schistosome Miracidia in the Presence of Compatible and Incompatible Snail Hosts. PeerJ 2020, 8, e9487. [Google Scholar] [CrossRef]
- Żbikowska, E.; Stanicka, A.; Cichy, A.; Żbikowski, J. Can Potamopyrgus Antipodarum (Gastropoda) Affect the Prevalence of Trichobilharzia szidati in Lymnaea stagnalis Populations? Knowl. Manag. Aquat. Ecosyst. 2021, 15, 422. [Google Scholar] [CrossRef]
- Stanicka, A.; Migdalski, Ł.; Szopieray, K.; Cichy, A.; Jermacz, Ł.; Lombardo, P.; Żbikowska, E. Invaders as Diluents of the Cercarial Dermatitis Etiological Agent. Pathogens 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Laidemitt, M.R.; Anderson, L.C.; Wearing, H.J.; Mutuku, M.W.; Mkoji, G.M.; Loker, E.S. Antagonism between Parasites within Snail Hosts Impacts the Transmission of Human Schistosomiasis. eLife 2019, 8, e50095. [Google Scholar] [CrossRef] [PubMed]
- Adema, C.M.; Loker, E.S. Digenean-Gastropod Host Associations Inform on Aspects of Specific Immunity in Snails. Dev. Comp. Immunol. 2015, 48, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Prevalence of Schistosome Infections within Molluscan Populations: Observed Patterns and Theoretical Predictions. Parasitology 1979, 79, 63–94. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, E.; Marszewska, A. Thermal Preferences of Bird Schistosome Snail Hosts Increase the Risk of Swimmer’s Itch. J. Therm. Biol. 2018, 78, 22–26. [Google Scholar] [CrossRef] [PubMed]
- McMullen, D.B.; Beaver, P.C. Studies on Schistosome Dermatitis. IX. The Life Cycles of Three Dermatitis-Producing Schistosomes from Birds and a Discussion of the Subfamily Bilharziellinae (Trematoda: Schistosomatidae). Am. J. Hyg. 1945, 42, 128–154. [Google Scholar]
- Horák, P.; Kolářová, L.; Adema, C. Biology of the Schistosome Genus Trichobilharzia. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 52, pp. 155–233. ISBN 978-0-12-031752-3. [Google Scholar]
- Cort, W.W.; Mcmullen, D.B.; Olivier, L.; Brackett, S. Studies on Schistosome Dermatitis: VII. Seasonal Incidence of Cercaria stagnicolae Talbot, 1936, in Relation to the Life Cycle of Its Snail Host, Stagnicola emarginata (Soweeby). Am. J. Epidemiol. 1940, 32, 33–69. [Google Scholar] [CrossRef]
- Clampitt, P. Comparative Ecology of the Snails Physa gyrina and Physa integra (Basommatophora: Physidae). Malacologia 1970, 10, 113–151. [Google Scholar]
- Hodasi, J.K.M. The Effects of Low Temperature on Lymnaea trunculata. Z. Parasitenkd. 1976, 48, 281–286. [Google Scholar] [CrossRef]
- Rudolfová, J.; Hampl, V.; Bayssade-Dufour, C.; Lockyer, A.E.; Littlewood, D.T.J.; Horák, P. Validity Reassessment of Trichobilharzia Species Using Lymnaea stagnalis as the Intermediate Host. Parasitol. Res. 2005, 95, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bourns, T.K.R.; Ellis, J.C.; Rau, M.E. Migration and Development of Trichobilharzia ocellata (Trematoda: Schistosomatidae) in Its Duck Hosts. Can. J. Zool. 1973, 51, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Brant, S.V.; Loker, E.S. Molecular Systematics of the Avian Schistosome Genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. J. Parasitol. 2009, 95, 941–963. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.E.; Bourns, T.K.R.; Ellis, J.C. Egg Production by Trichobilharzia ocellata (Trematoda: Schistosomatidae) after Initial and Challenge Infection in Ducks. Can. J. Zool. 1975, 53, 642–650. [Google Scholar] [CrossRef]
- Ellis, J.C.; Bourns, T.K.R.; Rau, M.E. Migration, Development, and Condition of Trichobilharzia ocellata (Trematoda: Schistosomatidae) in Homologous Challenge Infections. Can. J. Zool. 1975, 53, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, E.A.; Huyer, A.R.; Mooij, J.H. Maintenance of the Life Cycle of Trichobilharzia ocellata via the Duck Anas platyrhynchos and the Pond Snail Lymnaea stagnalis. Neth. J. Zool. 1983, 34, 414–417. [Google Scholar] [CrossRef]
- Soldánová, M.; Selbach, C.; Sures, B. The Early Worm Catches the Bird? Productivity and Patterns of Trichobilharzia szidati Cercarial Emission from Lymnaea stagnalis. PLoS ONE 2016, 11, e0149678. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild–Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Ogawa, S.; Yamamoto, Y.; Yamada, M.; Mase, M.; Nakamura, K. Pathology of Whooper Swans (Cygnus cygnus) Infected with H5N1 Avian Influenza Virus in Akita, Japan, in 2008. J. Vet. Med. Sci. 2009, 71, 1377–1380. [Google Scholar] [CrossRef][Green Version]
- Rudko, S.P.; Reimink, R.R.; Peter, B.; White, J.; Hanington, P.C. Democratizing Water Monitoring: Implementation of a Community-Based qPCR Monitoring Program for Recreational Water Hazards. PLoS ONE 2020, 15, e0229701. [Google Scholar] [CrossRef]
- Kamel, B.; Laidemitt, M.R.; Lu, L.; Babbitt, C.; Weinbaum, O.L.; Mkoji, G.M.; Loker, E.S. Detecting and Identifying Schistosoma Infections in Snails and Aquatic Habitats: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009175. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Mull, B.J.; Brant, S.V.; Loker, E.S.; Collinson, J.; Secor, W.E.; Hill, V.R. Real-Time PCR and Sequencing Assays for Rapid Detection and Identification of Avian Schistosomes in Environmental Samples. Appl. Environ. Microbiol. 2015, 81, 4207–4215. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, K.G.; Gordon, C.A.; McManus, D.P. DNA Diagnostics for Schistosomiasis Control. Trop. Med. Infect. Dis. 2018, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Born-Torrijos, A.; Poulin, R.; Raga, J.A.; Holzer, A.S. Estimating Trematode Prevalence in Snail Hosts Using a Single-Step Duplex PCR: How Badly Does Cercarial Shedding Underestimate Infection Rates? Parasit. Vectors 2014, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Huver, J.R.; Koprivnikar, J.; Johnson, P.T.J.; Whyard, S. Development and Application of an eDNA Method to Detect and Quantify a Pathogenic Parasite in Aquatic Ecosystems. Ecol. Appl. 2015, 25, 991–1002. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Hellström, M.; Kariuki, H.C.; Olsen, A.; Thomsen, P.F.; Mejer, H.; Willerslev, E.; Mwanje, M.T.; Madsen, H.; Kristensen, T.K.; et al. Environmental DNA for Improved Detection and Environmental Surveillance of Schistosomiasis. Proc. Natl. Acad. Sci. USA 2019, 116, 8931–8940. [Google Scholar] [CrossRef]
- Alzaylaee, H.; Collins, R.A.; Rinaldi, G.; Shechonge, A.; Ngatunga, B.; Morgan, E.R.; Genner, M.J. Schistosoma Species Detection by Environmental DNA Assays in African Freshwaters. PLoS Negl. Trop. Dis. 2020, 14, e0008129. [Google Scholar] [CrossRef]
- Archer, J.; Barksby, R.; Pennance, T.; Rostron, P.; Bakar, F.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; et al. Analytical and Clinical Assessment of a Portable, Isothermal Recombinase Polymerase Amplification (RPA) Assay for the Molecular Diagnosis of Urogenital Schistosomiasis. Molecules 2020, 25, 4175. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Lo, N.C.; Gurarie, D.; Yoon, N.; Coulibaly, J.T.; Bendavid, E.; Andrews, J.R.; King, C.H. Impact and Cost-Effectiveness of Snail Control to Achieve Disease Control Targets for Schistosomiasis. Proc. Natl. Acad. Sci. USA 2018, 115, E584–E591. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Lafferty, K.D.; Kuris, A.M.; Hsieh, M.H.; De Leo, G.A. To Reduce the Global Burden of Human Schistosomiasis, Use ‘Old Fashioned’ Snail Control. Trends Parasitol. 2018, 34, 23–40. [Google Scholar] [CrossRef]
- Knopp, S.; Mohammed, K.A.; Ali, S.M.; Khamis, I.S.; Ame, S.M.; Albonico, M.; Gouvras, A.; Fenwick, A.; Savioli, L.; Colley, D.G.; et al. Study and Implementation of Urogenital Schistosomiasis Elimination in Zanzibar (Unguja and Pemba Islands) Using an Integrated Multidisciplinary Approach. BMC Public Health 2012, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Froelich, K.L.; Reimink, R.L.; Rudko, S.P.; VanKempen, A.P.; Hanington, P.C. Evaluation of Targeted Copper Sulfate (CuSO4) Application for Controlling Swimmer’s Itch at a Freshwater Recreation Site in Michigan. Parasitol. Res. 2019, 118, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Slootweg, R.; Malek, E.A.; McCullough, F.S. The Biological Control of Snail Intermediate Hosts of Schistosomiasis by Fish. Rev. Fish Biol. Fish. 1994, 4, 67–90. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Wood, C.L.; Jones, I.J.; Chamberlin, A.J.; Jouanard, N.; Faye, D.S.; Kuris, A.M.; Riveau, G.; De Leo, G.A.; Sokolow, S.H. Potential Biological Control of Schistosomiasis by Fishes in the Lower Senegal River Basin. Am. J. Trop. Med. Hyg. 2019, 100, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Blankespoor, C.L.; Reimink, R.L.; Blankespoor, H.D. Efficacy of Praziquantel in Treating Natural Schistosome Infections in Common Mergansers. J. Parasitol. 2001, 87, 424–426. [Google Scholar] [CrossRef]
- Reimink, R.L.; DeGoede, J.A.; Blankespoor, H.D. Efficacy of Praziquantel in Natural Populations of Mallards Infected with Avian Schistosomes. J. Parasitol. 1995, 81, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Sturrock, R.F.; Kariuki, H.C.; Hamburger, J. Transmission Control for Schistosomiasis—Why It Matters Now. Trends Parasitol. 2006, 22, 575–582. [Google Scholar] [CrossRef]
- Blankespoor, H.D.; Reimink, R.L. The Control of Swimmer’s Itch in Michigan: Past, Present, and Future. Mich. Acad. 1991, 24, 7–23. [Google Scholar]
- Hanington, P.C.; Reimink, R.L. Preventing Swimmer’s Itch with 2020 Vision. Freshwater Solutions LLC. Available online: http://www.intermediatelake.org/itch/2020_vision.pdf (accessed on 19 April 2022).
- Lindblade, K.A. The Epidemiology of Cercarial Dermatitis and Its Association with Limnological Characteristics of a Northern Michigan Lake. J. Parasitol. 1998, 84, 19–23. [Google Scholar] [CrossRef]
- Wulff, C.; Haeberlein, S.; Haas, W. Cream Formulations Protecting against Cercarial Dermatitis by Trichobilharzia. Parasitol. Res. 2007, 101, 91–97. [Google Scholar] [CrossRef]
- Maier, T.; Wheeler, N.J.; Namigai, E.K.O.; Tycko, J.; Grewelle, R.E.; Woldeamanuel, Y.; Klohe, K.; Perez-Saez, J.; Sokolow, S.H.; De Leo, G.A.; et al. Gene Drives for Schistosomiasis Transmission Control. PLoS Negl. Trop. Dis. 2019, 13, e0007833. [Google Scholar] [CrossRef] [PubMed]
- Dheilly, N.M.; Lucas, P.; Blanchard, Y.; Rosario, K. A World of Viruses Nested within Parasites: Unraveling Viral Diversity within Parasitic Flatworms (Platyhelminthes). bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.C.; Mota, E.A.; Pereira, R.V.; Oliveira, V.F.; Costa, M.P.; de Gomes, M.S.; Jannotti-Passos, L.K.; Borges, W.C.; Guerra-Sá, R. Differential Expression Profiles of miRNAs and Their Putative Targets in Schistosoma mansoni during Its Life Cycle. Mem. Inst. Oswaldo Cruz 2021, 116, e200326. [Google Scholar] [CrossRef] [PubMed]
- Galaway, F.; Yu, R.; Constantinou, A.; Prugnolle, F.; Wright, G.J. Resurrection of the Ancestral RH5 Invasion Ligand Provides a Molecular Explanation for the Origin of P. falciparum Malaria in Humans. PLoS Biol. 2019, 17, e3000490. [Google Scholar] [CrossRef]
- Siao, M.C.; Borner, J.; Perkins, S.L.; Deitsch, K.W.; Kirkman, L.A. Evolution of Host Specificity by Malaria Parasites through Altered Mechanisms Controlling Genome Maintenance. mBio 2020, 11, e03272-19. [Google Scholar] [CrossRef]
- Mourier, T.; de Alvarenga, D.A.M.; Kaushik, A.; de Pina-Costa, A.; Douvropoulou, O.; Guan, Q.; Guzmán-Vega, F.J.; Forrester, S.; de Abreu, F.V.S.; Júnior, C.B.; et al. The Genome of the Zoonotic Malaria Parasite Plasmodium simium Reveals Adaptations to Host Switching. BMC Biol. 2021, 19, 219. [Google Scholar] [CrossRef]
- Luo, X.; Cui, K.; Wang, Z.; Li, Z.; Wu, Z.; Huang, W.; Zhu, X.-Q.; Ruan, J.; Zhang, W.; Liu, Q. High-Quality Reference Genome of Fasciola gigantica: Insights into the Genomic Signatures of Transposon-Mediated Evolution and Specific Parasitic Adaption in Tropical Regions. PLoS Negl. Trop. Dis. 2021, 15, e0009750. [Google Scholar] [CrossRef]
- Berger, D.J.; Crellen, T.; Lamberton, P.H.L.; Allan, F.; Tracey, A.; Noonan, J.D.; Kabatereine, N.B.; Tukahebwa, E.M.; Adriko, M.; Holroyd, N.; et al. Whole-Genome Sequencing of Schistosoma mansoni Reveals Extensive Diversity with Limited Selection despite Mass Drug Administration. Nat. Commun. 2021, 12, 4776. [Google Scholar] [CrossRef]
- Nikolakis, Z.L.; Hales, N.R.; Perry, B.W.; Schield, D.R.; Timm, L.E.; Liu, Y.; Zhong, B.; Kechris, K.J.; Carlton, E.J.; Pollock, D.D.; et al. Patterns of Relatedness and Genetic Diversity Inferred from Whole Genome Sequencing of Archival Blood Fluke Miracidia (Schistosoma japonicum). PLoS Negl. Trop. Dis. 2021, 15, e0009020. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S.; Mohmad, A.; Fular, A.; Parthasarathi, B.C.; Chaubey, A.K. Molecular Tools-Advances, Opportunities and Prospects for the Control of Parasites of Veterinary Importance. Int. J. Trop. Insect Sci. 2021, 41, 33–42. [Google Scholar] [CrossRef]
- Haas, W.; Pietsch, U. Migration of Trichobilharzia ocellata Schistosomula in the Duck and in the Abnormal Murine Host. Parasitol. Res. 1991, 77, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, E. Is There a Potential Danger of swimmer’s Itch in Poland? Parasitol. Res. 2002, 89, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Gordy, M.A.; Koprivnikar, J.; McPhail, B.; Hanington, P.C. Environmental and Ecological Factors Driving Trematode Parasite Community Assembly in Central Alberta Lakes. Int. J. Parasitol. Parasites Wildl. 2020, 13, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Al-Jubury, A.; Kania, P.; Bygum, A.; Buchmann, K. Temperature and Light Effects on Trichobilharzia szidati Cercariae with Implications for a Risk Analysis. Acta Vet. Scand. 2020, 62, 54. [Google Scholar] [CrossRef]
- Al-Jubury, A.; Duan, Y.; Kania, P.W.; Tracz, E.S.; Bygum, A.; Jørgensen, L.v.G.; Horák, P.; Buchmann, K. Avian Schistosome Species in Danish Freshwater Lakes: Relation to Biotic and Abiotic Factors. J. Helminthol. 2021, 95, e22. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Climate Change Effects on Trematodiases, with Emphasis on Zoonotic Fascioliasis and Schistosomiasis. Vet. Parasitol. 2009, 163, 264–280. [Google Scholar] [CrossRef]
- Hesselschwerdt, J.; Wantzen, K.M. Global Warming May Lower Thermal Barriers against Invasive Species in Freshwater Ecosystems—A Study from Lake Constance. Sci. Total Environ. 2018, 645, 44–50. [Google Scholar] [CrossRef]
- Peirce, J.P.; Pellett, J.J.; Sandland, G.J. A Mathematical Model for the Control of Swimmer’s Itch. Nat. Resour. Model. 2020, 33, e12275. [Google Scholar] [CrossRef]
- Nono, J.K.; Kamdem, S.D.; Musaigwa, F.; Nnaji, C.A.; Brombacher, F. Influence of Schistosomiasis on Host Vaccine Responses. Trends Parasitol. 2022, 38, 67–79. [Google Scholar] [CrossRef]
- Pointier, J.-P.; David, P.; Jarne, P. The Biological Control of the Snail Hosts of Schistosomes: The Role of Competitor Snails and Biological Invasions. In Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; pp. 215–238. ISBN 978-1-4419-7028-2. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loker, E.S.; DeJong, R.J.; Brant, S.V. Scratching the Itch: Updated Perspectives on the Schistosomes Responsible for Swimmer’s Itch around the World. Pathogens 2022, 11, 587. https://doi.org/10.3390/pathogens11050587
Loker ES, DeJong RJ, Brant SV. Scratching the Itch: Updated Perspectives on the Schistosomes Responsible for Swimmer’s Itch around the World. Pathogens. 2022; 11(5):587. https://doi.org/10.3390/pathogens11050587
Chicago/Turabian StyleLoker, Eric S., Randall J. DeJong, and Sara V. Brant. 2022. "Scratching the Itch: Updated Perspectives on the Schistosomes Responsible for Swimmer’s Itch around the World" Pathogens 11, no. 5: 587. https://doi.org/10.3390/pathogens11050587
APA StyleLoker, E. S., DeJong, R. J., & Brant, S. V. (2022). Scratching the Itch: Updated Perspectives on the Schistosomes Responsible for Swimmer’s Itch around the World. Pathogens, 11(5), 587. https://doi.org/10.3390/pathogens11050587





