Detection of Leishmania spp. in Chronic Dermatitis: Retrospective Study in Exposed Horse Populations
Abstract
1. Introduction
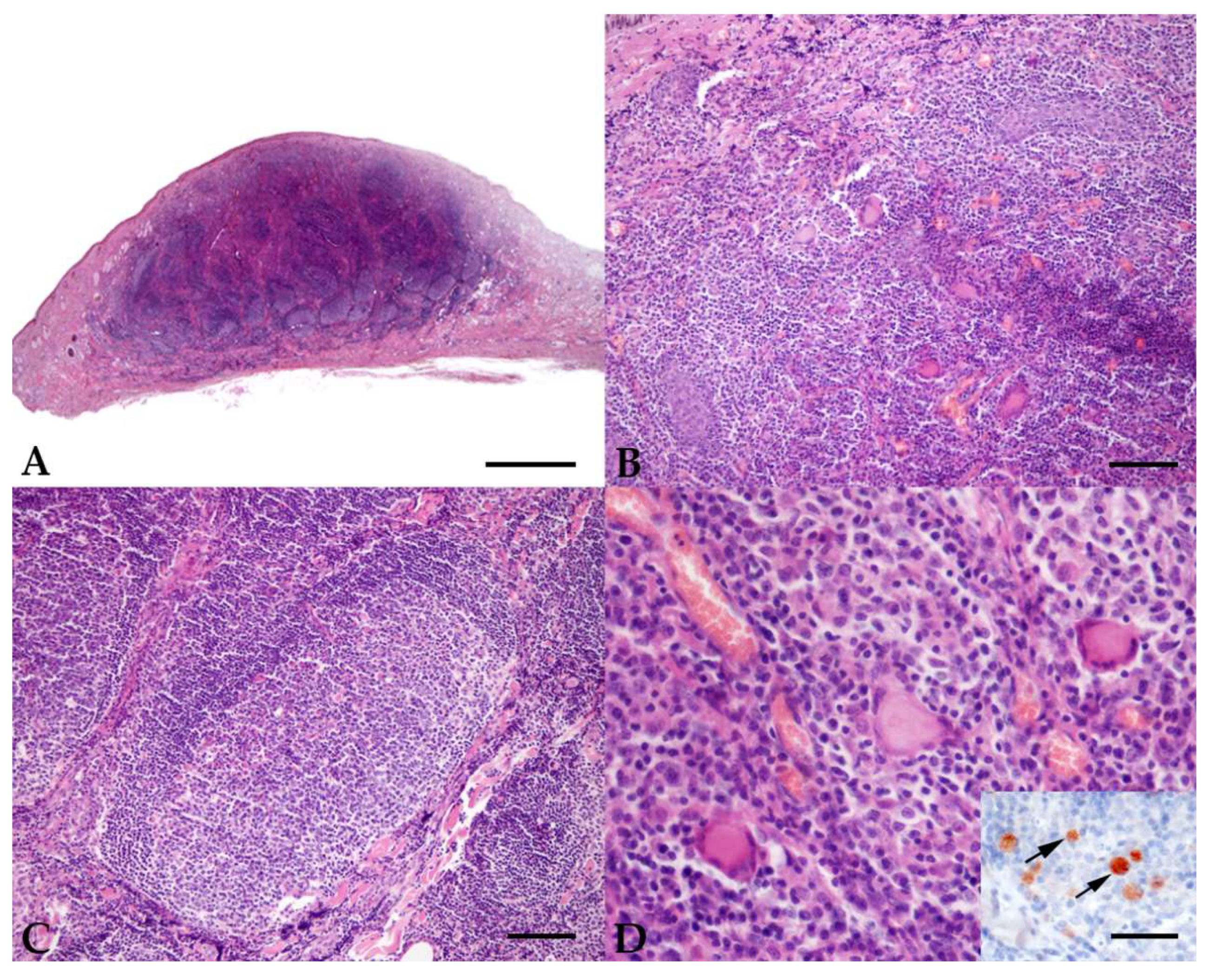
2. Results
3. Discussion
4. Materials and Methods
4.1. Skin Sampling Collection
4.2. Histopathology and Immunohistochemistry
4.3. Molecular Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Leishmaniasis. 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis (accessed on 30 December 2021).
- Maroli, M.; Rossi, L.; Baldelli, R.; Capelli, G.; Ferroglio, E.; Genchi, C.; Gramiccia, M.; Mortarino, M.; Pietrobelli, M.; Gradoni, L. The northward spread of leishmaniasis in Italy: Evidence from retrospective and ongoing studies on canine reservoir and phlebotomine vectors. Trop. Med. Int. Health 2008, 13, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Morosetti, G.; Toson, M.; Trevisiol, K.; Idrizi, I.; Natale, A.; Lucchese, L.; Michelutti, A.; Ceschi, P.; Lorenzi, G.; Piffer, C.; et al. Canine leishmaniosis in the Italian northeastern Alps: A survey to assess serological prevalence in dogs and distribution of phlebotomine sand flies in the Autonomous Province of Bolzano—South Tyrol, Italy. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100432. [Google Scholar] [CrossRef] [PubMed]
- Lobsiger, L.; Müller, N.; Schweizer, T.; Frey, C.F.; Wiederkehr, D.; Zumkehr, B.; Gottstein, B. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet. Parasitol. 2010, 169, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Ferroglio, E.; Solano-Gallego, L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014, 113, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Iatta, R.; Furlanello, T.; Colella, V.; Tarallo, V.D.; Latrofa, M.S.; Brianti, E.; Trerotoli, P.; Decaro, N.; Lorusso, E.; Schunack, B.; et al. A nationwide survey of Leishmania Infantum infection in cats and associated risk factors in Italy. PLoS Negl. Trop. Dis. 2019, 13, e0007594. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Sassi, A. Infection of the equine population by Leishmania parasites. Equine Vet. J. 2020, 52, 28–33. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Persichetti, M.F. Feline leishmaniosis: Is the cat a small dog? Vet. Parasitol. 2018, 251, 131–137. [Google Scholar] [CrossRef]
- Mazza, S. Leishmaniasis cutánea en el caballo y nueva observación de la misma en el perro. Bol. Univ. B. Aires 1927, 3, 462–464. [Google Scholar]
- Bonfante-Garrido, R.; Melendez, E.C.; Torres, R.A.; Morillo, N.C.; Arredondo, C.C.; Urdaneta, I. Enzootic equine cutaneous leishmaniasis in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 471. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Fernandez, E.; De Fernandez, R.; Deane, L.M. Study of an Outbreak of Cutaneous Leishmaniasis in Venezuela. The Role of Domestic Animals. Mem. Inst. Oswaldo Cruz. 1984, 79, 181–195. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Ferreira, E.R.; Deane, L.M. Cutaneous Leishmaniasis is frequent in equines from an endemic area in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 1986, 81, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Vexenat, J.A.; Barretto, A.C.; de Cassia, O.C.; Rosa, A. Infecção Experimental de Lutzomyia Whitmani Em Cães Infectados Com Leishmania Braziliensis Braziliensis. Mem. Inst. Oswaldo Cruz 1986, 81, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Stechele, M.; Hetzel, U.; Domingo, M.; Schönian, G.; Zahner, H.; Burkhardt, E. Cutaneous Leishmaniosis in a Horse in Southern Germany Caused by Leishmania Infantum. Vet. Parasitol. 2002, 109, 9–17. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Fernández-Bellon, H.; Serra, P.; Gállego, M.; Ramis, A.; Fondevila, D.; Ferrer, L. Cutaneous Leishmaniosis in three horses in Spain. Equine Vet. J. 2003, 35, 320–323. [Google Scholar] [CrossRef]
- Rolào, N.; Martins, M.J.; Joào, A.; Campino, L. Equine Infection with Leishmania in Portugal. Parasite 2005, 12, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.M.; Ferreira, E.R.; Grimaldi, G.; Momem, H. Human, Canine and Equine Leishmaniasis Caused by Leishmania braziliensis braziliensis in an Endemic Area in the State of Rio de Janeiro. Mem. Inst. Oswaldo Cruz 1987, 82, 143. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Ortiz-Santiago, B.; Segalès, J.; Dunstan, R.W. Cutaneous Leishmaniasis in Two Horses. Vet. Pathol. 1996, 33, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, O.A.; Serrano, M.G.; Camargo, E.P.; Teixeira, M.M.G.; Shaw, J.J. An Appraisal of the Taxonomy and Nomenclature of Trypanosomatids Presently Classified as Leishmania and Endotrypanum. Parasitology 2018, 145, 430–442. [Google Scholar] [CrossRef]
- Müller, N.; Welle, M.; Lobsiger, L.; Stoffel, M.H.; Boghenbor, K.K.; Hilbe, M.; Gottstein, B.; Frey, C.F.; Geyer, C.; von Bomhard, W. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet. Parasitol. 2009, 166, 346–351. [Google Scholar] [CrossRef]
- Reuss, S.M.; Dunbar, M.D.; Calderwood Mays, M.B.; Owen, J.L.; Mallicote, M.F.; Archer, L.L.; Wellehan, J.F., Jr. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg. Infect. Dis. 2012, 18, 1545–1547. [Google Scholar] [CrossRef]
- Limeira, C.H.; Alves, C.J.; De Azevedo, S.S.; De Souza, C.; Santos, A.B.; De Melo, M.A.; Soares, R.R.; Da Costa Barnabé, N.N.; De Queiroz Rodrigues, G. Clinical aspects and diagnosis of leishmaniasis in equids: A systematic review and meta-analysis. Rev. Bras. Parasitol. Vet. 2019, 28, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.C.; Campos, M.P.; Popielarczyk, M.; Kiupel, M. Cutaneous leishmaniosis caused by Leishmania martiniquensis in a horse in Florida. J. Comp. Pathol. 2019, 173, 13–18. [Google Scholar] [CrossRef]
- Falqueto, A.; Malta, J.B.V.; Sessa, P.A. Cutaneous leishmaniasis in a horse (Equus caballus) from endemic area in the state of Espirito Santo, Brazil. Mem. Inst. Oswaldo Cruz 1987, 82, 443. [Google Scholar] [CrossRef] [PubMed]
- Vedovello, F.D.; Jorge, F.A.; Lonardoni, M.V.C.; Teodoro, U.; Silveira, T.G.V. American cutaneous leishmaniasis in horses from endemic areas in the North-Central mesoregion of Paraná State, Brazil. Zoonoses Public Health 2008, 55, 149–155. [Google Scholar]
- Soares, I.R.; Silva, S.O.; Moraghi Moreira, F.; Gavião Prado, L.; Fantini, P.; de Pino Albuquerque Maranhão, R.; da Silva Filho, J.M.; Norma Melo, M.; Palhares, M.S. First evidence of autochthonous cases of Leishmania (Leishmania) infantum in horse (Equus Caballus) in the Americas and mixed infection of Leishmania infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2013, 197, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Santos, E.G.O.; Marzochi, M.C.A.; Urtado, W.; Queiros, F.; Chicarino, J.; Pacheco, R.S. Leishmaniasis dissemineted by Leishmania braziliensis in a mare (Equus cabalus) immunotherapy and chemotherapy assays. Mem. Inst. Oswaldo Cruz. 1994, 89, 217–220. [Google Scholar] [CrossRef]
- Fernández-Bellon, H.; Solano-Gallego, L.; Bardagí, M.; Alberola, J.; Ramis, A.; Ferrer, L. Immuneresponse to Leishmania infantum in healthy horses in Spain. Vet. Parasitol. 2006, 135, 181–185. [Google Scholar] [CrossRef]
- Sgorbini, M.; Bonelli, F.; Pizzolli, I.; Tognetti, R.; Corazza, M. Seroprevalence of Leishmania sp. infection in healthy horses housed in endemic areas in Tuscany. J. Equine Vet. Sci. 2014, 34, 572–574. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Bertero, F.; Moretta, I.; Morganti, G.; Mortarino, M.; Villa, L.; Zanzani, S.A.; Morandi, B.; Rinnovati, R.; Vitale, F.; et al. Detecting antibodies to Leishmania infantum in horses from areas with different epizooticity levels of canine leishmaniosis and a retrospective revision of Italian data. Parasit. Vectors 2020, 13, 530. [Google Scholar] [CrossRef]
- Di Muccio, T.; Scalone, A.; Bruno, A.; Marangi, M.; Grande, R.; Armignacco, O.; Gradoni, L.; Gramiccia, M. Epidemiology of imported leishmaniasis in Italy: Implications for a European endemic country. PLoS ONE 2015, 10, e0129418. [Google Scholar]
- Ferroglio, E.; Battisti, E.; Zanet, S.; Bolla, C.; Concialdi, E.; Trisciuoglio, A.; Khalili, S.; Biglino, A. Epidemiological evaluation of Leishmania infantum zoonotic transmission risk in the recently established endemic area of Northwestern Italy. Zoonoses Public Health 2018, 65, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sauda, F.; Malandrucco, L.; Macrì, G.; Scarpulla, M.; De Liberato, C.; Terracciano, G.; Fichi, G.; Berrilli, F.; Perrucci, S. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in Central Italy. Parasite 2018, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasit. Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.P.; Sousa, S.; Dubey, J.P.; Ribeiro, A.J.; Silvestre, R.; Cotovio, M.; Schallig, H.D.F.H.; Cardoso, L.; Cordeiro-Da-Silva, A. Prevalence of antibodies to Leishmania infantum and Toxoplasma gondii in horses from the North of Portugal. Parasit. Vectors 2013, 6, 9–12. [Google Scholar] [CrossRef]
- Postigo, J.A. Leishmaniasis in the world health organization eastern Mediterranean region. Int. J. Antimicrob. Agents 2010, 36, S62–S65. [Google Scholar] [CrossRef]
- Ntais, P.; Sifaki-Pistola, D.; Christodoulou, V.; Messaritakis, I.; Pratlong, F.; Poupalos, G.; Antoniou, M. Leishmaniases in Greece. Am. J. Trop. Med. Hyg. 2013, 89, 906–915. [Google Scholar] [CrossRef]
- Ortega-García, M.V.; Salguero, F.J.; García, N.; Domínguez, M.; Moreno, I.; Berrocal, A. Equine infection with Leishmania spp. in Costa Rica: Study of five cases. Vet. Med. Sci. 2021, 7, 2234–2239. [Google Scholar] [CrossRef]
- Porcellato, I.; Morganti, G.; Antognoni, M.T.; Walczak, K.M.; De Arcangeli, S.; Furlanello, T.; Quattrone, C.B.; Veronesi, F.; Brachelente, C. Comparison of immunohistochemical and qPCR methods from granulomatous dermatitis lesions for detection of Leishmania in dogs living in endemic areas: A preliminary study. Parasit. Vectors 2022, 24, 104. [Google Scholar] [CrossRef]
- Gama, A.; Elias, J.; Ribeiro, A.J.; Alegria, N.; Schallig, H.D.; Silva, F.; Santarém, N.; Cardoso, L.; Cotovio, M. Cutaneous leishmaniosis in a horse from northern Portugal. Vet. Parasitol. 2014, 200, 189–192. [Google Scholar] [CrossRef]
- Carragher, D.M.; Rangel-Moreno, J.; Randall, T.D. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008, 20, 26–42. [Google Scholar] [CrossRef]
- Yavuzer, R.; Akyürek, N.; Ozmen, S.; Demirtaş, Y.; Ataoğlu, O. Leishmania cutis with B-cell cutaneous hyperplasia. Plast. Reconstr. Surg. 2001, 108, 2177–2178. [Google Scholar] [CrossRef] [PubMed]
- Flaig, M.J.; Rupec, R.A. Cutaneous pseudolymphoma in association with Leishmania donovani. Br. J. Dermatol. 2007, 157, 1042–1043. [Google Scholar] [CrossRef]
- Recalcati, S.; Vezzoli, P.; Girgenti, V.; Venegoni, L.; Veraldi, S.; Berti, E. Cutaneous lymphoid hyperplasia associated with Leishmania panamensis infection. Acta Derm. Venereol. 2010, 90, 418–419. [Google Scholar]
- Baneth, G.; Aroch, I. Canine Leishmaniasis: A Diagnostic and Clinical Challenge. Vet. J. 2008, 175, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.K.; Diakou, A.; Kanzoura, V.; Papadopoulos, E.; Gajadhar, A.A.; Theodoropoulos, G. A seroepidemiological study of exposure to Toxoplasma, Leishmania, Echinococcus and Trichinella in equids in Greece and analysis of risk factors. Vet. Parasitol. 2010, 170, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, G.; Habluetzel, A.; Khoury, C.; Maroli, M. Host preferences of phlebotomine sand flies at a hypoendemic focus of canine leishmaniasis in central Italy. Acta Trop. 2003, 88, 109–116. [Google Scholar] [CrossRef]
- Francino, O.; Altet, L.; Sánchez-Robert, E.; Rodriguez, A.; Solano-Gallego, L.; Alberola, J.; Ferrer, L.; Sánchez, A.; Roura, X. Advantages of Real-Time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet. Parasitol. 2006, 137, 214–221. [Google Scholar] [CrossRef]
- El Tai, N.O.; Osman, O.F.; El Fari, M.; Presber, W.; Schönian, G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 575–579. [Google Scholar] [CrossRef]
- Vitale, F.; Reale, S.; Vitale, M.; Petrotta, E.; Torina, A.; Caracappa, S. TaqMan-Based detection of Leishmania infantum DNA using canine samples. Ann. N. Y. Acad. Sci. 2004, 1026, 139–143. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
| Gene Target | Primers | Product (bp) | Annealing t° | Cycling Protocol | Cycle Numbers | Reference |
|---|---|---|---|---|---|---|
| k-DNA | F 5′-AACTTTTCTGGTCCTCCGGG-3′ R 5′-CCCCCAGTTTCCCGCCC-3′ | 120 | 58 °C | denaturation 94 °C for 60 s annealing 58 °C for 30 s extension 72 °C for 30 s | 35 | Francino et al., 2006 [48] |
| ITS-1 | F 5′-CTGGATCATTTTCCGATG-3′ R 5′-TGATACCACTTATCGCACTT-3′ | 330 | 51 °C | denaturation 94 °C for 30 s annealing 51 °C for 45 s extension 72 °C for 60 s | 35 | El Tai et al., 2000 [49] |
| k-DNA (qPCR) | F 5′-GGCGTTCTGCGAAAACCG-3′ R 5′-AAAATGGCATTTTCGGGCC-3′ probe 5′ Fam-TGGGTGCAGAAATCCCGTTCA-3′ BHQ1 | 68 | 60 °C | denaturation 95 °C for 15 s annealing 60 °C for 60 s extension 72 °C for 30 s | 40 | Vitale et al., 2004 [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzonis, A.L.; Morganti, G.; Porcellato, I.; Roccabianca, P.; Avallone, G.; Gavaudan, S.; Canonico, C.; Rigamonti, G.; Brachelente, C.; Veronesi, F. Detection of Leishmania spp. in Chronic Dermatitis: Retrospective Study in Exposed Horse Populations. Pathogens 2022, 11, 634. https://doi.org/10.3390/pathogens11060634
Gazzonis AL, Morganti G, Porcellato I, Roccabianca P, Avallone G, Gavaudan S, Canonico C, Rigamonti G, Brachelente C, Veronesi F. Detection of Leishmania spp. in Chronic Dermatitis: Retrospective Study in Exposed Horse Populations. Pathogens. 2022; 11(6):634. https://doi.org/10.3390/pathogens11060634
Chicago/Turabian StyleGazzonis, Alessia Libera, Giulia Morganti, Ilaria Porcellato, Paola Roccabianca, Giancarlo Avallone, Stefano Gavaudan, Cristina Canonico, Giulia Rigamonti, Chiara Brachelente, and Fabrizia Veronesi. 2022. "Detection of Leishmania spp. in Chronic Dermatitis: Retrospective Study in Exposed Horse Populations" Pathogens 11, no. 6: 634. https://doi.org/10.3390/pathogens11060634
APA StyleGazzonis, A. L., Morganti, G., Porcellato, I., Roccabianca, P., Avallone, G., Gavaudan, S., Canonico, C., Rigamonti, G., Brachelente, C., & Veronesi, F. (2022). Detection of Leishmania spp. in Chronic Dermatitis: Retrospective Study in Exposed Horse Populations. Pathogens, 11(6), 634. https://doi.org/10.3390/pathogens11060634










