Experimental Hut Trials Reveal That CYP6P9a/b P450 Alleles Are Reducing the Efficacy of Pyrethroid-Only Olyset Net against the Malaria Vector Anopheles funestus but PBO-Based Olyset Plus Net Remains Effective
Abstract
:1. Introduction
2. Results
2.1. Insecticide Susceptibility of Lab Strain and Crosses with WHO Cone and Tube Test
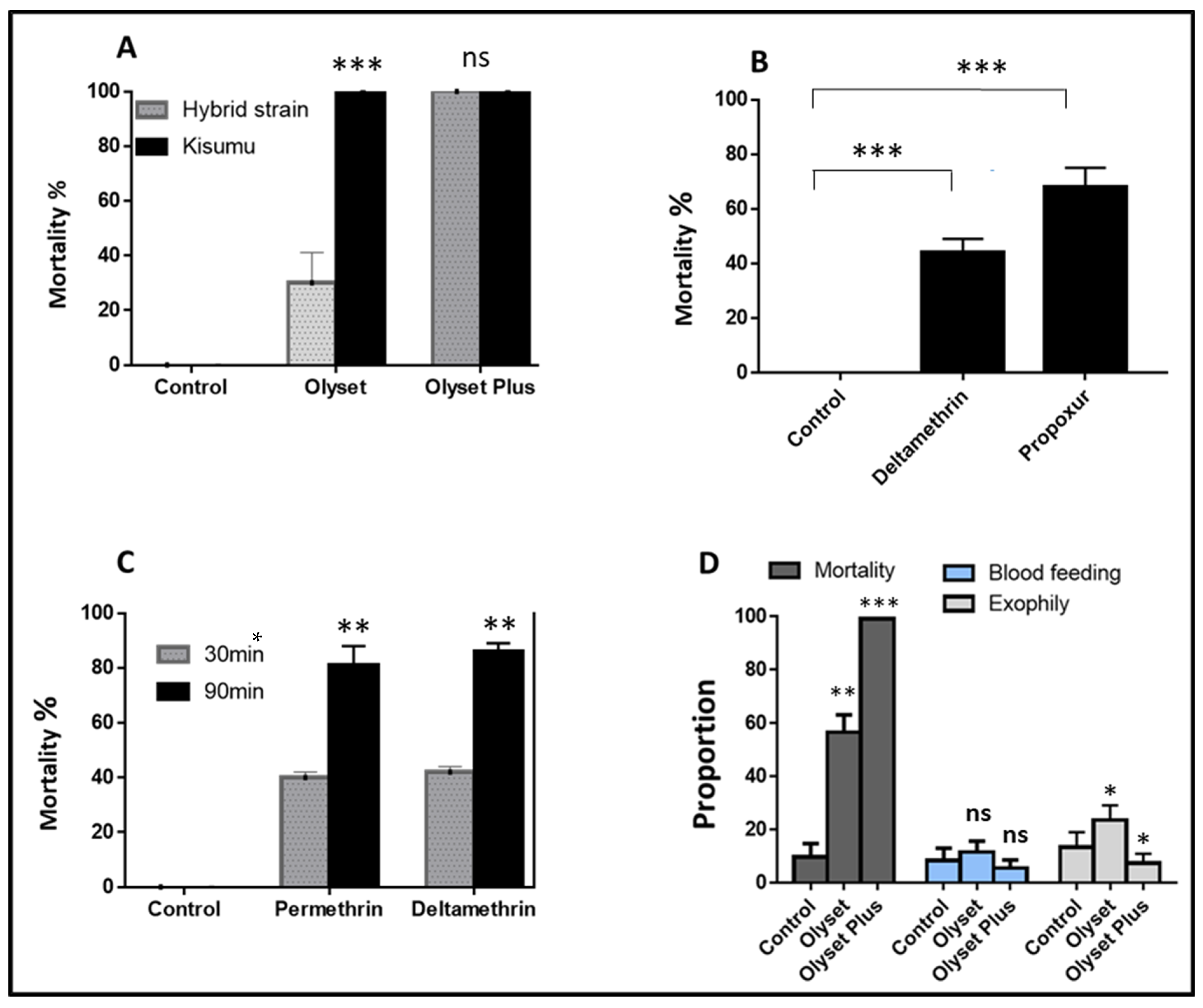
2.1.1. Quality Control and Performance of the Nets against the Hybrid Strain
2.1.2. Performance of Nets against the Hybrid Strain Using Experimental Hut
2.2. Validating the Role of CYP6P9a/b and 6.5 kb SV in Pyrethroid Resistance in the Hybrid FUMOZ-FANG Strains before the Experimental Hut Trials
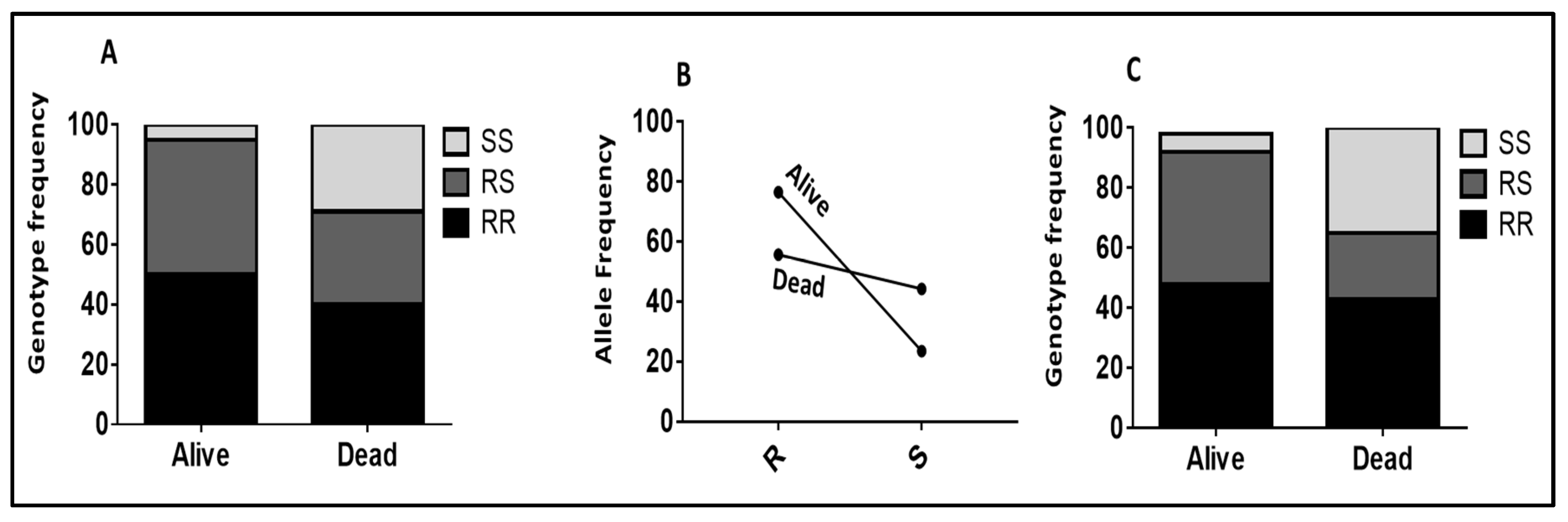
2.2.1. Role of CYP6P9a_R Using WHO Tube Assay Samples
2.2.2. Validating the Role of CYP6P9b_R Using Sample from WHO Tube Assays
2.2.3. Validation of the Role of CYP6P9a and CYP6P9b in Conferring Resistance Using Samples from Cone Assays
2.2.4. Validating the Role of 6.5 kb SV in Conferring Resistance Using Samples from Cone Assays
2.3. Impact of CYP6P9a on the Efficacy of Olyset and Olyset plus Nets Using Experimental Hut Trial
2.3.1. Impact on Mosquito Mortality
2.3.2. CYP6P9a Impacting the Blood-Feeding
2.4. Impact of CYP6P9b on the Efficacy of Olyset and Olyset plus Nets Using Experimental Hut Trial
2.4.1. The Impact of CYP6P9b on Mortality
2.4.2. CYP6P9b Impacting the Blood-Feeding
2.5. Impact of 6.5 kb SV on the Efficacy of Olyset and Olyset plus Nets Using Experimental Hut Trial
2.5.1. The Impact of 6.5. kb SV on Mortality
2.5.2. The Impact of 6.5. kb SV on Blood-Feeding
2.6. Combined Impact of CYP6P9a and CYP6Pb on the Efficacy of Olyset and Olyset plus Nets Using Experimental Hut Trial
2.7. Combined Impact of the Triple Resistance Alleles CYP6P9a/CYP6P9b/6.5 kb SV on the Efficacy of Olyset and Olyset plus Nets Using Experimental Hut Trial
3. Discussion
3.1. PBO-Based Nets (Olyset Plus) Exhibit Greater Efficacy than Pyrethroid-Only Nets (Olyset) in the Context of P450-Based Resistance
3.2. P450 Resistance Can Reduce the Efficacy of Pyrethroid-Only Nets Significantly Better Than PBO-Based Nets
3.3. Resistance Escalation with Multiple Resistance Alleles Present a Greater Risk of Control Failure
4. Materials and Methods
4.1. Study Site
4.2. Laboratory Strain: FUMOZ/FANG Crossing
4.3. Susceptibility Profile of the Hybrid FUMOZ/FANG Strain to Pyrethroids
4.4. Experimental Hut Design
4.4.1. Hut Treatment/Arm Comparison
4.4.2. Quality Control of Bed Nets Used in the Study
4.4.3. The Experimental Hut Trial
4.5. Bed Nets Performance Assessment
- (i)
- Exophily. The proportion of mosquitoes found exited in the veranda trap Exophily (%) = 100 × (Ev/Et), where Ev is the total number of mosquitoes found in veranda and Et is the total number of mosquitoes in the hut.
- (ii)
- Blood-feeding rate (BFR). This rate was calculated as follows: blood-feeding rate = (N mosquitoes fed) × 100/total N mosquitoes, where N mosquitoes fed was the number of mosquitoes fed and a total N mosquito was the total number of mosquitoes collected.
- (iii)
- Blood-feeding inhibition (BFI). The reduction in blood-feeding in comparison with the control hut. Blood-feeding inhibition is an indicator of personal protection (PP). More precisely, the personal protection effect of each bed net is the reduction in the blood-feeding percentage induced by the net when compared to the control. The protective effect of each bed net can be calculated as follows: personal protection (%) = 100 × (Bu – Bt)/Bu, where Bu is the total number of blood-fed mosquitoes in the huts with untreated nets and Bt is the total number of blood-fed mosquitoes in the huts with treated nets [47].
- (iv)
- Immediate and delay mortality. The proportion of mosquitoes entering the hut that are found dead in the morning (immediate mortality) or after being caught alive and held for 24 h with access to sugar solution (delay mortality) [47]. In this study, we presented the overall mortality calculated as follows: mortality (%) = 100 × (Mt/MT), where Mt is the total number of mosquitoes found dead in the hut and MT is the total number of mosquitoes collected in the hut [28,47].
- (v)
- As mosquitoes were rather released in the huts, the deterrence, i.e., the reduction in the entry rate of mosquitoes in the treated huts relative to control, could not be determined here.
4.6. Impact of the Duplicated CYP6P9a and CYP6P9b P450 Genes on the Performance of Bed Nets
4.6.1. Genotyping of the CYP6P9a-R Marker Using PCR-RFLP
4.6.2. Genotyping of the CYP6P9b-R Maker Using PCR-RFLP
4.6.3. PCR Assay to Detect the 6.5 kb SV
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Weiss, D.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.; Henry, A.; Eckhoff, P. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbel, V.; Darriet, F.; Chandre, F.; Hougard, J.-M. Insecticide mixtures for mosquito net impregnation against malaria vectors. Parasite 2002, 9, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouassi, B.L.; Edi, C.; Tia, E.; Konan, L.Y.; Akré, M.A.; Koffi, A.A.; Ouattara, A.F.; Tanoh, A.M.; Zinzindohoue, P.; Kouadio, B. Susceptibility of Anopheles gambiae from Côte d’Ivoire to insecticides used on insecticide-treated nets: Evaluating the additional entomological impact of piperonyl butoxide and chlorfenapyr. Malar. J. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tungu, P.K.; Michael, E.; Sudi, W.; Kisinza, W.W.; Rowland, M. Efficacy of interceptor® G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: Experimental hut trials in north-eastern Tanzania. Malar. J. 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Menze, B.D.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Riveron, J.M.; Wondji, C.S. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar. J. 2018, 17, 317. [Google Scholar] [CrossRef]
- Riveron, J.M.; Huijben, S.; Tchapga, W.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Irving, H.; Cuamba, N.; Maquina, M.; Paaijmans, K. Escalation of pyrethroid resistance in the malaria vector Anopheles funestus induces a loss of efficacy of Piperonyl Butoxide–Based Insecticide-Treated Nets in Mozambique. J. Infect. Dis. 2019, 220, 467–475. [Google Scholar] [CrossRef]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide resistance in malaria vectors: An update at a global scale. In Towards Malaria Elimination-A Leap Forward; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- WHO. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Antonio-Nkondjio, C.; Fossog, B.T.; Kopya, E.; Poumachu, Y.; Djantio, B.M.; Ndo, C.; Tchuinkam, T.; Awono-Ambene, P.; Wondji, C.S. Rapid evolution of pyrethroid resistance prevalence in Anopheles gambiae populations from the cities of Douala and Yaoundé (Cameroon). Malar. J. 2015, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Antonio-Nkondjio, C.; Sonhafouo-Chiana, N.; Ngadjeu, C.; Doumbe-Belisse, P.; Talipouo, A.; Djamouko-Djonkam, L.; Kopya, E.; Bamou, R.; Awono-Ambene, P.; Wondji, C.S. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasites Vectors 2017, 10, 472. [Google Scholar] [CrossRef]
- Edi, C.V.; Djogbenou, L.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.; Poupardin, R.; Jones, C.M.; Essandoh, J.; Ketoh, G.K.; Paine, M.J. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014, 10, e1004236. [Google Scholar] [CrossRef]
- Piameu, M.; Nwane, P.; Toussile, W.; Mavridis, K.; Wipf, N.C.; Kouadio, P.F.; Mbakop, L.R.; Mandeng, S.; Ekoko, W.E.; Toto, J.C. Pyrethroid and Etofenprox Resistance in Anopheles gambiae and Anopheles coluzzii from Vegetable Farms in Yaoundé, Cameroon: Dynamics, Intensity and Molecular Basis. Molecules 2021, 26, 5543. [Google Scholar] [CrossRef] [PubMed]
- Toé, K.H.; N’Falé, S.; Dabiré, R.K.; Ranson, H.; Jones, C.M. The Recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genom. 2015, 16, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemingway, J. Insecticide resistance in malaria vectors: A new approach to an old subject. Parassitologia 1999, 41, 315–318. [Google Scholar] [PubMed]
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghen, K.; Van Bortel, W.; Roelants, P.; Backeljau, T.; Coosemans, M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar. J. 2006, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Martinez-Torres, D.; Chandre, F.; Williamson, M.; Darriet, F.; Berge, J.B.; Devonshire, A.L.; Guillet, P.; Pasteur, N.; Pauron, D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 1998, 7, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Ranson, H.; Jensen, B.; Vulule, J.; Wang, X.; Hemingway, J.; Collins, F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Donnelly, M.J.; Williamson, M.S.; Ranson, H.; Ball, A.; Vontas, J.; Field, L.M. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar. J. 2007, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Gueye, O.; Tchouakui, M.; Dia, A.K.; Faye, M.B.; Ahmed, A.A.; Wondji, M.J.; Nguiffo, D.N.; Mugenzi, J.; Tripet, F.; Konaté, L. Insecticide Resistance Profiling of Anopheles coluzzii and Anopheles gambiae Populations in the Southern Senegal: Role of Target Sites and Metabolic Resistance Mechanisms. Genes 2020, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, M.J.; Corbel, V.; Weetman, D.; Wilding, C.S.; Williamson, M.S.; Black, I.V.W.C. Does kdr genotype predict Insecticide-Resistance phenotype in mosquitoes? Trends Parasitol. 2009, 25, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Okorie, P.N.; Ademowo, O.; Irving, H.; Kelly-Hope, L.A.; Wondji, C.S. Insecticide susceptibility of A nopheles coluzzii and A nopheles gambiae mosquitoes in I badan, Southwest N igeria. Med. Vet. Entomol. 2015, 29, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irving, H.; Wondji, C.S. Investigating knockdown resistance (kdr) mechanism against pyrethroids/DDT in the malaria vector Anopheles funestus across Africa. BMC Genet. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weedall, G.D.; Riveron, J.M.; Hearn, J.; Irving, H.; Kamdem, C.; Fouet, C.; White, B.J.; Wondji, C.S. An Africa-Wide genomic evolution of insecticide resistance in the malaria vector Anopheles funestus involves selective sweeps, copy number variations, gene conversion and transposons. PLoS Genet. 2020, 16, e1008822. [Google Scholar] [CrossRef] [PubMed]
- Mugenzi, L.M.; Menze, B.D.; Tchouakui, M.; Wondji, M.J.; Irving, H.; Tchoupo, M.; Hearn, J.; Weedall, G.D.; Riveron, J.M.; Cho-Ngwa, F. A 6.5-kb intergenic structural variation enhances P450-mediated resistance to pyrethroids in malaria vectors lowering bed net efficacy. Mol. Ecol. 2020, 29, 4395–4411. [Google Scholar] [CrossRef]
- Mugenzi, L.M.; Menze, B.D.; Tchouakui, M.; Wondji, M.J.; Irving, H.; Tchoupo, M.; Hearn, J.; Weedall, G.D.; Riveron, J.M.; Wondji, C.S. Cis-regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.M.; Hemingway, J.; Ranson, H.; Albert, A. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014, 15, R27. [Google Scholar] [CrossRef] [Green Version]
- Weedall, G.D.; Mugenzi, L.M.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.J.; Tchoupo, M.; Djouaka, R. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Plan for Insecticide Resistance Management; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Gleave, K.; Lissenden, N.; Chaplin, M.; Choi, L.; Ranson, H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- Martin, J.L.; Mosha, F.W.; Lukole, E.; Rowland, M.; Todd, J.; Charlwood, J.D.; Mosha, J.F.; Protopopoff, N. Personal protection with PBO-pyrethroid synergist-treated nets after 2 years of household use against pyrethroid-resistant Anopheles in Tanzania. Parasites Vectors 2021, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pennetier, C.; Bouraima, A.; Chandre, F.; Piameu, M.; Etang, J.; Rossignol, M.; Sidick, I.; Zogo, B.; Lacroix, M.-N.; Yadav, R. Efficacy of Olyset® Plus, a new long-lasting insecticidal net incorporating permethrin and piperonil-butoxide against multi-resistant malaria vectors. PLoS ONE 2013, 8, e75134. [Google Scholar] [CrossRef]
- Toe, K.; Müller, P.; Badolo, A.; Traore, A.; Sagnon, N.; Dabiré, R.; Ranson, H. Do bednets including piperonyl butoxide offer additional protection against populations of Anopheles gambiae sl. that are highly resistant to pyrethroids? An experimental hut evaluation in Burkina Fasov. Med. Vet. Entomol. 2018, 32, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wondji, C.; Hearn, J.; Irving, H.; Wondji, M.; Weedall, G. RNAseq-based gene expression profiling of the Anopheles funestus pyrethroid-resistant strain FUMOZ highlights the predominant role of the duplicated CYP6P9a/b cytochrome P450s. G3 2022, 12, 352. [Google Scholar] [CrossRef]
- Keserü, G.M.; Kolossváry, I.; Bertók, B. Piperonyl butoxide-mediated inhibition of cytochrome P450-catalysed insecticide metabolism: A rational approach. Pestic. Sci. 1999, 55, 1004–1006. [Google Scholar]
- Lindsay, S.W.; Thomas, M.B.; Kleinschmidt, I. Threats to the effectiveness of insecticide-treated bednets for malaria control: Thinking beyond insecticide resistance. Lancet Glob. Health 2021, 9, e1325–e1331. [Google Scholar] [CrossRef]
- Glunt, K.D.; Abílio, A.P.; Bassat, Q.; Bulo, H.; Gilbert, A.E.; Huijben, S.; Manaca, M.N.; Macete, E.; Alonso, P.; Paaijmans, K.P. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malar. J. 2015, 14, 298. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.M.; Chiumia, M.; Menze, B.D.; Barnes, K.G.; Irving, H.; Ibrahim, S.S.; Weedall, G.D.; Mzilahowa, T.; Wondji, C.S. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: A major concern for malaria vector control. Malar. J. 2015, 14, 344. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.M.; Watsenga, F.; Irving, H.; Irish, S.R.; Wondji, C.S. High Plasmodium infection rate and reduced bed net efficacy in multiple insecticide-resistant malaria vectors in Kinshasa, Democratic Republic of Congo. J. Infect. Dis. 2018, 217, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Menze, B.D.; Kouamo, M.F.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Kusimo, M.O.; Mouhamadou, C.S.; Riveron, J.M.; Wondji, C.S. An Experimental Hut Evaluation of PBO-Based and Pyrethroid-Only Nets against the Malaria Vector Anopheles funestus Reveals a Loss of Bed Nets Efficacy Associated with GSTe2 Metabolic Resistance. Genes 2020, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Amenya, D.A.; Naguran, R.; Lo, T.C.; Ranson, H.; Spillings, B.L.; Wood, O.R.; Brooke, B.D.; Coetzee, M.; Koekemoer, L.L. Over expression of a cytochrome P450 (CYP6P9) in a major African malaria vector, Anopheles Funestus, resistant to pyrethroids. Insect Mol. Biol. 2008, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.; Wondji, C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2013, 110, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protopopoff, N.; Mosha, J.F.; Lukole, E.; Charlwood, J.D.; Wright, A.; Mwalimu, C.D.; Manjurano, A.; Mosha, F.W.; Kisinza, W.; Kleinschmidt, I.; et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet 2018, 391, 1577–1588. [Google Scholar] [PubMed] [Green Version]
- WHO. Guidelines for Laboratory and Field-Testing of Long-Lasting Insecticidal Nets; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 2005, 19, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar] [CrossRef]
- Badolo, A.; Guelbéogo, W.M.; Tiono, A.B.; Traoré, A.; Sirima, S.B. Laboratory evaluation of Fendona 6SC® treated bednets and Interceptor® long-lasting nets against Anopheles gambiae sl in Burkina Faso. Parasitol. Res. 2014, 113, 1069–1075. [Google Scholar] [CrossRef]
- Chouaibou, M.; Simard, F.; Chandre, F.; Etang, J.; Darriet, F.; Hougard, J.M. Efficacy of bifenthrin-impregnated bednets against Anopheles funestus and pyrethroid-resistant Anopheles gambiae in North Cameroon. Malar. J. 2006, 5, 77. [Google Scholar] [CrossRef] [Green Version]
- Lowry, R. VassarStats: Website for statistical computation. 2015. View Artic. 2014. Available online: http://vassarstats.net/ (accessed on 18 May 2022).





| Treatment Arm | Description | Manufacturer |
|---|---|---|
| Untreated | 100% polyester with no insecticide | Local market |
| Olyset | 8.6 × 10−4 kg/m2 (2%) of permethrin incorporated into polyethylene | Sumitomo Chemical |
| Olyset Plus | 8.6 × 10−4 kg/m2 (2%) of permethrin and 4.3 × 10−4 kg/m2 (1%) of PBO incorporated into polyethylene | Sumitomo Chemical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menze, B.D.; Mugenzi, L.M.J.; Tchouakui, M.; Wondji, M.J.; Tchoupo, M.; Wondji, C.S. Experimental Hut Trials Reveal That CYP6P9a/b P450 Alleles Are Reducing the Efficacy of Pyrethroid-Only Olyset Net against the Malaria Vector Anopheles funestus but PBO-Based Olyset Plus Net Remains Effective. Pathogens 2022, 11, 638. https://doi.org/10.3390/pathogens11060638
Menze BD, Mugenzi LMJ, Tchouakui M, Wondji MJ, Tchoupo M, Wondji CS. Experimental Hut Trials Reveal That CYP6P9a/b P450 Alleles Are Reducing the Efficacy of Pyrethroid-Only Olyset Net against the Malaria Vector Anopheles funestus but PBO-Based Olyset Plus Net Remains Effective. Pathogens. 2022; 11(6):638. https://doi.org/10.3390/pathogens11060638
Chicago/Turabian StyleMenze, Benjamin D., Leon M. J. Mugenzi, Magellan Tchouakui, Murielle J. Wondji, Micareme Tchoupo, and Charles S. Wondji. 2022. "Experimental Hut Trials Reveal That CYP6P9a/b P450 Alleles Are Reducing the Efficacy of Pyrethroid-Only Olyset Net against the Malaria Vector Anopheles funestus but PBO-Based Olyset Plus Net Remains Effective" Pathogens 11, no. 6: 638. https://doi.org/10.3390/pathogens11060638
APA StyleMenze, B. D., Mugenzi, L. M. J., Tchouakui, M., Wondji, M. J., Tchoupo, M., & Wondji, C. S. (2022). Experimental Hut Trials Reveal That CYP6P9a/b P450 Alleles Are Reducing the Efficacy of Pyrethroid-Only Olyset Net against the Malaria Vector Anopheles funestus but PBO-Based Olyset Plus Net Remains Effective. Pathogens, 11(6), 638. https://doi.org/10.3390/pathogens11060638







