Mycobacterium bovis Tuberculosis in Two Goat Farms in Multi-Host Ecosystems in Sicily (Italy): Epidemiological, Diagnostic, and Regulatory Considerations
Abstract
:1. Introduction
2. Results
2.1. Description of the Outbreaks
2.2. Intradermotuberculin Test
2.3. Gross Pathology
2.4. Bacteriological and Molecular Investigations
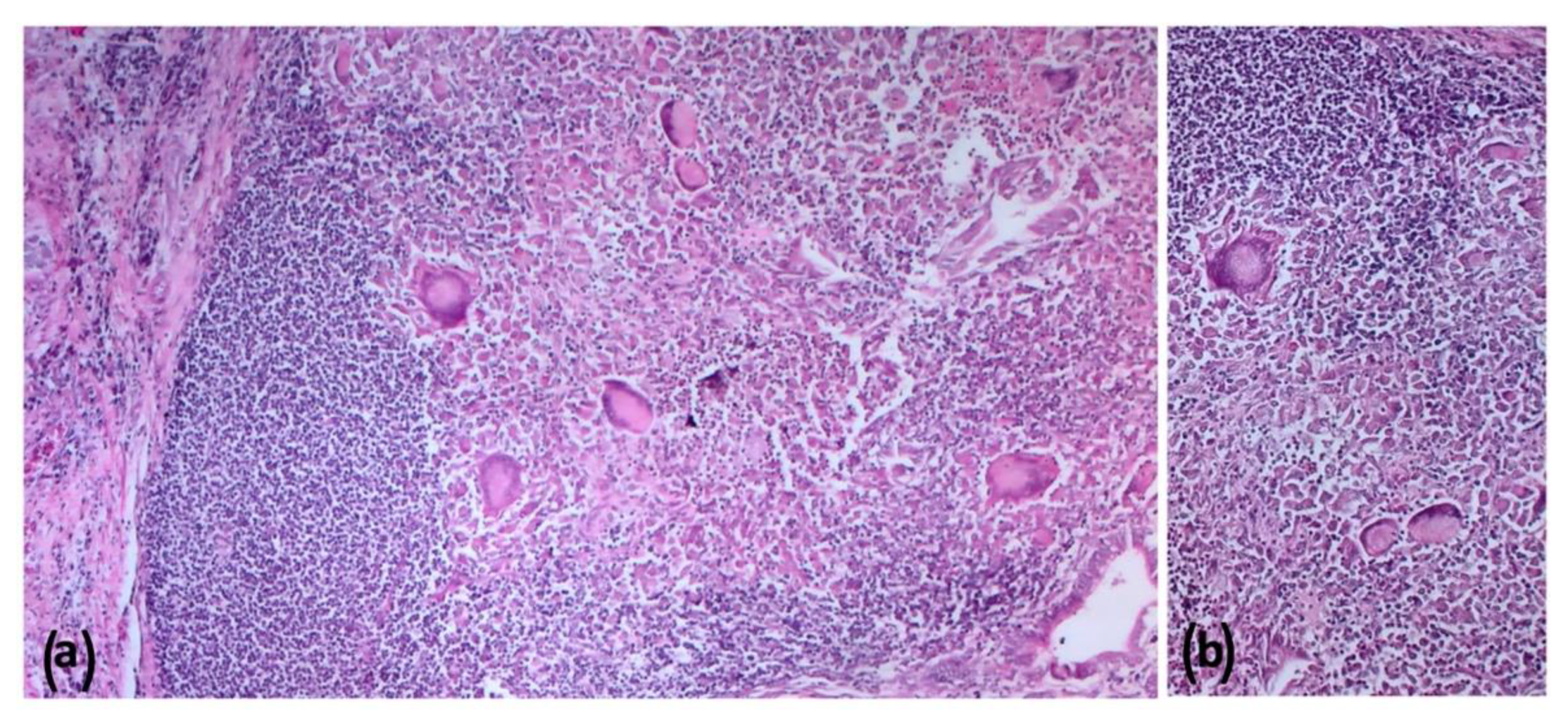
2.5. Histopathologic Findings
3. Discussion
4. Materials and Methods
4.1. Anamnesis, Clinical, and Epidemiological Investigations
4.2. Single Intradermal Cervical Test (SICT)
4.3. Post-Mortem Examination
4.4. Bacteriological and Molecular Examinations
4.5. Histology and Immunohistochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gortázar, C.; Delahay, R.J.; Mcdonald, R.A.; Boadella, M.; Wilson, G.J.; Gavier-Widen, D.; Acevedo, P. The Status of Tuberculosis in European Wild Mammals: The Status of TB in European Wild Mammals. Mammal Rev. 2012, 42, 193–206. [Google Scholar] [CrossRef]
- Crispell, J.; Benton, C.H.; Balaz, D.; De Maio, N.; Ahkmetova, A.; Allen, A.; Biek, R.; Presho, E.L.; Dale, J.; Hewinson, G.; et al. Combining Genomics and Epidemiology to Analyse Bi-Directional Transmission of Mycobacterium Bovis in a Multi-Host System. eLife 2019, 8, e45833. [Google Scholar] [CrossRef] [PubMed]
- Haydon, D.; Cleaveland, S.; Taylor, L.; Laurenson, K.M. Identifying Reservoirs of Infection: A Conceptual and Practical Challenge. Emerg. Infect. Dis. 2002, 8, 1468–1473. [Google Scholar] [CrossRef]
- Santos, N.; Richomme, C.; Nunes, T.; Vicente, J.; Alves, P.C.; de la Fuente, J.; Correia-Neves, M.; Boschiroli, M.-L.; Delahay, R.; Gortázar, C. Quantification of the Animal Tuberculosis Multi-Host Community Offers Insights for Control. Pathogens 2020, 9, 421. [Google Scholar] [CrossRef]
- Marianelli, C.; Cifani, N.; Capucchio, M.T.; Fiasconaro, M.; Russo, M.; La Mancusa, F.; Pasquali, P.; Di Marco, V. A Case of Generalized Bovine Tuberculosis in a Sheep. J. Vet. Diagn. Invest. 2010, 22, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, V.; Mazzone, P.; Capucchio, M.T.; Boniotti, M.B.; Aronica, V.; Russo, M.; Fiasconaro, M.; Cifani, N.; Corneli, S.; Biasibetti, E.; et al. Epidemiological Significance of the Domestic Black Pig (Sus Scrofa) in Maintenance of Bovine Tuberculosis in Sicily. J. Clin. Microbiol. 2012, 50, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Amato, B.; Mignacca, S.A.; Pacciarini, M.L.; Vitale, M.; Antoci, S.; Cucinotta, S.; Puleio, R.; Biasibetti, E.; Fiasconaro, M.; Capucchio, M.T.; et al. An Outbreak of Bovine Tuberculosis in a Fallow Deer Herd (Dama Dama) in Sicily. Res. Vet. Sci. 2016, 106, 116–120. [Google Scholar] [CrossRef]
- Amato, B.; Capucchio, T.M.; Biasibetti, E.; Mangano, E.; Boniotti, B.M.; Pacciarini, L.M.; Migliore, S.; Vitale, M.; Fiasconaro, M.; Di Marco Lo Presti, V. Pathology and Genetic Findings in a Rare Case of Mycobacterium Caprae Infection in a Sow. Vet. Microbiol. 2017, 205, 71–74. [Google Scholar] [CrossRef]
- Amato, B.; Di Marco Lo Presti, V.; Gerace, E.; Capucchio, M.T.; Vitale, M.; Zanghì, P.; Pacciarini, M.L.; Marianelli, C.; Boniotti, M.B. Molecular Epidemiology of Mycobacterium Tuberculosis Complex Strains Isolated from Livestock and Wild Animals in Italy Suggests the Need for a Different Eradication Strategy for Bovine Tuberculosis. Transbound. Emerg. Dis. 2018, 65, e416–e424. [Google Scholar] [CrossRef]
- Marianelli, C.; Amato, B.; Boniotti, M.B.; Vitale, M.; Pruiti Ciarello, F.; Pacciarini, M.L.; Di Marco Lo Presti, V. Genotype Diversity and Distribution of Mycobacterium Bovis from Livestock in a Small, High-Risk Area in Northeastern Sicily, Italy. PLoS Negl. Trop. Dis. 2019, 13, e0007546. [Google Scholar] [CrossRef] [Green Version]
- Liébana, E.; Aranaz, A.; Urquía, J.; Mateos, A.; Domínguez, L. Evaluation of the Gamma-Interferon Assay for Eradication of Tuberculosis in a Goat Herd. Aust. Vet. J. 1998, 76, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Evans, H.; Rolfe, S.; de la Rua-Domenech, R.; Crawshaw, T.; Higgins, R.J.; Schock, A.; Clifton-Hadley, R. Outbreak of Tuberculosis Caused by Mycobacterium bovis in Golden Guernsey Goats in Great Britain. Vet. Rec. 2009, 165, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.V.; Monteiro, M.; Carvalho, P.; Mendonça, P.; Albuquerque, T.; Botelho, A. Multihost Tuberculosis: Insights from the Portuguese Control Program. Vet. Med. Int. 2011, 2011, 795165. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, A.; Good, M.; Duignan, A.; Curtin, T.; More, S.J. Tuberculosis in Goats on a Farm in Ireland: Epidemiological Investigation and Control. Vet. Rec. 2011, 168, 485. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, G.; Boniotti, M.B.; Gaffuri, A.; Casto, B.; Zanoni, M.; Pacciarini, M.L. Tuberculosis Transmission by Mycobacterium Bovis in a Mixed Cattle and Goat Herd. Res. Vet. Sci. 2013, 95, 430–433. [Google Scholar] [CrossRef]
- Javed, M.T.; Munir, A.; Shahid, M.; Severi, G.; Irfan, M.; Aranaz, A.; Cagiola, M. Percentage of Reactor Animals to Single Comparative Cervical Intradermal Tuberculin (SCCIT) in Small Ruminants in Punjab Pakistan. Acta Trop. 2010, 113, 88–91. [Google Scholar] [CrossRef]
- Napp, S.; Allepuz, A.; Mercader, I.; Nofrarías, M.; López-Soria, S.; Domingo, M.; Romero, B.; Bezos, J.; Pérez de Val, B. Evidence of Goats Acting as Domestic Reservoirs of Bovine Tuberculosis. Vet. Rec. 2013, 172, 663. [Google Scholar] [CrossRef] [Green Version]
- Wangoo, A.; Johnson, L.; Gough, J.; Ackbar, R.; Inglut, S.; Hicks, D.; Spencer, Y.; Hewinson, G.; Vordermeier, M. Advanced Granulomatous Lesions in Mycobacterium Bovis-Infected Cattle Are Associated with Increased Expression of Type I Procollagen, Γδ (WC1+) T Cells and CD 68+ Cells. J. Comp. Pathol. 2005, 133, 223–234. [Google Scholar] [CrossRef]
- García-Jiménez, W.L.; Salguero, F.J.; Fernández-Llario, P.; Martínez, R.; Risco, D.; Gough, J.; Ortiz-Peláez, A.; Hermoso-de-Mendoza, J.; Gómez, L. Immunopathology of Granulomas Produced by Mycobacterium Bovis in Naturally Infected Wild Boar. Vet. Immunol. Immunopathol. 2013, 156, 54–63. [Google Scholar] [CrossRef]
- Seva, J.; Menchén, V.; Navarro, J.A.; Pallarés, F.J.; Villar, D.; Vásquez, F.; Bernabé, A. Caprine Tuberculosis Eradication Program: An Immunohistochemical Study. Small Rumin. Res. 2002, 46, 107–114. [Google Scholar] [CrossRef]
- Santos, N.; Geraldes, M.; Afonso, A.; Almeida, V.; Correia-Neves, M. Diagnosis of Tuberculosis in the Wild Boar (Sus Scrofa): A Comparison of Methods Applicable to Hunter-Harvested Animals. PLoS ONE 2010, 5, e12663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, D.A.V.; Siconelli, M.J.L.; Bürger, K.P.; Keid, L.B. Comparison between Tests for Tuberculosis Diagnosis in Slaughtered Bovines. Arq. Inst. Biológico 2018, 85, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gormley, E.; Corner, L.A.L.; Costello, E.; Rodriguez-Campos, S. Bacteriological Diagnosis and Molecular Strain Typing of Mycobacterium bovis and Mycobacterium caprae. Res. Vet. Sci. 2014, 97, S30–S43. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Issa, M.; Martins Soares Filho, P.; Fonseca Júnior, A.A.; Arrais Hodon, M.; Cristian dos Santos, L.; Karlisson Pimenta dos Reis, J.; Cerqueira Leite, R. Comparative Study of Mycobacterium Bovis Primary Isolation Methods. Braz. J. Microbiol. 2017, 48, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, H.P.; Yu, M.C.; Wu, M.H.; Lin, T.P.; Luh, K.T. Comparison of the BACTEC MGIT 960 with Löwenstein-Jensen Medium for Recovery of Mycobacteria from Clinical Specimens. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2000, 4, 866–870. [Google Scholar]
- Lekko, Y.M.; Ooi, P.T.; Omar, S.; Mazlan, M.; Ramanoon, S.Z.; Jasni, S.; Jesse, F.F.A.; Che-Amat, A. Mycobacterium Tuberculosis Complex in Wildlife: Review of Current Applications of Antemortem and Postmortem Diagnosis. Vet. World 2020, 13, 1822–1836. [Google Scholar] [CrossRef]
- Sanchez, J.; Tomás, L.; Ortega, N.; Buendía, A.J.; del Rio, L.; Salinas, J.; Bezos, J.; Caro, M.R.; Navarro, J.A. Microscopical and Immunological Features of Tuberculoid Granulomata and Cavitary Pulmonary Tuberculosis in Naturally Infected Goats. J. Comp. Pathol. 2011, 145, 107–117. [Google Scholar] [CrossRef]
- Ramos, D.F.; Tavares, L.; da Silva, P.E.A.; Dellagostin, O.A. Molecular Typing of Mycobacterium Bovis Isolates: A Review. Braz. J. Microbiol. 2014, 45, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Boniotti, M.B.; Goria, M.; Loda, D.; Garrone, A.; Benedetto, A.; Mondo, A.; Tisato, E.; Zanoni, M.; Zoppi, S.; Dondo, A.; et al. Molecular Typing of Mycobacterium bovis Strains Isolated in Italy from 2000 to 2006 and Evaluation of Variable-Number Tandem Repeats for Geographically Optimized Genotyping. J. Clin. Microbiol. 2009, 47, 636–644. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marco Lo Presti, V.; Capucchio, M.T.; Fiasconaro, M.; Puleio, R.; La Mancusa, F.; Romeo, G.; Biondo, C.; Ippolito, D.; Guarda, F.; Pruiti Ciarello, F. Mycobacterium bovis Tuberculosis in Two Goat Farms in Multi-Host Ecosystems in Sicily (Italy): Epidemiological, Diagnostic, and Regulatory Considerations. Pathogens 2022, 11, 649. https://doi.org/10.3390/pathogens11060649
Di Marco Lo Presti V, Capucchio MT, Fiasconaro M, Puleio R, La Mancusa F, Romeo G, Biondo C, Ippolito D, Guarda F, Pruiti Ciarello F. Mycobacterium bovis Tuberculosis in Two Goat Farms in Multi-Host Ecosystems in Sicily (Italy): Epidemiological, Diagnostic, and Regulatory Considerations. Pathogens. 2022; 11(6):649. https://doi.org/10.3390/pathogens11060649
Chicago/Turabian StyleDi Marco Lo Presti, Vincenzo, Maria Teresa Capucchio, Michele Fiasconaro, Roberto Puleio, Francesco La Mancusa, Giovanna Romeo, Carmelinda Biondo, Dorotea Ippolito, Franco Guarda, and Flavia Pruiti Ciarello. 2022. "Mycobacterium bovis Tuberculosis in Two Goat Farms in Multi-Host Ecosystems in Sicily (Italy): Epidemiological, Diagnostic, and Regulatory Considerations" Pathogens 11, no. 6: 649. https://doi.org/10.3390/pathogens11060649






