Reliable Field Assessment of Proliferative Kidney Disease in Wild Brown Trout, Salmo trutta, Populations: When Is the Optimal Sampling Period?
Abstract
:1. Introduction
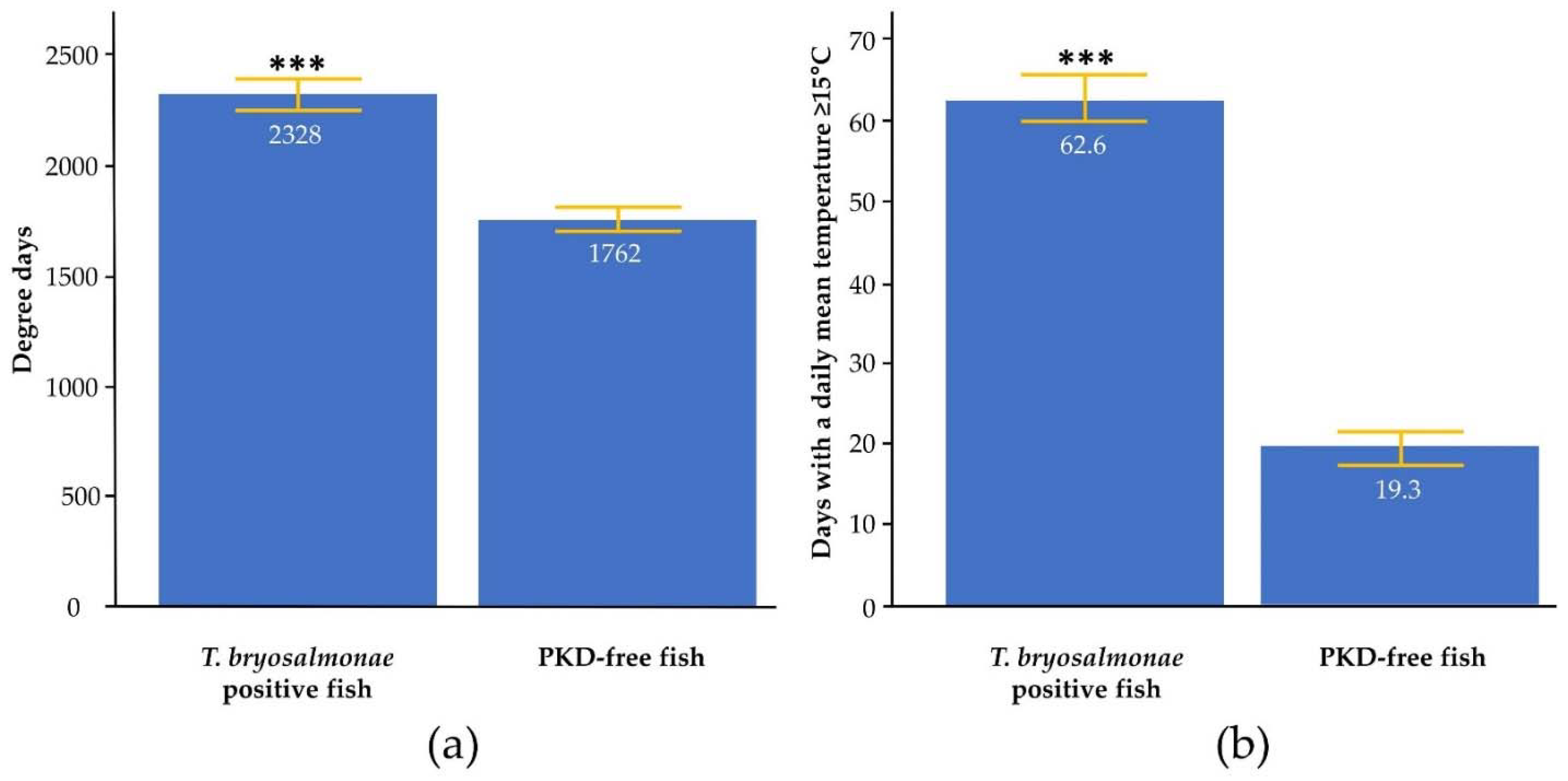
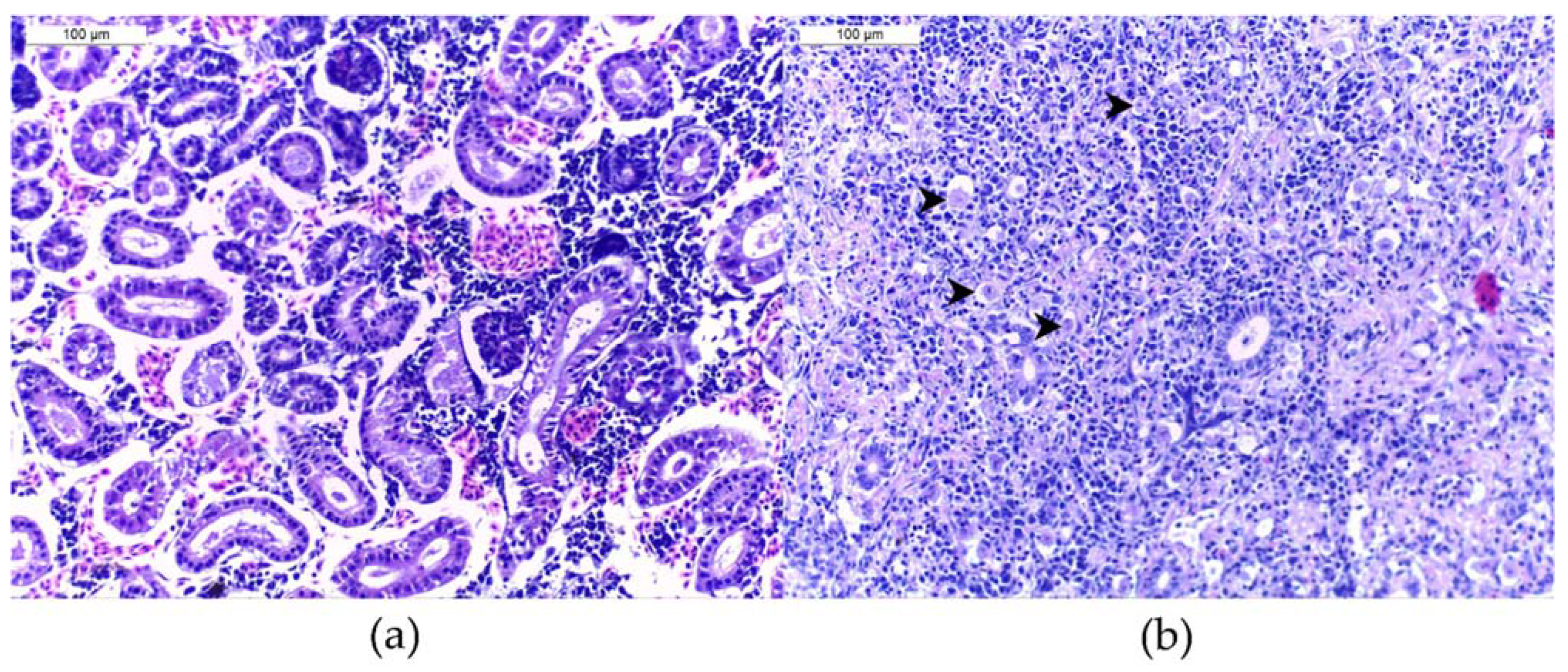
2. Results
3. Discussion
4. Materials and Methods
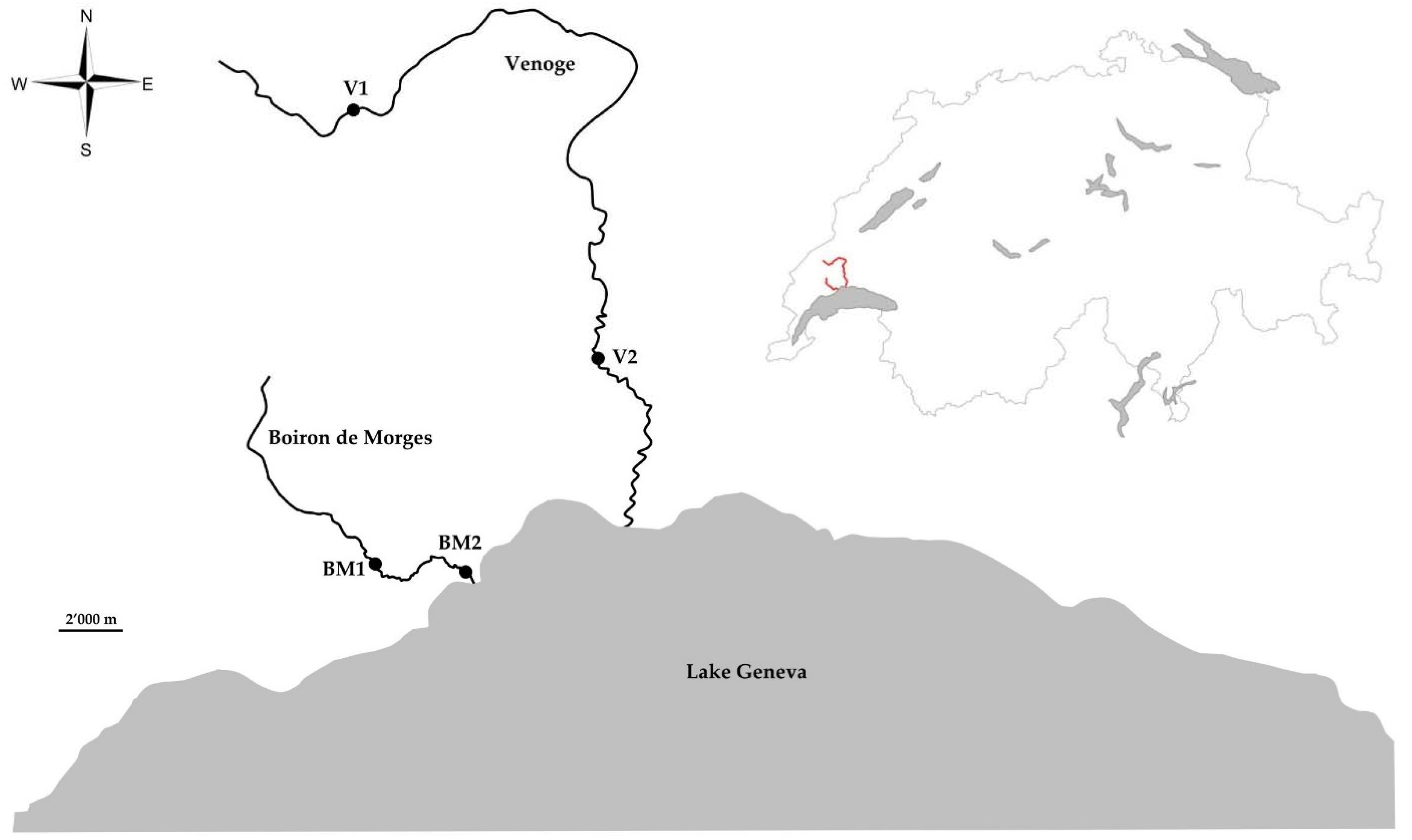
4.1. Fish Sampling
4.2. Histological Analysis
4.3. Water Temperature
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkhardt-Holm, P.; Peter, A.; Segner, H. Decline of fish catch in Switzerland. Aquat. Sci. 2002, 64, 36–54. [Google Scholar] [CrossRef]
- Burkhardt-Holm, P.; Giger, W.; Güttinger, H.; Ochsenbein, U.; Peter, A.; Scheurer, K.; Segner, H.; Staub, E.; Suter, M.J.-F. Where have all the fish gone? The reasons why fish catches in Swiss rivers are declining. Environ. Sci. Technol. 2005, 39, 441A–447A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, S.; Bernet, D.; Burkhardt-Holm, P.; Schmidt-Posthaus, H.; Vonlanthen, P.; Wahli, T.; Segner, H. Assessment of fish health status in four Swiss rivers showing a decline of brown trout catches. Aquat. Sci. 2007, 69, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt-Holm, P. Decline of brown trout (Salmo trutta) in Switzerland—How to assess potential causes in a multi-factorial cause-effect relationship. Mar. Environ. Res. 2008, 66, 181–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giantsis, I.A.; Sapounidis, A.; Koutrakis, E.; Apostolidis, A.P. Assessment of Stocking Activities on the Native Brown Trout Populations from Nestos River (Southern Balkans) Inferred by mtDNA RFLP and Sequencing Analyses. Appl. Sci. 2021, 11, 9034. [Google Scholar] [CrossRef]
- Borsuk, M.E.; Reichert, P.; Peter, A.; Schager, E.; Burkhardt-Holm, P. Assessing the decline of brown trout (Salmo trutta) in Swiss rivers using a Bayesian probability network. Ecol. Model. 2006, 192, 224–244. [Google Scholar] [CrossRef]
- Schager, E.; Peter, A.; Burkhardt-Holm, P. Status of young-of-the-year brown trout (Salmo trutta fario) in Swiss streams: Factors influencing YOY trout recruitment. Aquat. Sci. 2007, 69, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Wahli, T.; Bernet, D.; Steiner, P.A.; Schmidt-Posthaus, H. Geographic distribution of Tetracapsuloides bryosalmonae infected fish in Swiss rivers: An update. Aquat. Sci. 2007, 69, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Canning, E.; Curry, A.; Feist, S.; Longshaw, M.; Okamura, B. Tetracapsula bryosalmonae N.SP. for PKX organism, the cause of PKD in salmonid fish. Bull. Eur. Assoc. Fish Pathol. 1999, 19, 203–206. [Google Scholar]
- Anderson, C.L.; Canning, E.U.; Okamura, B. Molecular data implicate bryozoans as hosts for PKX (phylum Myxozoa) and identify a clade of bryozoan parasites within the Myxozoa. Parasitology 1999, 119, 555–561. [Google Scholar] [CrossRef]
- Morris, D.J.; Adams, A. Transmission of Tetracapsuloides bryosalmonae (Myxozoa: Malacosporea), the causative organism of salmonid proliferative kidney disease, to the freshwater bryozoan Fredericella sultana. Parasitology 2006, 133, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Tops, S.; Hartikainen, H.L.; Okamura, B. The effect of infection by Tetracapsuloides bryosalmonae (Myxozoa) and temperature on Fredericella sultana (Bryozoa). Int. J. Parasitol. 2009, 39, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Fontes, I.; Hartikainen, H.; Taylor, N.G.H.; Okamura, B. Conditional persistence and tolerance characterize endoparasite-colonial host interactions. Parasitology 2017, 144, 1052–1063. [Google Scholar] [CrossRef]
- Hartikainen, H.L.; Okamura, B. Castrating parasites and colonial hosts. Parasitology 2012, 139, 547–556. [Google Scholar] [CrossRef] [PubMed]
- McGurk, C.; Morris, D.J.; Auchinachie, N.A.; Adams, A. Development of Tetracapsuloides bryosalmonae (Myxozoa: Malacosporea) in bryozoan hosts (as examined by light microscopy) and quantitation of infective dose to rainbow trout (Oncorhynchus mykiss). Vet. Parasitol. 2006, 135, 249–257. [Google Scholar] [CrossRef]
- Feist, S.W.; Longshaw, M. Phylum Myxozoa. In Fish Diseases and Disorders. Protozoan and Metazoan Infections, 2nd ed.; Woo, P.T.K., Ed.; CAB International: Wallington, UK, 2006; Volume 1, pp. 230–296. [Google Scholar]
- Longshaw, M.; Le Deuff, R.M.; Harris, A.F.; Feist, S.W. Development of proliferative kidney disease in rainbow trout, Oncorhynchus mykiss (Walbaum), following short-term exposure to Tetracapsula bryosalmonae infected bryozoans. J. Fish Dis. 2002, 25, 443–449. [Google Scholar] [CrossRef]
- Grabner, D.; El-Matbouli, M. Transmission of Tetracapsuloides bryosalmonae (Myxozoa: Malacosporea) to Fredericella sultana (Bryozoa: Phylactolaemata) by various fish species. Dis. Aquat. Org. 2008, 79, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Bettge, K.; Wahli, T.; Segner, H.; Schmidt-Posthaus, H. Proliferative kidney disease in rainbow trout: Time- and temperature-related renal pathology and parasite distribution. Dis. Aquat. Org. 2009, 83, 67–76. [Google Scholar] [CrossRef]
- Bettge, K.; Segner, H.; Burki, R.; Schmidt-Posthaus, H.; Wahli, T. Proliferative kidney disease (PKD) of rainbow trout: Temperature- and time-related changes of Tetracapsuloides bryosalmonae DNA in the kidney. Parasitology 2009, 136, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Okamura, B.; Hartikainen, H.L.; Schmidt-Posthaus, H.; Wahli, T. Life cycle complexity, environmental change and the emerging status of salmonid proliferative kidney disease. Freshw. Biol. 2011, 56, 735–753. [Google Scholar] [CrossRef]
- Schmidt-Posthaus, H.; Hirschi, R.; Schneider, E. Proliferative kidney disease in brown trout: Infection level, pathology and mortality under field conditions. Dis. Aquat. Org. 2015, 114, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foott, J.S.; Hedrick, R.P. Seasonal occurrence of the infectious stage of proliferative kidney disease (PKD) and resistance of rainbow trout, Salmo gairdneri Richardson, to reinfection. J. Fish Biol. 1987, 30, 477–483. [Google Scholar] [CrossRef]
- De Kinkelin, P.; Loriot, B. A water temperature regime which prevents the occurrence of proliferative kidney disease (PKD) in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2001, 24, 489–493. [Google Scholar] [CrossRef]
- Gay, M.; Okamura, B.; De Kinkelin, P. Evidence that infectious stages of Tetracapsula bryosalmonae for rainbow trout Oncorhynchus mykiss are present throughout the year. Dis. Aquat. Org. 2001, 46, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.; Segner, H.; Casanova-Nakayama, A.; Wahli, T. Who needs the hotspot? The effect of temperature on the fish host immune response to Tetracapsuloides bryosalmonae the causative agent of proliferative kidney disease. Fish Shellfish Immunol. 2017, 63, 424–437. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.; Schmidt-Posthaus, H.; Segner, H.; Wahli, T.; Strepparava, N. Are brown trout Salmo trutta fario and rainbow trout Oncorhynchus mykiss two of a kind? A comparative study of salmonids to temperature-influenced Tetracapsuloides bryosalmonae infection. J. Fish Dis. 2018, 41, 191–198. [Google Scholar] [CrossRef]
- Palikova, M.; Papezikova, I.; Markova, Z.; Navratil, S.; Mares, J.; Mares, L.; Vojtek, L.; Hyrsl, P.; Jelinkova, E.; Schmidt-Posthaus, H. Proliferative kidney disease in rainbow trout (Oncorhynchus mykiss) under intensive breeding conditions: Pathogenesis and haematological and immune parameters. Vet. Parasitol. 2017, 238, 5–16. [Google Scholar] [CrossRef]
- Wahli, T.; Knuesel, R.; Bernet, D.; Segner, H.; Pugovkin, D.; Burkhardt-Holm, P.; Escher, M.; Schmidt-Posthaus, H. Proliferative kidney disease in Switzerland: Current state of knowledge. J. Fish Dis. 2002, 25, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.; Rubin, A.; Strepparava, N.; Segner, H.; Rubin, J.-F.; Wahli, T. Do fish get wasted? Assessing the influence of effluents on parasitic infection of wild fish. PeerJ 2018, 6, e5956. [Google Scholar] [CrossRef]
- Rubin, A.; de Coulon, P.; Bailey, C.; Segner, H.; Wahli, T.; Rubin, J.-F. Keeping an eye on wild brown trout (Salmo trutta) populations: Correlation between temperature, environmental parameters, and proliferative kidney disease. Front. Vet. Sci. 2019, 6, 281. [Google Scholar] [CrossRef]
- Borgwardt, F.; Unfer, G.; Auer, S.; Waldner, K.; El-Matbouli, M.; Bechter, T. Direct and Indirect Climate Change Impacts on Brown Trout in Central Europe: How Thermal Regimes Reinforce Physiological Stress and Support the Emergence of Diseases. Front. Environ. Sci. 2020, 8, 59. [Google Scholar] [CrossRef]
- Strepparava, N.; Segner, H.; Ros, A.; Hartikainen, H.; Schmidt-Posthaus, H.; Wahli, T. Temperature-related parasite infection dynamics: The case of proliferative kidney disease of brown trout. Parasitology 2018, 145, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Waldner, K.; Borkovec, M.; Borgwardt, F.; Unfer, G.; El-Matbouli, M. Effect of water temperature on the morbidity of Tetracapsuloides bryosalmonae (Myxozoa) to brown trout (Salmo trutta) under laboratory conditions. J. Fish Dis. 2021, 44, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Rehberger, K.; Wernicke von Siebenthal, E.; Bailey, C.; Bregy, P.; Fasel, M.; Herzog, E.L.; Neumann, S.; Schmidt-Posthaus, H.; Segner, H. Long-term exposure to low 17α-ethinylestradiol (EE2) concentrations disrupts both the reproductive and the immune system of juvenile rainbow trout, Oncorhynchus mykiss. Environ. Int. 2020, 142, 105836. [Google Scholar] [CrossRef]
- Bruneaux, M.; Visse, M.; Gross, R.; Pukk, L.; Saks, L.; Vasemägi, A. Parasite infection and decreased thermal tolerance: Impact of proliferative kidney disease on a wild salmonid fish in the context of climate change. Funct. Ecol. 2017, 31, 216–226. [Google Scholar] [CrossRef]
- Schmidt-Posthaus, H.; Bettge, K.; Forster, U.; Segner, H.; Wahli, T. Kidney pathology and parasite intensity in rainbow trout Oncorhynchus mykiss surviving proliferative kidney disease: Time course and influence of temperature. Dis. Aquat. Org. 2012, 97, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.M.; Kelly, L.A.; Teffer, A.K.; Miller, K.M.; Cooke, S.J. Disease ecology of wild fish: Opportunities and challenges for linking infection metrics with behaviour, condition, and survival. Can. J. Fish Aquat. Sci. 2021, 78, 995–1007. [Google Scholar] [CrossRef]
- Tops, S.; Baxa, D.V.; McDowell, T.S.; Hedrick, R.P.; Okamura, B. Evaluation of malacosporean life cycles through transmission studies. Dis. Aquat. Org. 2004, 60, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Waldner, K.; Bechter, T.; Auer, S.; Borgwardt, F.; El-Matbouli, M.; Unfer, G. A brown trout (Salmo trutta) population faces devastating consequences due to proliferative kidney disease and temperature increase: A case study from Austria. Ecol. Freshw. Fish 2020, 29, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Soliman, H.; Kumar, G.; El-Matbouli, M. Tetracapsuloides bryosalmonae persists in brown trout Salmo trutta for five years post exposure. Dis. Aquat. Org. 2018, 127, 151–156. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: Synthesis Report. Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021; 40p. [Google Scholar]
- Dash, M.; Vasemägi, A. Proliferative kidney disease (PKD) agent Tetracapsuloides bryosalmonae in brown trout populations in Estonia. Dis. Aquat. Org. 2014, 109, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Posthaus, H.; Schneider, E.; Schölzel, N.; Hirschi, R.; Stelzer, M.; Peter, A. The role of migration barriers for dispersion of Proliferative Kidney Disease—Balance between disease emergence and habitat connectivity. PLoS ONE 2021, 16, e0247482. [Google Scholar] [CrossRef] [PubMed]
- Fischnetz. Final Report of Project Fischnetz; Eawag: Dübendorf, Switzerland; BUWAL: Bern, Switzerland, 2004; 188p. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]

| Station | Year | Sampling Date | Number of Sampled Fish | % of T. bryosalmonae-Infected Fish | Infection Severity | Degree Days | Number of Days with a Daily Mean Temperature ≥15 °C |
|---|---|---|---|---|---|---|---|
| BM1 | 1 | 5 June | 25 | 0 | 0.0 | 737 | 0 |
| 8 July | 25 | 0 | 0.0 | 1144 | 1 | ||
| 22 August | 25 | 0 | 0.0 | 1814 | 18 | ||
| 12 September | 25 | 0 | 0.0 | 2099 | 20 | ||
| 8 November | 31 | 0 | 0.0 | 2777 | 20 | ||
| 2 | 7 July | 2 | 0 | 0.0 | 1391 | 11 | |
| 8 September | 25 | 0 | 0.0 | 2316 | 35 | ||
| 3 | 14 July | 4 | 0 | 0.0 | 1499 | 15 | |
| 31 August | 14 | 7 * | 1.0 * | 2271 * | 49 * | ||
| BM2 | 1 | 5 June | 25 | 0 | 0.0 | 749 | 0 |
| 8 July | 25 | 0 | 0.0 | 1188 | 5 | ||
| 22 August | 25 | 68 | 3.9 | 1931 | 49 | ||
| 12 September | 25 | 68 | 3.2 | 2240 | 57 | ||
| 8 November | 26 | 31 | 1.5 | 2950 | 57 | ||
| 2 | 7 July | 18 | 0 | 0.0 | 1417 | 28 | |
| 8 September | 25 | 88 | 4.6 | 2444 | 88 | ||
| 3 | 14 July | 25 | 68 | 3.3 | 1627 | 34 | |
| 31 August | 25 | 88 | 3.5 | 2488 | 82 | ||
| V1 | 1 | 19 June | 25 | 0 | 0.0 | 834 | 0 |
| 12 July | 25 | 0 | 0.0 | 1047 | 0 | ||
| 20 August | 25 | 0 | 0.0 | 1465 | 0 | ||
| 2 October | 25 | 0 | 0.0 | 1892 | 0 | ||
| 29 November | 26 | 0 | 0.0 | 2339 | 0 | ||
| 2 | 1 September | 26 | 0 | 0.0 | 1730 | 0 | |
| V2 | 1 | 19 June | 25 | 0 | 0.0 | 1056 | 3 |
| 12 July | 26 | 0 | 0.0 | 1397 | 13 | ||
| 20 August | 25 | 40 | 2.9 | 2064 | 50 | ||
| 2 October | 25 | 44 | 1.8 | 2661 | 63 | ||
| 29 November | 25 | 44 | 1.3 | 3278 | 63 | ||
| 2 | 1 September | 24 | 38 | 2.8 | 2366 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubin, A.; Bailey, C.; Strepparava, N.; Wahli, T.; Segner, H.; Rubin, J.-F. Reliable Field Assessment of Proliferative Kidney Disease in Wild Brown Trout, Salmo trutta, Populations: When Is the Optimal Sampling Period? Pathogens 2022, 11, 681. https://doi.org/10.3390/pathogens11060681
Rubin A, Bailey C, Strepparava N, Wahli T, Segner H, Rubin J-F. Reliable Field Assessment of Proliferative Kidney Disease in Wild Brown Trout, Salmo trutta, Populations: When Is the Optimal Sampling Period? Pathogens. 2022; 11(6):681. https://doi.org/10.3390/pathogens11060681
Chicago/Turabian StyleRubin, Aurélie, Christyn Bailey, Nicole Strepparava, Thomas Wahli, Helmut Segner, and Jean-François Rubin. 2022. "Reliable Field Assessment of Proliferative Kidney Disease in Wild Brown Trout, Salmo trutta, Populations: When Is the Optimal Sampling Period?" Pathogens 11, no. 6: 681. https://doi.org/10.3390/pathogens11060681






