Oral and Intragastric: New Routes of Infection by Leishmania braziliensis and Leishmania infantum?
Abstract
1. Introduction
2. Results
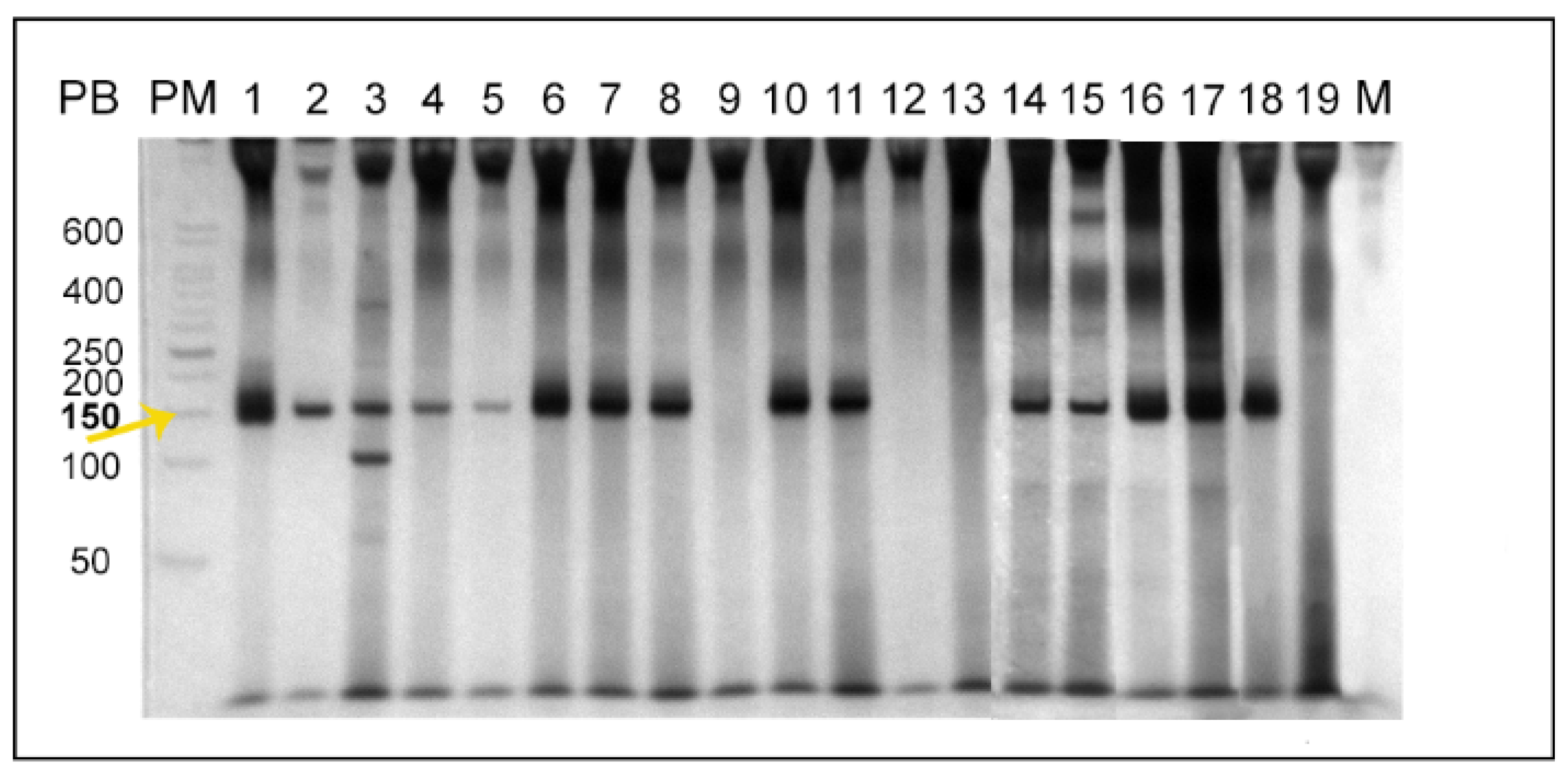
2.1. Leishmania braziliensis
2.2. Leishmania infantum
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Parasites and Infection
4.3. Obtaining Infected Macrophages
4.4. Lutzomyia Longipalpis Feed on Infected Hamsters
4.5. Euthanasia and Sample Collection
4.6. Tissue Imprints
4.7. Cultures
4.8. Multiplex PCR for the Detection of Leishmania sp. kDNA
4.9. Serological Diagnosis
4.10. Histopathological Diagnosis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and Poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; de Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Brandão-Filho, S.P.; Brito, M.E.; Carvalho, F.G.; Ishikaw, E.A.; Cupolillo, E.; Floeter-Winter, L.; Shaw, J.J. Wild and Synanthropic Hosts of Leishmania (Viannia) Braziliensis in the Endemic Cutaneous Leishmaniasis Locality of Amaraji, Pernambuco State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 291–296. [Google Scholar] [CrossRef]
- Santiago, M.E.B.; Vasconcelos, R.O.; Fattori, K.R.; Munari, D.P.; de Michelin, A.F.; Lima, V.M.F. An Investigation of Leishmania spp. in Didelphis spp. from Urban and Peri-Urban Areas in Bauru (São Paulo, Brazil). Vet. Parasitol. 2007, 150, 283–290. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Jansen, A.M. Wild and Synanthropic Reservoirs of Leishmania Species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- da Silva-Nunes, M.; Cavasini, C.E.; da Silva, N.S.; Galati, E.A.B. Epidemiologia Da Leishmaniose Tegumentar e Descrição Das Populações de Flebotomíneos No Município de Acrelândia, Acre, Brasil. Revista Brasileira de Epidemiologia 2008, 11, 241–251. [Google Scholar] [CrossRef][Green Version]
- Silva, J.G.D.E.; Werneck, G.L.; do Cruz, M.S.P.E.; Costa, C.H.N.; de Mendonça, I.L. Natural Infection of Lutzomyia Longipalpis by Leishmania sp. in Teresina, Piauí State, Brazil. Cadernos de Saúde Pública 2007, 23, 1715–1720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quinnell, R.J.; Courtenay, O. Transmission, Reservoir Hosts and Control of Zoonotic Visceral Leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.B.; de Marzochi, M.C.A.; de Carvalho, R.W.; Ribeiro, P.C.; dos Pontes, C.S.; Caetano, J.M.; de Meira, A.M. Absence of Lutzomyia Longipalpis in Some Endemic Visceral Leishmaniasis Areas in Rio de Janeiro Municipality. Cadernos de Saúde Pública 2003, 19, 1881–1885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinto, I.D.S.; dos Santos, C.B.; Grimaldi, G., Jr.; Ferreira, A.L.; Falqueto, A. American Visceral Leishmaniasis Dissociated from Lutzomyia Longipalpis (Diptera, Psychodidae) in the State of Espírito Santo, Brazil. Cadernos de Saúde Pública 2010, 26, 365–372. [Google Scholar] [CrossRef]
- Rêgo, F.D.; Souza, G.D.; Miranda, J.B.; Peixoto, L.V.; Andrade-Filho, J.D. Potential Vectors of Leishmania Parasites in a Recent Focus of Visceral Leishmaniasis in Neighborhoods of Porto Alegre, State of Rio Grande Do Sul, Brazil. J. Med. Entomol. 2020, 57, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Galvis-Ovallos, F.; Ueta, A.E.; Marques, G.D.O.; Sarmento, A.M.C.; Araujo, G.; Sandoval, C.; Tomokane, T.Y.; da Matta, V.L.R.; Laurenti, M.D.; Galati, E.A.B. Detection of Pintomyia Fischeri (Diptera: Psychodidae) with Leishmania Infantum (Trypanosomatida: Trypanosomatidae) Promastigotes in a Focus of Visceral Leishmaniasis in Brazil. J. Med. Entomol. 2021, 58, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, A.A.; Schantz, P.; Jackson, J.; Birkenheuer, A.; Tomlinson, L.; Gramiccia, M.; Levy, M.; Steurer, F.; Kollmar, E.; Hegarty, B.C.; et al. Visceral Leishmaniasis in a New York Foxhound Kennel. J. Vet. Intern. Med. 2002, 16, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Singh, S. Transfusion Transmitted Leishmaniasis: A Case Report and Review of Literature. Indian J. Med. Microbiol. 2006, 24, 165–170. [Google Scholar] [CrossRef]
- Rosypal, A.C.; Troy, G.C.; Zajac, A.M.; Frank, G.; Lindsay, D.S. Transplacental Transmission of a North American Isolate of Leishmania Infantum in an Experimentally Infected Beagle. J. Parasitol. 2005, 91, 970–972. [Google Scholar] [CrossRef]
- Zinchuk, A.; Nadraga, A. Congenital Visceral Leishmaniasis in Ukraine: Case Report. Ann. Trop. Paediatr. 2010, 30, 161–164. [Google Scholar] [CrossRef]
- Mescouto-Borges, M.R.M.; Maués, É.; Costa, D.L.; da Silva Pranchevicius, M.C.; Romero, G.A.S. Congenitally Transmitted Visceral Leishmaniasis: Report of Two Brazilian Human Cases. Braz. J. Infect. Dis. 2013, 17, 263–266. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Norman, F.F.; Cruz, I.; Alvar, J.; López-Vélez, R. Visceral Leishmaniasis and HIV Coinfection in the Mediterranean Region. PLoS Negl. Trop. Dis. 2014, 8, e3021. [Google Scholar] [CrossRef]
- Carrasco-Antón, N.; López-Medrano, F.; Fernández-Ruiz, M.; Carrillo, E.; Moreno, J.; García-Reyne, A.; Pérez-Ayala, A.; Rodríguez-Ferrero, M.L.; Lumbreras, C.; San-Juan, R.; et al. Environmental Factors as Key Determinants for Visceral Leishmaniasis in Solid Organ Transplant Recipients, Madrid, Spain. Emerg. Infect. Dis. 2017, 23, 1155–1159. [Google Scholar] [CrossRef]
- Gajurel, K.; Dhakal, R.; Deresinski, S. Leishmaniasis in Solid Organ and Hematopoietic Stem Cell Transplant Recipients. Clin. Transplant. 2017, 31, e12867. [Google Scholar] [CrossRef]
- Diniz, S.A.; Melo, M.S.; Borges, A.M.; Bueno, R.; Reis, B.P.; Tafuri, W.L.; Nascimento, E.F.; Santos, R.L. Genital Lesions Associated with Visceral Leishmaniasis and Shedding of Leishmania sp. in the Semen of Naturally Infected Dogs. Vet. Pathol. 2005, 42, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Quintal, A.P.N. Leishmaniose Visceral Em Hamsters Experimentalmente Infectados: Doença Venérea Em Machos Com Transmissão Sexual; Universidade Federal de Uberlândia: Uberlândia, Brazil, 2015. [Google Scholar]
- Coutinho, M.T.Z.; Bueno, L.L.; Sterzik, A.; Fujiwara, R.T.; Botelho, J.R.; de Maria, M.; Genaro, O.; Linardi, P.M. Participation of Rhipicephalus Sanguineus (Acari: Ixodidae) in the Epidemiology of Canine Visceral Leishmaniasis. Vet. Parasitol. 2005, 128, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.A.; Odorizzi, R.M.F.N.; Laurenti, M.D.; Galati, E.A.B.; Canavez, F.; Pereira-Chioccola, V.L. Detection of Leishmania (Leishmania) Infantum RNA in Fleas and Ticks Collected from Naturally Infected Dogs. Parasitol. Res. 2011, 109, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; D’Andrea, P.S.; Xavier, S.C.C.; Mangia, R.H.; Fernandes, O.; Jansen, A.M. Trypanosoma Cruzi Infection in Wild Mammals of the National Park “Serra Da Capivara” and Its Surroundings (Piaui, Brazil), an Area Endemic for Chagas Disease. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 379–388. [Google Scholar] [CrossRef]
- Rocha, M.N.; Corrêa, C.M.; Melo, M.N.; Beverley, S.M.; Martins-Filho, O.A.; Madureira, A.P.; Soares, R.P. An Alternative in Vitro Drug Screening Test Using Leishmania Amazonensis Transfected with Red Fluorescent Protein. Diagn. Microbiol. Infect. Dis. 2013, 75, 282–291. [Google Scholar] [CrossRef]
- Rueda, K.; Trujillo, J.E.; Carranza, J.C.; Vallejo, G.A. Transmisión Oral de Trypanosoma Cruzi: Una Nueva Situación Epidemiológica de La Enfermedad de Chagas En Colombia y Otros Países Suramericanos. Biomédica 2014, 3434, 631–641. [Google Scholar] [CrossRef]
- Baker, J.R. Bat Trypanosome Models for Trypanosoma Cruzi. Parasitol. Today 1985, 1, 111–113. [Google Scholar] [CrossRef]
- Roellig, D.M.; Ellis, A.E.; Yabsley, M.J. Oral Transmission of Trypanosoma Cruzi with Opposing Evidence for the Theory of Carnivory. J. Parasitol. 2009, 95, 360–364. [Google Scholar] [CrossRef]
- Franzén, O.; Talavera-López, C.; Ochaya, S.; Butler, C.E.; Messenger, L.A.; Lewis, M.D.; Llewellyn, M.S.; Marinkelle, C.J.; Tyler, K.M.; Miles, M.A.; et al. Comparative Genomic Analysis of Human Infective Trypanosoma Cruzi Lineages with the Bat-Restricted Subspecies T. Cruzi Marinkellei. BMC Genom. 2012, 13, 531. [Google Scholar] [CrossRef]
- Eisenstein, M. Disease: Poverty and Pathogens. Nature 2016, 531, S61–S63. [Google Scholar] [CrossRef]
- Balouz, V.; Agüero, F.; Buscaglia, C.A. Chagas Disease Diagnostic Applications: Present Knowledge and Future Steps. Adv. Parasitol. 2017, 97, 1–45. [Google Scholar] [CrossRef]
- Maraghi, S.; Wallbanks, K.R.; Molyneux, D.H. Oral Transmission of Trypanosomes of the Subgenus Herpetosoma from Small Mammals. Parasitol. Res. 1995, 81, 693–695. [Google Scholar] [CrossRef]
- Howie, S.; Guy, M.; Fleming, L.; Bailey, W.; Noyes, H.; Faye, J.A.; Pepin, J.; Greenwood, B.; Whittle, H.; Molyneux, D.; et al. A Gambian Infant with Fever and an Unexpected Blood Film. PLoS Med. 2006, 3, 1508–1512. [Google Scholar] [CrossRef]
- Pumhom, P.; Morand, S.; Tran, A.; Jittapalapong, S.; Desquesnes, M. Trypanosoma from Rodents as Potential Source of Infection in Human-Shaped Landscapes of South-East Asia. Vet. Parasitol. 2015, 208, 174–180. [Google Scholar] [CrossRef]
- Camargo, E.P. Phytomonas and Other Trypanosomatid Parasites of Plants and Fruit. Adv. Parasitol. 1998, 42, 29–112. [Google Scholar]
- Maslov, D.A.; Votýpka, J.; Yurchenko, V.; Lukeš, J. Diversity and Phylogeny of Insect Trypanosomatids: All That is Hidden Shall Be Revealed. Trends Parasitol. 2013, 29, 43–52. [Google Scholar] [CrossRef]
- Espinosa, O.A.; Serrano, M.G.; Camargo, E.P.; Teixeira, M.M.G.; Shaw, J.J. An Appraisal of the Taxonomy and Nomenclature of Trypanosomatids Presently Classified as Leishmania and Endotrypanum. Parasitology 2018, 145, 430–442. [Google Scholar] [CrossRef]
- Wilson, V.C.L.C.; Southgate, B.A. Lizard Leishmania. In Biology of the Kinetoplastida; Lumsden, W.H.R., Evans, D.A., Eds.; Academic Press: London, UK, 1979; Volume 2, pp. 241–268. [Google Scholar]
- Bates, P.A. Transmission of Leishmania Metacyclic Promastigotes by Phlebotomine Sand Flies. Int. J. Parasitol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef]
- Karkamo, V.; Kaistinen, A.; Näreaho, A.; Dillard, K.; Vainio-Siukola, K.; Vidgrén, G.; Tuoresmäki, N.; Anttila, M. The First Report of Autochthonous Non-Vector-Borne Transmission of Canine Leishmaniosis in the Nordic Countries. Acta Vet. Scand. 2014, 56, 84. [Google Scholar] [CrossRef]
- Daval, N.; Marchal, C.; Guillaumot, L.; Hüe, T.; Ravel, C.; Keck, N.; Kasbari, M. First Report of Autochthonous Non-Vectorial Canine Leishmaniasis in New Caledonia, South-Western Pacific: Implications for New Control Measures and Recommendations on Importation of Dogs. Parasites Vectors 2016, 9, 108. [Google Scholar] [CrossRef][Green Version]
- Naucke, T.J.; Amelung, S.; Lorentz, S. First Report of Transmission of Canine Leishmaniosis through Bite Wounds from a Naturally Infected Dog in Germany. Parasites Vectors 2016, 9, 256. [Google Scholar] [CrossRef]
- Gomes-Silva, A.; Valverde, J.G.; Ribeiro-Romão, R.P.; Plácido-Pereira, R.M.; Da-Cruz, A.M. Golden Hamster (Mesocricetus Auratus) as an Experimental Model for Leishmania (Viannia) Braziliensis Infection. Parasitology 2013, 140, 771–779. [Google Scholar] [CrossRef]
- Saini, S.; Rai, A.K. Hamster, a Close Model for Visceral Leishmaniasis: Opportunities and Challenges. Parasite Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef]
- Reis, N.R.; Shibatta, O.A.; Peracchi, A.L.; Pedro, W.A.; Lima, I.P. Sobre Os Morcegos Brasileiros; Universidade Estadual de Londrina: Londrina, Brazil, 2007; ISBN 9788590639510. [Google Scholar]
- Santos, T. Análise Parcimoniosa de Endemismo (Pae) Dos Mamíferos Terrestres Do Novo Mundo. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2012. [Google Scholar]
- Dyce, K.M.; Wensing, C.J.G.; Sack, W.O. Tratado de Anatomia Veterinária; Elsevier: Rio de Janeiro, Brazil, 2010; ISBN 978-85-352-3672-9. [Google Scholar]
- Tavares, N.M.; Santos, D.M.; de Oliveira, C.I.; Brodskyn, C.I. Estratégias de Vacinação Contra Leishmaniose Visceral e Cutânea: Lições Dos Modelos Experimentais. Gazeta Médica da Bahia 2009, 79, 110–121. [Google Scholar]
- Travi, B.L.; Osorio, Y.; Melby, P.C.; Chandrasekar, B.; Arteaga, L.; Saravia, N.G. Gender is a Major Determinant of the Clinical Evolution and Immune Response in Hamsters Infected with Leishmania spp. Infect. Immun. 2002, 70, 2288–2296. [Google Scholar] [CrossRef]
- Requena, J.M.; Soto, M.; Doria, M.D.; Alonso, C. Immune and Clinical Parameters Associated with Leishmania Infantum Infection in the Golden Hamster Model. Vet. Immunol. Immunopathol. 2000, 76, 269–281. [Google Scholar] [CrossRef]
- de Lima Celeste, J.L.; Moura, A.P.V.; França-Silva, J.C.; De Sousa, G.M.; Silva, S.O.; Melo, M.N.; Tafuri, W.L.; Souza, C.C.; De Andrade, H.M. Experimental Mixed Infection of Leishmania (Leishmania) Amazonensis and Leishmania (L.) Infantum in Hamsters (Mesocricetus Auratus). Parasitology 2017, 144, 1191–1202. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Murty, V.L.N.; Piotrowski, J.; Slomiany, A. Salivary Mucins in Oral Mucosal Defense. Gen. Pharmacol. 1996, 27, 761–771. [Google Scholar] [CrossRef]
- Asoh, T.; Saito, M.; Villanueva, S.Y.A.M.; Kanemaru, T.; Gloriani, N.; Yoshida, S. Natural Defense by Saliva and Mucosa against Oral Infection by Leptospira. Can. J. Microbiol. 2014, 60, 383–389. [Google Scholar] [CrossRef]
- da Silva, A.L.; Williams, P.; Melo, M.N.; Mayrink, W. Susceptibility of Laboratory-Reared Female Lutzomyia Longipalpis (Lutz & Neiva, 1912) to Infection by Different Species and Strains of Leishmania Ross, 1903. Memorias do Instituto Oswaldo Cruz 1990, 85, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Salomón, O.D.; Feliciangeli, M.D.; Quintana, M.G.; Afonso, M.M.D.S.; Rangel, E.F. Lutzomyia Longipalpis Urbanisation and Control. Memorias do Instituto Oswaldo Cruz 2015, 110, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, R.S.; Filho, A.A.P.; Moraes, J.L.P.; Kuppinger, O.; Rebêlo, J.M.M. Experimental Infection of Lutzomyia (Nyssomyia) Whitmani (Diptera: Psychodidae: Phlebotominae) with Leishmania (Viannia) Braziliensis and Leishmania (L.) Amazonensis, Etiological Agents of American Tugumentary Leishmaniasis. J. Med. Entomol. 2016, 53, 206–209. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Oshiro, E.T.; Fernandes, W.S.; Murat, P.G.; de Medeiros, M.J.; Souza, A.I.; de Oliveira, A.G.; Galati, E.A.B. Experimental Infection and Transmission of Leishmania by Lutzomyia Cruzi (Diptera: Psychodidae): Aspects of the Ecology of Parasite-Vector Interactions. PLoS Negl. Trop. Dis. 2017, 11, e0005401. [Google Scholar] [CrossRef] [PubMed]
- Doehl, J.S.P.; Bright, Z.; Dey, S.; Davies, H.; Magson, J.; Brown, N.; Romano, A.; Dalton, J.E.; Pinto, A.I.; Pitchford, J.W.; et al. Skin Parasite Landscape Determines Host Infectiousness in Visceral Leishmaniasis. Nat. Commun. 2017, 8, 57. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Cupolillo, E.; Marchevsky, R.S.; Jansen, A.M. Thrichomys Laurentius (Rodentia; Echimyidae) as a Putative Reservoir of Leishmania Infantum and L. braziliensis: Patterns of Experimental Infection. PLoS Negl. Trop. Dis. 2010, 4, e589. [Google Scholar] [CrossRef]
- Alexander, J.; Russell, D.G. The Interaction of Leishmania Species with Macrophages. Adv. Parasitol. 1992, 31, 175–254. [Google Scholar] [CrossRef]
- Sánchez, M.D.B.; Serrano, X. A Alimentação Nas Diferentes Fases Da Vida Do Cão; Universitat Autònoma de Barcelona: Bellaterra, Spain, 1997; 45p, Available online: https://ddd.uab.cat/record/166829 (accessed on 19 August 2020).
- Case, L.; Daristotle, L.; Hayek, M.; Raasch, M.F. Canine and Feline Nutrition; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hoekstra, R.; Vazire, S. Aspiring to Greater Intellectual Humility in Science. Nat. Hum. Behav. 2021, 5, 1602–1607. [Google Scholar] [CrossRef]
- Giemsa, G. Eine Vereinfachung Und Vervollkommnung Meiner Methylenblau-Eosin-Färbemethode Zur Erzielung Der Romanowsky-Nocht’schen Chromatinfärbung. Centralblatt für Bakteriologie 1904, 32, 307–313. [Google Scholar]
- Lallo, M.A.; Bondan, E.F.; Xavier, J.G.; Hirschfeld, M.P.M. Stain Techniques for Detection of Encephalitozoon Cuniculi in Tissue Sections. Ciência Rural 2010, 40, 2406–2410. [Google Scholar] [CrossRef]
- Cássia-Pires, R.; Boité, M.C.; D’Andrea, P.S.; Herrera, H.M.; Cupolillo, E.; Jansen, A.M.; Roque, A.L.R. Distinct Leishmania Species Infecting Wild Caviomorph Rodents (Rodentia: Hystricognathi) from Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3389. [Google Scholar] [CrossRef]
- de Cássia-Pires, R.; de Melo, M.D.F.A.D.; da Hora Barbosa, R.; Roque, A.L.R. Multiplex PCR as a Tool for the Diagnosis of Leishmania spp. KDNA and the Gapdh Housekeeping Gene of Mammal Hosts. PLoS ONE 2017, 12, e0173922. [Google Scholar] [CrossRef]
- Camargo, M.E. Improved Technique of Indirect Immunofluorescence for Serological Diagnosis for Toxoplasmosis. Revista do Instituto de Medicina Tropical de São Paulo 1964, 6, 117–118. [Google Scholar]
- Brandão, E.M.V.; Xavier, S.C.C.; Carvalhaes, J.G.; D’Andrea, P.S.; Lemos, F.G.; Azevedo, F.C.; Cássia-Pires, R.; Jansen, A.M.; Roque, A.L.R. Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens 2019, 8, 190. [Google Scholar] [CrossRef]


| Group | Route of Infection | Culture (Tissue) | Imprint (Tissue) | Serology (Titre) | PCR (Tissue) | Histology |
|---|---|---|---|---|---|---|
| Lb1 | Intradermal (Control Group) | Positive (site of inoculation, spleen, and liver) | Positive (site of inoculation, spleen, and liver) | Positive (1/320) | Positive (site of inoculation, intact skin, spleen, and liver) | Negative |
| Lb2 | Intragastric | Positive (spleen and liver) | Positive (spleen) | Positive (1/160) | Positive (spleen and liver) | Negative |
| Lb3 | Oral—Axenic culture | Negative | Negative | Negative | Negative | Negative |
| Lb4 | Oral—Culture of macrophages (L. braziliensis) | Negative | Negative | Positive (1/1280) | Positive (stomach, spleen, and liver) | ND * |
| Lb5 | Oral—Sandflies | Negative | Negative | Negative | Negative | Negative |
| Lb6 | Oral—Fragment of dermal lesion | Negative | Negative | Negative | Negative | Negative |
| Lb7 | Oral—Fragment of spleen | Negative | Negative | Negative | Negative | Negative |
| Li1 | Intraperitoneal (Control Group) | Positive (intact skin, spleen and liver) | Negative | Positive (1/160) | Positive (intact skin, spleen and liver) | ND * |
| Li2 | Intragastric | Positive (spleen) | Negative | Positive (1/40) | Positive (intact skin, spleen and liver) | ND * |
| Li3 | Oral—Axenic culture | Negative | Negative | Negative | Negative | ND * |
| Li4 | Oral—Culture of macrophages (L. infantum) | Negative | Negative | Negative | Negative | ND * |
| Group | Route of Infection | Infected Hamsters (n) |
|---|---|---|
| Lb1 | Intradermal (Control Group) | 2 |
| Lb2 | Intragastric—Axenic culture | 2 |
| Lb3 | Oral—Axenic culture | 2 |
| Lb4 | Oral—Culture of macrophages infected with L. braziliensis | 2 |
| Lb5 | Oral—Lutzomyia longipalpis fed directly on skin lesions from animals of group Lb1 | 2 |
| Lb6 | Oral—Fragment of dermal lesion from animals of group Lb1 | 2 |
| Lb7 | Oral—Fragment of spleen with macroscopic lesions from an animal of group Lb2 | 2 |
| Li1 | Intraperitoneal (Control Group) | 2 |
| Li2 | Intragastric—Axenic culture | 6 |
| Li3 | Oral—Axenic culture | 6 |
| Li4 | Oral—Culture of macrophages infected with L. infantum | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reimann, M.M.; Torres-Santos, E.C.; Souza, C.S.F.d.; Andrade-Neto, V.V.; Jansen, A.M.; Brazil, R.P.; Roque, A.L.R. Oral and Intragastric: New Routes of Infection by Leishmania braziliensis and Leishmania infantum? Pathogens 2022, 11, 688. https://doi.org/10.3390/pathogens11060688
Reimann MM, Torres-Santos EC, Souza CSFd, Andrade-Neto VV, Jansen AM, Brazil RP, Roque ALR. Oral and Intragastric: New Routes of Infection by Leishmania braziliensis and Leishmania infantum? Pathogens. 2022; 11(6):688. https://doi.org/10.3390/pathogens11060688
Chicago/Turabian StyleReimann, Mayra M., Eduardo Caio Torres-Santos, Celeste S. F. de Souza, Valter V. Andrade-Neto, Ana Maria Jansen, Reginaldo P. Brazil, and André Luiz R. Roque. 2022. "Oral and Intragastric: New Routes of Infection by Leishmania braziliensis and Leishmania infantum?" Pathogens 11, no. 6: 688. https://doi.org/10.3390/pathogens11060688
APA StyleReimann, M. M., Torres-Santos, E. C., Souza, C. S. F. d., Andrade-Neto, V. V., Jansen, A. M., Brazil, R. P., & Roque, A. L. R. (2022). Oral and Intragastric: New Routes of Infection by Leishmania braziliensis and Leishmania infantum? Pathogens, 11(6), 688. https://doi.org/10.3390/pathogens11060688








