The Predominance of Klebsiella aerogenes among Carbapenem-Resistant Enterobacteriaceae Infections in Japan
Abstract
:1. Introduction
2. Results
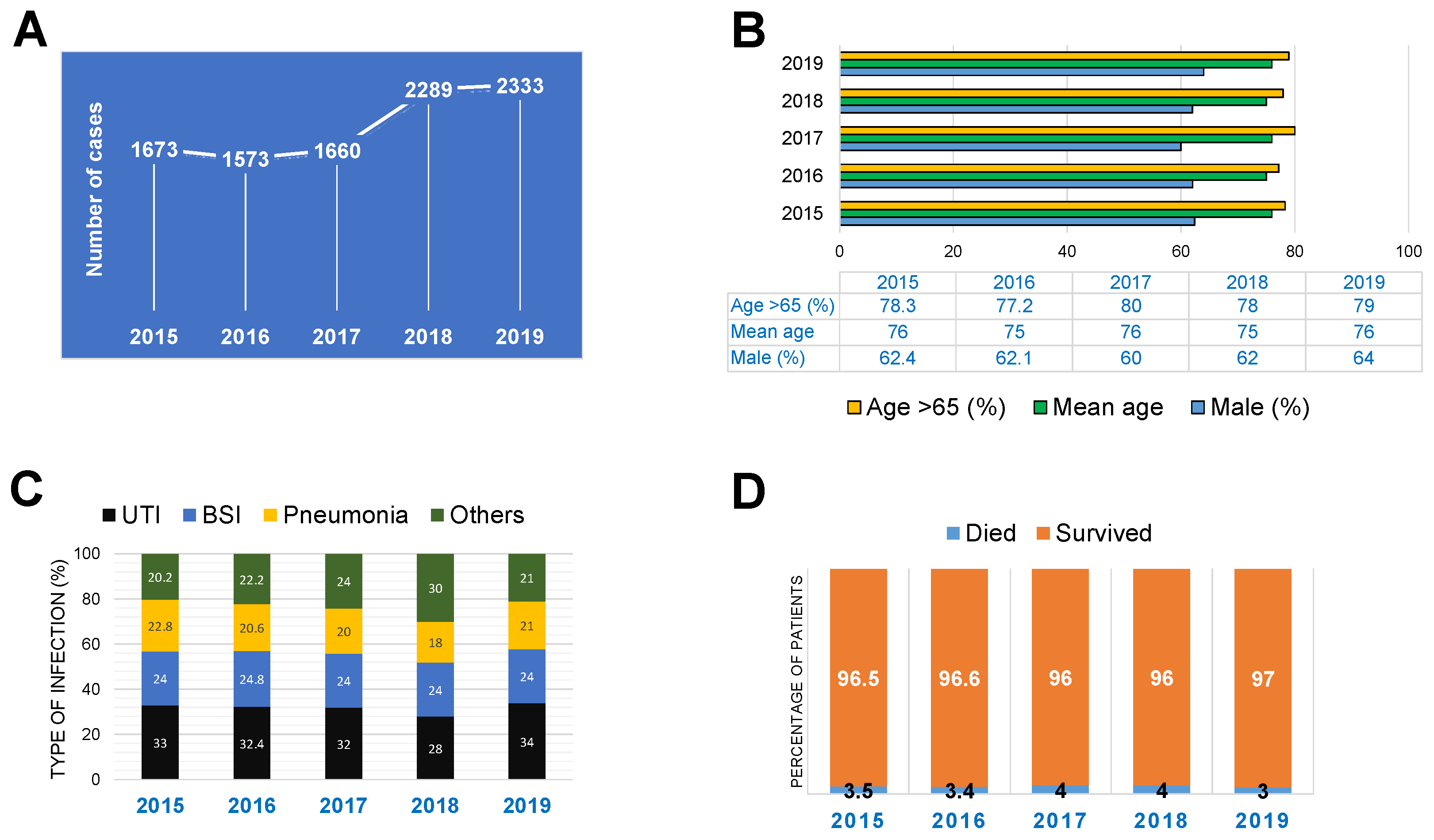
2.1. Epidemiological and Clinical Characteristics of CRE Infections in Japan
2.2. Microbiological Characteristics of CRE Infections
3. Discussion
4. Materials and Methods
Data Management and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic resistance in Enterobacteriaceae: Mechanisms and clinical implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.T.; Espinoza, H.V.; Espinoza, J.L. Emerging superbugs: The threat of Carbapenem Resistant Enterobacteriaceae. AIMS Microbiol. 2020, 6, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante-Mangoni, E.; Andini, R.; Zampino, R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin. Microbiol. Infect. 2019, 25, 943–950. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macareño-Castro, J.; Solano-Salazar, A.; Dong, L.T.; Mohiuddin, M.; Espinoza, J.L. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. J. Infect. 2022, 84, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections Caused by Carbapenem-Resistant. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischendorf, J.; de Avila, R.A.; Safdar, N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am. J. Infect. Control 2016, 44, 539–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindall, B.J.; Sutton, G.; Garrity, G.M. Enterobacter aerogenes Hormaeche and Edwards 1960 (Approved Lists 1980) and Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980) share the same nomenclatural type (ATCC 13048) on the Approved Lists and are homotypic synonyms, with consequences for the name Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980). Int. J. Syst. Evol. Microbiol. 2017, 67, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Marín, R.; Lepe, J.A.; Gasch-Blasi, O.; Rodríguez-Martínez, J.M.; Calvo-Montes, J.; Lara-Contreras, R.; Martín-Gandul, C.; Tubau-Quintano, F.; Cano-García, M.E.; Rodríguez-López, F.; et al. Clinical characteristics and outcome of bacteraemia caused by Enterobacter cloacae and Klebsiella aerogenes: More similarities than differences. J. Glob. Antimicrob. Resist. 2021, 25, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wesevich, A.; Sutton, G.; Ruffin, F.; Park, L.P.; Fouts, D.E.; Fowler, V.G.; Thaden, J.T. Newly Named Klebsiella aerogenes (formerly Enterobacter aerogenes) Is Associated with Poor Clinical Outcomes Relative to Other. J. Clin. Microbiol. 2020, 58, e00582-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, H.; Zhao, Y.; Shang, L.; Di, L.; Lyu, C.; Liu, J. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosn. J. Basic. Med. Sci. 2019, 19, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.J.; Hsieh, C.F.; Chang, P.C.; Chen, J.J.; Lin, Y.H.; Lai, C.C.; Chao, C.M.; Chuang, Y.C. Clinical Significance of Community- and Healthcare-Acquired Carbapenem-Resistant Enterobacteriaceae Isolates. PLoS ONE 2016, 11, e0151897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, K.; Matsumoto, A.; Tetsuka, N.; Morioka, H.; Iguchi, M.; Ishiguro, N.; Nagamori, T.; Takahashi, S.; Saito, N.; Tokuda, K.; et al. Clinical characteristics and treatment outcomes of carbapenem-resistant Enterobacterales infections in Japan. J. Glob. Antimicrob. Resist. 2022, 29, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, O.A.; Igwe, H.A. Carbapenem-resistant. AIMS Public Health 2020, 7, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.M.; Larsson, M.; Olson, L.; Hoang, N.T.B.; Le, N.K.; Khu, D.T.K.; Nguyen, H.D.; Vu, T.V.; Trinh, T.H.; Le, T.Q.; et al. High prevalence of colonisation with carbapenem-resistant Enterobacteriaceae among patients admitted to Vietnamese hospitals: Risk factors and burden of disease. J. Infect. 2019, 79, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabino, S.; Soares, S.; Ramos, F.; Moretti, M.; Zavascki, A.P.; Rigatto, M.H. A Cohort Study of the Impact of Carbapenem-Resistant. mSphere 2019, 4, e00052-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Tansarli, G.S.; Karageorgopoulos, D.E.; Vardakas, K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014, 20, 1170–1175. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamio, K.; Espinoza, J.L. The Predominance of Klebsiella aerogenes among Carbapenem-Resistant Enterobacteriaceae Infections in Japan. Pathogens 2022, 11, 722. https://doi.org/10.3390/pathogens11070722
Kamio K, Espinoza JL. The Predominance of Klebsiella aerogenes among Carbapenem-Resistant Enterobacteriaceae Infections in Japan. Pathogens. 2022; 11(7):722. https://doi.org/10.3390/pathogens11070722
Chicago/Turabian StyleKamio, Kosuke, and J. Luis Espinoza. 2022. "The Predominance of Klebsiella aerogenes among Carbapenem-Resistant Enterobacteriaceae Infections in Japan" Pathogens 11, no. 7: 722. https://doi.org/10.3390/pathogens11070722
APA StyleKamio, K., & Espinoza, J. L. (2022). The Predominance of Klebsiella aerogenes among Carbapenem-Resistant Enterobacteriaceae Infections in Japan. Pathogens, 11(7), 722. https://doi.org/10.3390/pathogens11070722






