HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity
Abstract
:1. Introduction
2. Clinical Phenotypes
2.1. Epidermodysplasia Verruciformis
2.2. Profuse Warts (PWs)
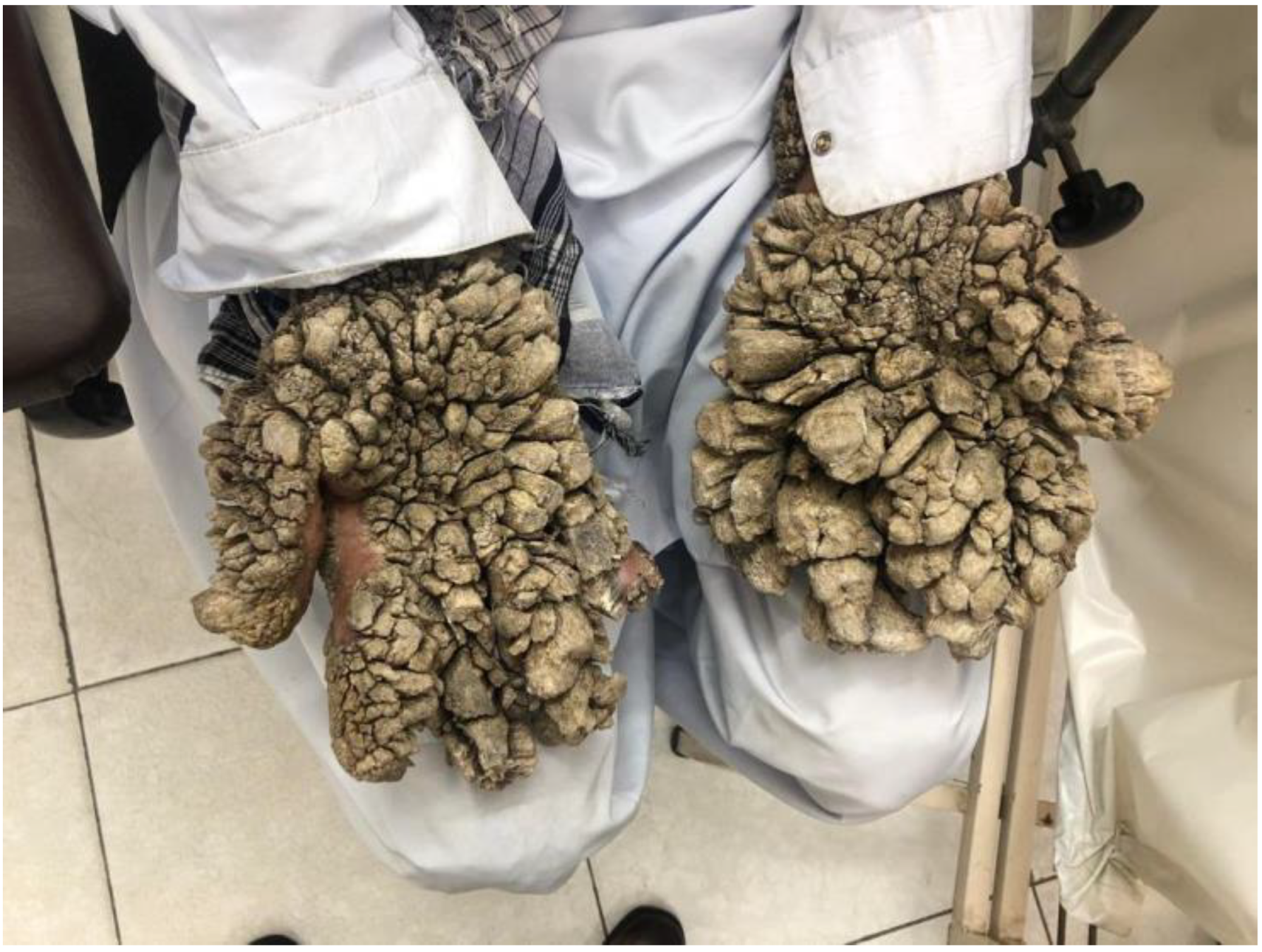
2.3. Tree Man Syndrome
3. Immunological Phenotypes and Inborn Errors of Immunity
3.1. No immunological Phenotype in Blood (Skin-Intrinsic Immunity Disorder)
3.2. Immunological Phenotype with Qualitative or/and Quantitative T Cells Defects Only
3.3. Immunological Phenotype with Several Impaired Leukocyte Subsets
4. Warts and IEI: Diagnostic Strategy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernard, H.-U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.-M. Classification of Papillomaviruses (PVs) Based on 189 PV Types and Proposal of Taxonomic Amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PaVE: Papilloma Virus Genome Database. Available online: https://pave.niaid.nih.gov/#home (accessed on 22 October 2021).
- Massing, A.M.; Epstein, W.L. Natural History of Warts. A Two-Year Study. Arch. Dermatol. 1963, 87, 306–310. [Google Scholar] [CrossRef]
- Kainz, J.T.; Kozel, G.; Haidvogl, M.; Smolle, J. Homoeopathic versus Placebo Therapy of Children with Warts on the Hands: A Randomized, Double-Blind Clinical Trial. Dermatology 1996, 193, 318–320. [Google Scholar] [CrossRef]
- Loo, S.K.; Tang, W.Y. Warts (Non-Genital). BMJ Clin. Evid. 2009, 2009, 1710. [Google Scholar] [PubMed]
- Béziat, V. Human genetic dissection of papillomavirus-driven diseases: New insight into their pathogenesis. Hum. Genet. 2020, 139, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Rapaport, F.; Hu, J.; Titeux, M.; Bonnet des Claustres, M.; Bourgey, M.; Griffin, H.; Bandet, É.; Ma, C.S.; Sherkat, R.; et al. Humans with Inherited T Cell CD28 Deficiency Are Susceptible to Skin Papillomaviruses but Are Otherwise Healthy. Cell 2021, 184, 3812–3828.e30. [Google Scholar] [CrossRef] [PubMed]
- Zambruno, G. Epidermodysplasie Verruciforme, Orpha.Net. Available online: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=FR&data_id=8531&MISSING%20CONTENT=Epidermodisplasia-verruciforme&search=Disease_Search_Simple&title=Epidermodisplasia-verruciforme (accessed on 11 July 2022).
- de Jong, S.J.; Imahorn, E.; Itin, P.; Uitto, J.; Orth, G.; Jouanguy, E.; Casanova, J.-L.; Burger, B. Epidermodysplasia Verruciformis: Inborn Errors of Immunity to Human Beta-Papillomaviruses. Front. Microbiol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Orth, G. Génétique et Sensibilité Aux Papillomavirus: Le Modèle de l’épidermodysplasie Verruciforme. Bull. Acad. Natl. Méd. 2010, 194, 923–941. [Google Scholar] [CrossRef]
- Leung, L. Recalcitrant Nongenital Warts. Aust. Fam. Physician 2011, 40, 40–42. [Google Scholar] [PubMed]
- Uitto, J.; Saeidian, A.H.; Youssefian, L.; Saffarian, Z.; Casanova, J.-L.; Béziat, V.; Jouanguy, E.; Vahidnezhad, H. Recalcitrant Warts, Epidermodysplasia Verruciformis, and the Tree-Man Syndrome: Phenotypic Spectrum of Cutaneous Human Papillomavirus Infections at the Intersection of Genetic Variability of Viral and Human Genomes. J. Invest. Dermatol. 2022, 142, 1265–1269. [Google Scholar] [CrossRef]
- Emanuel, P. Verruca Vulgaris Pathology. Available online: https://dermnetnz.org/topics/verruca-vulgaris-pathology (accessed on 11 June 2022).
- de Jong, S.J.; Créquer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The Human CIB1–EVER1–EVER2 Complex Governs Keratinocyte-Intrinsic Immunity to β-Papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, S.E.; Kilic, S.S.; Aytekin, C.; Kumar, A.; Porras, O.; Kainulainen, L.; Kostyuchenko, L.; Genel, F.; Kütükcüler, N.; Karaca, N.; et al. DOCK8 Deficiency: Clinical and Immunological Phenotype and Treatment Options—A Review of 136 Patients. J. Clin. Immunol. 2015, 35, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, H.; Appaswamy, G.; Kotlarz, D.; Diestelhorst, J.; Beier, R.; Schäffer, A.A.; Gertz, E.M.; Schambach, A.; Kreipe, H.H.; Pfeifer, D.; et al. The phenotype of human STK4 deficiency. Blood 2012, 119, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Alazami, A.M.; Al-Helale, M.; Alhissi, S.; Al-Saud, B.; Alajlan, H.; Monies, D.; Shah, Z.; Abouelhoda, M.; Arnaout, R.; Al-Dhekri, H.; et al. Novel CARMIL2 Mutations in Patients with Variable Clinical Dermatitis, Infections, and Combined Immunodeficiency. Front. Immunol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousfiha, A.; Jeddane, L.; Picard, C.; Al-Herz, W.; Ailal, F.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 2020, 40, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Crequer, A.; Troeger, A.; Patin, E.; Ma, C.S.; Picard, C.; Pedergnana, V.; Fieschi, C.; Lim, A.; Abhyankar, A.; Gineau, L.; et al. Human RHOH Deficiency Causes T Cell Defects and Susceptibility to EV-HPV Infections. J. Clin. Invest. 2012, 122, 3239–3247. [Google Scholar] [CrossRef]
- OMIM—Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 20 October 2021).
- Crequer, A.; Picard, C.; Patin, E.; D’Amico, A.; Abhyankar, A.; Munzer, M.; Debré, M.; Zhang, S.-Y.; de Saint-Basile, G.; Fischer, A.; et al. Inherited MST1 Deficiency Underlies Susceptibility to EV-HPV Infections. PLoS ONE 2012, 7, e44010. [Google Scholar] [CrossRef] [PubMed]
- Guerouaz, N.; Ismaili, N.; Bousfiha, M.A.; Ailal, F.; Picard, C.; Hassam, B.; Senouci, K. Le déficit en DOCK8 (dedicator of cytokinesis 8 gene): À propos d’un nouveau cas. Ann Dermatol Venerol. Ann. Dermatol. Vénéreol. 2014, 141, S502–S503. Available online: https://www.sciencedirect.com/science/article/pii/B9780123742797140238 (accessed on 20 October 2021). [CrossRef]
- Yee, C.S.; Massaad, M.J.; Bainter, W.; Ohsumi, T.K.; Föger, N.; Chan, A.C.; Akarsu, N.A.; Aytekin, C.; Ayvaz, D.Ç.; Tezcan, I.; et al. Recurrent Viral Infections Associated with a Homozygous CORO1A Mutation That Disrupts Oligomerization and Cytoskeletal Association. J. Allergy Clin. Immunol. 2016, 137, 879–888.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béziat, V.; Jouanguy, E. Human inborn errors of immunity to oncogenic viruses. Curr. Opin. Immunol. 2021, 72, 277–285. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.S.; Ling, Y.; Bousfiha, A.; Camcioglu, Y.; Jacquot, S.; Payne, K.; Crestani, E.; Roncagalli, R.; Belkadi, A.; et al. Dual T Cell- and B Cell-Intrinsic Deficiency in Humans with Biallelic RLTPR Mutations. J. Exp. Med. 2016, 213, 2413–2435. [Google Scholar] [CrossRef] [PubMed]
- Dotta, L.; Notarangelo, L.D.; Moratto, D.; Kumar, R.; Porta, F.; Soresina, A.; Lougaris, V.; Plebani, A.; Smith, C.E.; Norlin, A.C.; et al. Long-Term Outcome of WHIM Syndrome in 18 Patients: High Risk of Lung Disease and HPV-Related Malignancies. J. Allergy Clin. Immunol. Pract. 2019, 7, 1568–1577. [Google Scholar] [CrossRef] [Green Version]
- Volk, T.; Pannicke, U.; Reisli, I.; Bulashevska, A.; Ritter, J.; Björkman, A.; Schäffer, A.A.; Fliegauf, M.; Sayar, E.H.; Salzer, U.; et al. DCLRE1C (ARTEMIS) Mutations Causing Phenotypes Ranging from Atypical Severe Combined Immunodeficiency to Mere Antibody Deficiency. Hum. Mol. Genet. 2015, 24, 7361–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eränkö, E.; Ilander, M.; Tuomiranta, M.; Mäkitie, A.; Lassila, T.; Kreutzman, A.; Klemetti, P.; Mustjoki, S.; Hannula-Jouppi, K.; Ranki, A. Immune Cell Phenotype and Functional Defects in Netherton Syndrome. Orphanet J. Rare Dis. 2018, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Leiding, J.W.; Holland, S.M. Warts and All: HPV in Primary Immunodeficiencies. J. Allergy Clin. Immunol. 2012, 130, 1030–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| HPV Phenotype | Gene/protein (Mode of Inheritance) | Clinical Phenotypes | T Cell Counts | T Function | Other Immunological Features | Reference |
|---|---|---|---|---|---|---|
| Isolated EV | TMC6/EVER1 (AR) | EV | Normal | Normal | None | [9,14] |
| TMC8/EVER2 (AR) | EV | Normal with slightly high proportions for skin-homing subsets | Normal | None | ||
| CIB1 (AR) | EV | Normal | Normal | None |
| HPV Phenotype | Gene/Protein (Mode of Inheritance) | Other Clinical Phenotypes | T Cell Counts | T Function | Other Immunological Features | Reference |
|---|---|---|---|---|---|---|
| Syndromic EV | RHOH (AR) | Cutaneous viral infections, bronchopulmonary disease, Burkitt lymphoma | Low naïve CD4+ Tc, high memory CD4+ and CD8+ Tc counts, low proportions of skin-homing Tc subsets | Mildly impaired antigen-induced Tc proliferation, no anti-CD3-induced proliferation | - | [18,19] |
| Syndromic EV or profuse warts | STK4 (AR) | Bacterial, candida infections, EBV lymphoproliferation, lymphoma, congenital heart disease | Low Tc Low terminal differentiated effector memory cells Low naïve Tc | Poor proliferation Impaired mitogen (PHA, PMA/ ionomycin)- and antigen (candida, tetanus toxoid, tuberculin)- induced proliferation | Intermittent neutropenia, autoimmune cytopenia, low Bc | [16,20,21] |
| Syndromic EV or profuse warts | DOCK8 (AR) | Cutaneous staphylococcal and viral infections, severe eczema, severe atopy | Low Tc CD4+ | Poor production of antiviral cytokines (TNFα, IFNγ) | Hyper IgE, hyper eosinophilia Low IgM | [15,22] |
| Syndromic EV or chronic warts | CORO1A (AR) | Severe varicella, molluscum contagiosum and aggressive EBV infection | Low Tc | - | Defective number and/or cytolytic activity of NK cells, hypogammaglobulinemia, and defective antibody responses | [18,23] |
| Syndromic EV | RASGRP1 (AR) | Recurrent pneumonia, herpes virus infections, EBV-associated lymphoma | Low Tc | Tc: poor activation, proliferation, motility | Increased IgA, Bc: poor activation, proliferation, motility | [6,18,24] |
| Syndromic EV | LCK (AR) | Failure to thrive, severe diarrhea, opportunistic infections | Low CD4+ Low Tregs, restricted Tc repertoire | Poor TCR signaling | Autoimmunity, high IgM | [18,20,24] |
| Syndromic EV | TPP2 (AR) | Evans syndrome (immune thrombocytopenic purpura and autoimmune hemolytic anemia), progressive Leukopenia, mild viral infections, mild developmental delay | Normal or slightly low CD4+ Tc counts | Senescent CD8+ Tc (impaired proliferation, enhanced staurosporine-induced apoptosis) | Premature immunosenescence (Tc and Bc and antinuclear antibodies), normal IgA and IgE levels, IgG and IgM levels high | [18,24] |
| Profuse warts | CARMIL2 (AR) | Recurrent bacterial, fungal and mycobacterial infections, molluscum contagiosum, EBV lymphoproliferative syndrome and other malignancy, atopy | Low Tregs, high frequency of naïve CD4+, but normal CD4+ overall | Poor Tc dependent antibody response Poor Tc function | Low frequency of memory B cells Ig normal or low | [17,25] |
| Warts | IKBKG/NFκB essential modulator (XL) | Opportunistic Infections: P. jirovecii, common, NTM, histoplasma, HSV, CMV, MCV infections | Tc normal or low | TCR activation impaired | Low memory and isotype switched Bc, monocyte dysfunction, low IgG, some elevated IgG, IgM | [18,20,24] |
| Tree man syndrome or common warts | CD28 (AR) | None | Low Tregs, Low central memory CD4 and CD8 T cells | Abolished CD28 costimulation response, impaired T cell proliferation upon antigens stimulation | Low NK cells | [7] |
| Disease Name | Gene/Protein (Mode of Inheritance) | Other Clinical Phenotypes | T Cell Counts | T Function | Other Immunological Features | Reference |
|---|---|---|---|---|---|---|
| WHIM syndrome | CXCR4 gof (AD) | Warts, genital dysplasia pneumonia, cellulitis, sinusitis, urinary tract infection, thrombophlebitis, omphalitis, osteomyelitis, soft tissue abscesses, HSV infections, VZV infections | Low | Low LPA/LPM Cutaneous anergy | Low IgG and IgA, normal antibody responses neutropenia low Bc: - Low CD27+ Bc - Low IgD+ and IgD-Bc | [26] |
| MonoMac syndrome DCML Emberger syndrome or WILD syndrome | GATA2 (AD) | Warts, susceptibility to mycobacteria, histoplasmosis, lymphedema, pulmonary alveolar proteinosis, myelodysplasia | Variable, Low Tc | Variable, impaired T cell proliferation upon mitogen stimulation | Monocytopenia, Low Bc Low NK | [18,24] |
| LAD syndrome | LAD1/ITGB2 (AR) | Warts, delayed cord separation with omphalitis, no pus formation, lack in inflammation is observed in infection area, periodontitis | Leukocytosis | - | Low CD18+ neutropenia | [18,20,24] |
| Warts | CD154/CD40L, tumor necrosis factor surface family 5 (XL) | P. jirovecii pneumonia, chronic watery diarrhea due to infection with cryptosporidium, liver and biliary tract disease with sclerosing cholangitis due to cryptosporidium parvum, and infections with hepatitis B and C viruses as well as CMV can result in liver and biliary tract tumors | Variable | Defect in CD40L production | Neutropenia, autoimmunity with immune TNFSF5 thrombocytopenia, hemolytic anemia, and immune mediated nephritis | [18,20] |
| Syndromic EV | DCLRE1C/ ARTEMIS (hypomorphic, AR) | Recurrent respiratory and gastrointestinal infections | Low CD4+ Tc | Impaired proliferative response and reduced counts of naïve T cells, restricted T cell receptor repertoire | Low B cell numbers and serum IgA levels increased sensitivity to ionizing radiation of fibroblasts | [18,27] |
| Warts | SPINK5 (AR) | Congenital icthyosis, bamboo hair, atopic diathesis, bacterial infections | Normal T cell counts, the proportion of naïve CD4+ T cells is reduced and the proportion of CD8+ T central memory elevated | - | Switched and non-switched Bc are reduced hyper IgE and IgA Other Ig: variably decreased impaired NK cytotoxicity | [18,20,28] |
| Warts | ADA (XL) | Chondrosternal dysplasia, deafness, pulmonary alveolar proteinosis, cognitive defects | Low Tc | - | Low Bc absent or reduced ADA activity (<1% of normal) | [18,20,24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kettani, A.; Ailal, F.; El Bakkouri, J.; Zerouali, K.; Béziat, V.; Jouanguy, E.; Casanova, J.-L.; Bousfiha, A.A. HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity. Pathogens 2022, 11, 857. https://doi.org/10.3390/pathogens11080857
El Kettani A, Ailal F, El Bakkouri J, Zerouali K, Béziat V, Jouanguy E, Casanova J-L, Bousfiha AA. HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity. Pathogens. 2022; 11(8):857. https://doi.org/10.3390/pathogens11080857
Chicago/Turabian StyleEl Kettani, Assiya, Fatima Ailal, Jalila El Bakkouri, Khalid Zerouali, Vivien Béziat, Emmanuelle Jouanguy, Jean-Laurent Casanova, and Ahmed Aziz Bousfiha. 2022. "HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity" Pathogens 11, no. 8: 857. https://doi.org/10.3390/pathogens11080857
APA StyleEl Kettani, A., Ailal, F., El Bakkouri, J., Zerouali, K., Béziat, V., Jouanguy, E., Casanova, J.-L., & Bousfiha, A. A. (2022). HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity. Pathogens, 11(8), 857. https://doi.org/10.3390/pathogens11080857







