LC3-Associated Phagocytosis in Bacterial Infection
Abstract
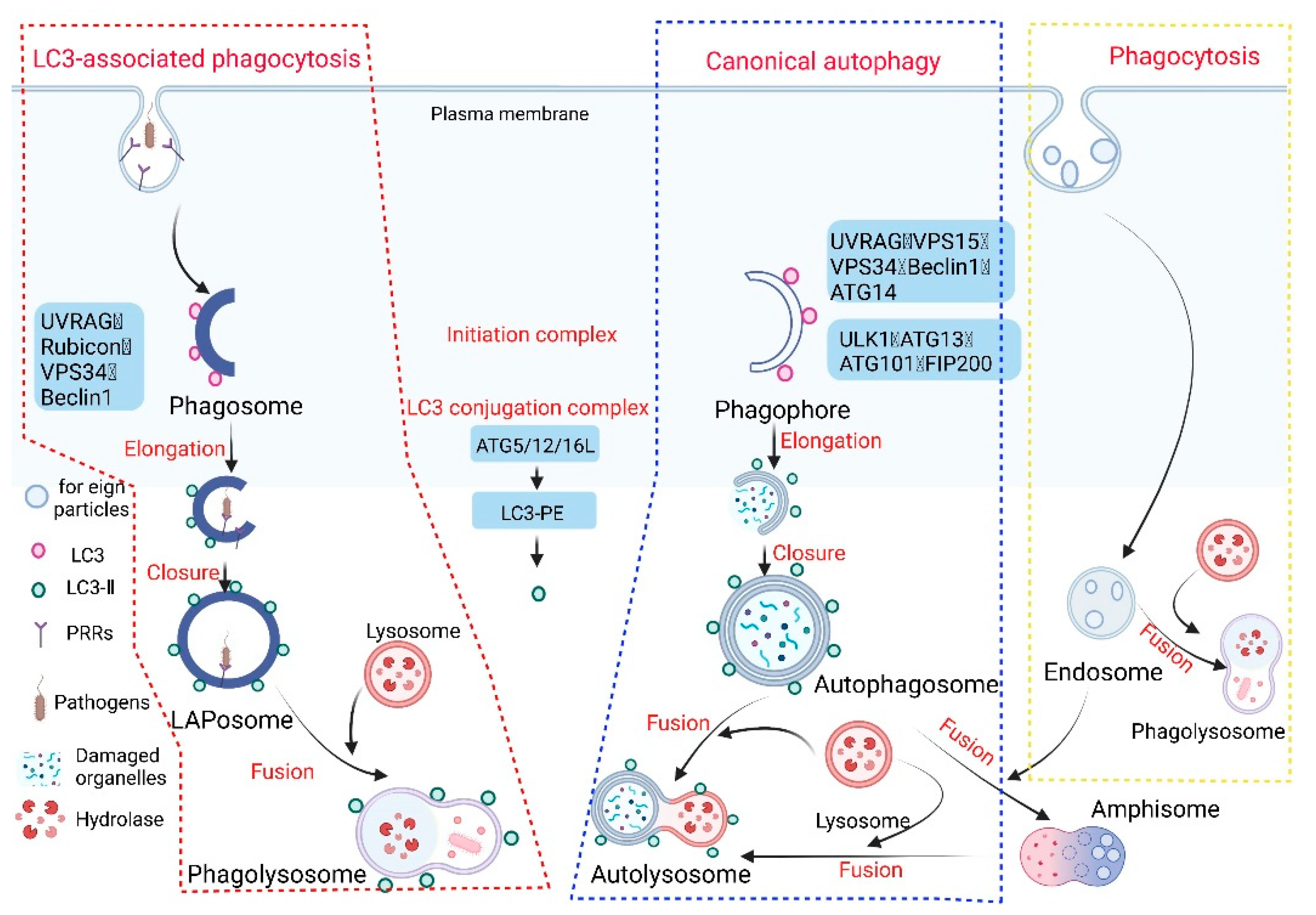
:1. Introduction
2. Biological Functions of LAP
3. LAP and Bacterial Infection
3.1. Legionella
3.1.1. Legionella dumoffii
3.1.2. Legionella pneumophila
3.2. Burkholderia pseudomallei
3.3. Listeria monocytogenes
3.3.1. LAP and Mitochondrial Ca2+ Signal Transduction
3.3.2. LAP and β2 Integrin Mac-1
3.4. Streptococcus pneumoniae
3.5. Mycobacteria
3.5.1. Mycobacterium tuberculosis
3.5.2. Mycobacterium marinum
3.6. Shigella flexneri
3.7. Yersinia pseudotuberculosis
3.8. Salmonella Typhimurium
3.9. Staphylococcus aureus
4. Potential Therapeutic Strategies
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Brumell, J.H. Autophagy in Immunity Against Intracellular Bacteria. Curr. Top. Microbiol. Immunol. 2009, 335, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Sosale, N.G.; Spinler, K.R.; Alvey, C.; Discher, D.E. Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific ‘Marker of Self’ CD47, and target physical properties. Curr. Opin. Immunol. 2015, 35, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the Dead: Apoptotic Cell Sensing, Recognition, Engulfment, and Digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Pao, K.C.; Rape, M. Tug of War in the Xenophagy World. Trends Cell Biol. 2019, 29, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Morishita, H.; Mizushima, N. Diverse Cellular Roles of Autophagy. Annu. Rev. Cell Dev. Biol. 2019, 35, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Almendinger, J.; Oberst, A.; Ness, R.; Dillon, C.P.; Fitzgerald, P.; Hengartner, M.O.; Green, D.R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17396–17401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauwels, A.-M.; Trost, M.; Beyaert, R.; Hoffmann, E. Patterns, Receptors, and Signals: Regulation of Phagosome Maturation. Trends Immunol. 2017, 38, 407–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.G.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Henault, J.; Martinez, J.; Riggs, J.M.; Tian, J.; Mehta, P.; Clarke, L.; Sasai, M.; Latz, E.; Brinkmann, M.M.; Iwasaki, A.; et al. Noncanonical Autophagy Is Required for Type I Interferon Secretion in Response to DNA-Immune Complexes. Immunity 2012, 37, 986–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprinaki, D.; Beasy, G.; Zhekova, A.; Wittmann, A.; James, S.; Dicks, J.; Iwakura, Y.; Saijo, S.; Wang, X.; Chow, C.-W.; et al. LC3-Associated Phagocytosis Is Required for Dendritic Cell Inflammatory Cytokine Response to Gut Commensal Yeast Saccharomyces cerevisiae. Front. Immunol. 2017, 8, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Taura, M.; Iwasaki, A. The interaction between IKKα and LC3 promotes type I interferon production through the TLR9-containing LAPosome. Sci. Signal. 2018, 11, eaan4144. [Google Scholar] [CrossRef]
- Shahnazari, S.; Yen, W.-L.; Birmingham, C.L.; Shiu, J.; Namolovan, A.; Zheng, Y.T.; Nakayama, K.; Klionsky, D.J.; Brumell, J.H. A Diacylglycerol-Dependent Signaling Pathway Contributes to Regulation of Antibacterial Autophagy. Cell Host Microbe 2010, 8, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, K.; Saitoh, T.; Tabata, K.; Omori, H.; Satoh, T.; Kurotori, N.; Maejima, I.; Shirahama-Noda, K.; Ichimura, T.; Isobe, T.; et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 2009, 11, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005, 106, 2543–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, J.M.; Mansour, M.K.; Khan, N.S.; Seward, M.; Puranam, S.; Tanne, A.; Sokolovska, A.; Becker, C.E.; Acharya, M.; Baird, M.A.; et al. Dectin-1–Dependent LC3 Recruitment to Phagosomes Enhances Fungicidal Activity in Macrophages. J. Infect. Dis. 2014, 210, 1844–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herb, M.; Gluschko, A.; Schramm, M. LC3-associated phagocytosis—The highway to hell for phagocytosed microbes. Semin. Cell Dev. Biol. 2020, 101, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, Q.; Li, X.; Yan, Y.; Backer, J.M.; Chait, B.T.; Heintz, N.; Yue, Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1–phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 2009, 11, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Yang, C.-S.; Lee, J.-S.; Rodgers, M.; Min, C.-K.; Lee, J.-Y.; Kim, H.J.; Lee, K.-H.; Kim, C.-J.; Oh, B.; Zandi, E.; et al. Autophagy Protein Rubicon Mediates Phagocytic NADPH Oxidase Activation in Response to Microbial Infection or TLR Stimulation. Cell Host Microbe 2012, 11, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.; Malireddi, R.; Lu, Q.; Cunha, L.D.; Pelletier, S.; Gingras, S.; Orchard, R.; Guan, J.-L.; Tan, H.; Peng, J.; et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 2015, 17, 893–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Green, D.R. Autophagy-Independent Functions of the Autophagy Machinery. Cell 2019, 177, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, B.L.; Green, D.R. LC3-associated phagocytosis at a glance. J. Cell Sci. 2019, 132, 222984. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, S.; Philips, J.A. LC3-associated phagocytosis: Host defense and microbial response. Curr. Opin. Immunol. 2019, 60, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Kagan, J.C. Cytosolic detection of phagosomal bacteria—Mechanisms underlying PAMP exodus from the phagosome into the cytosol. Mol. Microbiol. 2021, 116, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Gluschko, A.; Herb, M.; Wiegmann, K.; Krut, O.; Neiss, W.F.; Utermöhlen, O.; Krönke, M.; Schramm, M. The β2 Integrin Mac-1 Induces Protective LC3-Associated Phagocytosis of Listeria monocytogenes. Cell Host Microbe 2018, 23, 324–337.e5. [Google Scholar] [CrossRef] [Green Version]
- Köster, S.; Upadhyay, S.; Chandra, P.; Papavinasasundaram, K.; Yang, G.; Hassan, A.; Grigsby, S.J.; Mittal, E.; Park, H.S.; Jones, V.; et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc. Natl. Acad. Sci. USA 2017, 114, E8711–E8720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckmann, B.L.; Boada-Romero, E.; Cunha, L.D.; Magne, J.; Green, D.R. LC3-Associated Phagocytosis and Inflammation. J. Mol. Biol. 2017, 429, 3561–3576. [Google Scholar] [CrossRef]
- Hubber, A.; Kubori, T.; Coban, C.; Matsuzawa, T.; Ogawa, M.; Kawabata, T.; Yoshimori, T.; Nagai, H. Bacterial secretion system skews the fate of Legionella-containing vacuoles towards LC3-associated phagocytosis. Sci. Rep. 2017, 7, 44795. [Google Scholar] [CrossRef] [Green Version]
- Rolando, M.; Escoll, P.; Nora, T.; Botti, J.; Boitez, V.; Bedia, C.; Daniels, C.; Abraham, G.; Stogios, P.J.; Skarina, T.; et al. Legionella pneumophila S1P-lyase targets host sphingolipid metabolism and restrains autophagy. Proc. Natl. Acad. Sci. USA 2016, 113, 1901–1906. [Google Scholar] [CrossRef] [Green Version]
- Broek, C.W.V.; Stevens, J.M. Type III Secretion in the Melioidosis Pathogen Burkholderia pseudomallei. Front. Cell. Infect. Microbiol. 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Utaisincharoen, P.; Arjcharoen, S.; Lengwehasatit, I.; Limposuwan, K.; Sirisinha, S. Burkholderia pseudomallei invasion and activation of epithelial cells requires activation of p38 mitogen-activated protein kinase. Microb. Pathog. 2005, 38, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cullinane, M.; Treerat, P.; Ramm, G.; Prescott, M.; Adler, B.; Boyce, J.D.; Devenish, R.J. The Burkholderia pseudomallei Type III Secretion System and BopA Are Required for Evasion of LC3-Associated Phagocytosis. PLoS ONE 2011, 6, e17852. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Rizzuto, R.; Pozzan, T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef]
- Lam, G.Y.; Cemma, M.; Muise, A.M.; Higgins, D.E.; Brumell, J.H. Host and bacterial factors that regulate LC3 recruitment to Listeria monocytogenesduring the early stages of macrophage infection. Autophagy 2013, 9, 985–995. [Google Scholar] [CrossRef] [Green Version]
- Lam, G.Y.; Fattouh, R.; Muise, A.M.; Grinstein, S.; Higgins, D.E.; Brumell, J.H. Listeriolysin O Suppresses Phospholipase C-Mediated Activation of the Microbicidal NADPH Oxidase to Promote Listeria monocytogenes Infection. Cell Host Microbe 2011, 10, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Inomata, M.; Xu, S.; Chandra, P.; Meydani, S.N.; Takemura, G.; Philips, J.A.; Leong, J.M. Macrophage LC3-associated phagocytosis is an immune defense against Streptococcus pneumoniae that diminishes with host aging. Proc. Natl. Acad. Sci. USA 2020, 117, 33561–33569. [Google Scholar] [CrossRef]
- Vilchez, D.; Saez, I.; Dillin, A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014, 5, 5659. [Google Scholar] [CrossRef]
- Li, P.; Shi, J.; He, Q.; Hu, Q.; Wang, Y.Y.; Zhang, L.J.; Chan, W.T.; Chen, W.-X. Streptococcus pneumoniae Induces Autophagy through the Inhibition of the PI3K-I/Akt/mTOR Pathway and ROS Hypergeneration in A549 Cells. PLoS ONE 2015, 10, e0122753. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Matsuda, R.; Takada, N.; Tomokiyo, M.; Yamamoto, S.; Shizukuishi, S.; Yamaji, T.; Yoshikawa, Y.; Yoshida, M.; Tanida, I.; et al. Molecular mechanisms of Streptococcus pneumoniae -targeted autophagy via pneumolysin, Golgi-resident Rab41, and Nedd4-1-mediated K63-linked ubiquitination. Cell. Microbiol. 2018, 20, e12846. [Google Scholar] [CrossRef] [PubMed]
- González-Juarbe, N.; Gilley, R.P.; Hinojosa, C.A.; Bradley, K.M.; Kamei, A.; Gao, G.; Dube, P.H.; Bergman, M.A.; Orihuela, C.J. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLOS Pathog. 2015, 11, e1005337. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, A.; Etna, M.P.; Giacomini, E.; Pardini, M.; Remoli, M.E.; Corazzari, M.; Falasca, L.; Goletti, D.; Gafa, V.; Simeone, R.; et al. ESX-1 dependent impairment of autophagic flux byMycobacterium tuberculosisin human dendritic cells. Autophagy 2012, 8, 1357–1370. [Google Scholar] [CrossRef] [Green Version]
- Ouimet, M.; Koster, S.; Sakowski, E.; Ramkhelawon, B.; Van Solingen, C.; Oldebeken, S.; Karunakaran, D.; Portal-Celhay, C.; Sheedy, F.J.; Ray, T.D.; et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 2016, 17, 677–686. [Google Scholar] [CrossRef]
- Saini, N.K.; Baena, A.; Ng, T.W.; Venkataswamy, M.M.; Kennedy, S.C.; Kunnath-Velayudhan, S.; Carreño, L.J.; Xu, J.; Chan, J.; Larsen, M.H.; et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat. Microbiol. 2016, 1, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.-M.; Jeon, B.-Y.; Lee, H.-M.; Jin, H.S.; Yuk, J.-M.; Song, C.-H.; Lee, S.-H.; Lee, Z.-W.; Cho, S.-N.; Kim, J.M.; et al. Mycobacterium tuberculosis Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling. PLoS Pathog. 2010, 6, e1001230. [Google Scholar] [CrossRef] [Green Version]
- Van der Vaart, M.; Korbee, C.J.; Lamers, G.E.; Tengeler, A.C.; Hosseini, R.; Haks, M.C.; Ottenhoff, T.H.; Spaink, H.P.; Meijer, A.H. The DNA Damage-Regulated Autophagy Modulator DRAM1 Links Mycobacterial Recognition via TLR-MYD88 to Autophagic Defense. Cell Host Microbe 2014, 15, 753–767. [Google Scholar] [CrossRef] [Green Version]
- Köster, S.; Klevorn, T.; Papavinasasundaram, K.; Sassetti, C.M.; Portal-Celhay, C.; Philips, J.A. Consequence of enhanced LC3-trafficking for a live, attenuated M. tuberculosis vaccine. Vaccine 2018, 36, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Lerena, M.C.; Colombo, M.I. Mycobacterium marinum induces a marked LC3 recruitment to its containing phagosome that depends on a functional ESX-1 secretion system. Cell Microbiol. 2011, 13, 814–835. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, S.; Van Der Vaart, M.; Meijer, A.H. Autophagy and Lc3-Associated Phagocytosis in Zebrafish Models of Bacterial Infections. Cells 2020, 9, 2372. [Google Scholar] [CrossRef] [PubMed]
- Torraca, V.; Masud, S.; Spaink, H.P.; Meijer, A.H. Macrophage-pathogen interactions in infectious diseases: New therapeutic insights from the zebrafish host model. Dis. Model. Mech. 2014, 7, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, A.H. Protection and pathology in TB: Learning from the zebrafish model. Semin. Immunopathol. 2016, 38, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhu, L.; Jones, V.; Wang, C.; Hua, Y.; Shi, X.; Feng, X.; Jackson, M.; Niu, C.; Gao, Q. CpsA, a LytR-CpsA-Psr Family Protein in Mycobacterium marinum, Is Required for Cell Wall Integrity and Virulence. Infect. Immun. 2015, 83, 2844–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weddle, E.; Agaisse, H. Spatial, Temporal, and Functional Assessment of LC3-Dependent Autophagy in Shigella flexneri Dissemination. Infect. Immun. 2018, 86, e00134-18. [Google Scholar] [CrossRef] [Green Version]
- Campbell-Valois, F.-X.; Sachse, M.; Sansonetti, P.J.; Parsot, C. Escape of Actively Secreting Shigella flexneri from ATG8/LC3-Positive Vacuoles Formed during Cell-To-Cell Spread Is Facilitated by IcsB and VirA. mBio 2015, 6, e02567-14. [Google Scholar] [CrossRef] [Green Version]
- De Lichtenberg, U.; Jensen, L.J.; Fausbøll, A.; Jensen, T.S.; Bork, P.; Brunak, S. Comparison of computational methods for the identification of cell cycle-regulated genes. Bioinformatics 2005, 21, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Lemarignier, M.; Pizarro-Cerdá, J. Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species. Biomolecules 2020, 10, 1637. [Google Scholar] [CrossRef]
- Ligeon, L.-A.; Moreau, K.; Barois, N.; Bongiovanni, A.; Lacorre, D.-A.; Werkmeister, E.; Proux-Gillardeaux, V.; Galli, T.; Lafont, F. Role of VAMP3 and VAMP7 in the commitment ofYersinia pseudotuberculosisto LC3-associated pathways involving single- or double-membrane vacuoles. Autophagy 2014, 10, 1588–1602. [Google Scholar] [CrossRef] [Green Version]
- Moreau, K.; Lacas-Gervais, S.; Fujita, N.; Sebbane, F.; Yoshimori, T.; Simonet, M.; Lafont, F. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell. Microbiol. 2010, 12, 1108–1123. [Google Scholar] [CrossRef]
- Masud, S.; van der Burg, L.; Storm, L.; Prajsnar, T.K.; Meijer, A.H. Rubicon-Dependent Lc3 Recruitment to Salmonella-Containing Phagosomes Is a Host Defense Mechanism Triggered Independently From Major Bacterial Virulence Factors. Front. Cell. Infect. Microbiol. 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-Y.; Wang, L.-D.; Li, J.-L.; Xu, G.-M.; He, M.-L.; Li, Y.-Y.; Huang, R. Salmonella spv locus suppresses host innate immune responses to bacterial infection. Fish Shellfish Immunol. 2016, 58, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Ye, D.; Li, J.; Liu, J.; Deng, F. Knockdown of zebrafish Nanog increases primordial germ cells during early embryonic development. Dev. Growth Differ. 2016, 58, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Liu, Y.; Zuo, L.; Li, Y.; Wu, S.; Huang, R. Salmonella spv locus affects type I interferon response and the chemotaxis of neutrophils via suppressing autophagy. Fish Shellfish Immunol. 2019, 87, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Masud, S.; Prajsnar, T.K.; Torraca, V.; Lamers, G.E.M.; Benning, M.; Van Der Vaart, M.; Meijer, A.H. Macrophages target Salmonella by Lc3-associated phagocytosis in a systemic infection model. Autophagy 2019, 15, 796–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prajsnar, T.K.; Serba, J.J.; Dekker, B.M.; Gibson, J.F.; Masud, S.; Fleming, A.; Johnston, S.A.; Renshaw, S.A.; Meijer, A.H. The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils. Autophagy 2021, 17, 888–902. [Google Scholar] [CrossRef] [Green Version]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubber, A.; Roy, C.R. Modulation of Host Cell Function by Legionella pneumophila Type IV Effectors. Annu. Rev. Cell Dev. Biol. 2010, 26, 261–283. [Google Scholar] [CrossRef] [Green Version]
- Choy, A.; Dancourt, J.; Mugo, B.; O’Connor, T.J.; Isberg, R.R.; Melia, T.J.; Roy, C.R. The Legionella Effector RavZ Inhibits Host Autophagy Through Irreversible Atg8 Deconjugation. Science 2012, 338, 1072–1076. [Google Scholar] [CrossRef] [Green Version]
- Ensminger, A.W.; Isberg, R.R. Legionella pneumophila Dot/Icm translocated substrates: A sum of parts. Curr. Opin. Microbiol. 2009, 12, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Isberg, R.R.; O’Connor, T.J.; Heidtman, M. The Legionella pneumophila replication vacuole: Making a cosy niche inside host cells. Nat. Rev. Genet. Microbiol. 2008, 7, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullinane, M.; Gong, L.; Li, X.; Adler, N.-L.; Tra, T.; Wolvetang, E.; Prescott, M.; Boyce, J.D.; Devenish, R.J.; Adler, B. Stimulation of autophagy suppresses the intracellular survival ofBurkholderia pseudomalleiin mammalian cell lines. Autophagy 2008, 4, 744–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muangsombut, V.; Suparak, S.; Pumirat, P.; Damnin, S.; Vattanaviboon, P.; Thongboonkerd, V.; Korbsrisate, S. Inactivation of Burkholderia pseudomallei bsaQ results in decreased invasion efficiency and delayed escape of bacteria from endocytic vesicles. Arch. Microbiol. 2008, 190, 623–631. [Google Scholar] [CrossRef]
- Stevens, M.P.; Friebel, A.; Taylor, L.A.; Wood, M.W.; Brown, P.J.; Hardt, W.-D.; Galyov, E.E. A Burkholderia pseudomallei Type III Secreted Protein, BopE, Facilitates Bacterial Invasion of Epithelial Cells and Exhibits Guanine Nucleotide Exchange Factor Activity. J. Bacteriol. 2003, 185, 4992–4996. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Roversi, P.; Espina, M.; Olive, A.; Deane, J.E.; Birket, S.; Field, T.; Picking, W.D.; Blocker, A.J.; Galyov, E.E.; et al. Self-chaperoning of the Type III Secretion System Needle Tip Proteins IpaD and BipD. J. Biol. Chem. 2007, 282, 4035–4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, J.; Grishin, N.V. The Rho GTPase inactivation domain inVibrio choleraeMARTX toxin has a circularly permuted papain-like thiol protease fold. Proteins Struct. Funct. Bioinform. 2009, 77, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayath, C.A.; Hussey, S.; El Hajjami, N.; Nagra, K.; Philpott, D.; Allaoui, A. Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microbes Infect. 2010, 12, 956–966. [Google Scholar] [CrossRef]
- Banoth, B.; Cassel, S.L. Mitochondria in innate immune signaling. Transl. Res. 2018, 202, 52–68. [Google Scholar] [CrossRef]
- Abuaita, B.H.; Schultz, T.L.; O’Riordan, M.X. Mitochondria-Derived Vesicles Deliver Antimicrobial Reactive Oxygen Species to Control Phagosome-Localized Staphylococcus aureus. Cell Host Microbe 2018, 24, 625–636.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Li, T.; Kong, L.; Li, X.; Wu, S.; Attri, K.S.; Li, Y.; Gong, W.; Zhao, B.; Li, L.; Herring, L.E.; et al. Listeria monocytogenes upregulates mitochondrial calcium signalling to inhibit LC3-associated phagocytosis as a survival strategy. Nat. Microbiol. 2021, 6, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Marquis, H.; Jones, S.; Johnston, N.C.; Portnoy, D.A.; Goldfine, H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 1995, 63, 4231–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.A.O.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birmingham, C.L.; Canadien, V.; Gouin, E.; Troy, E.B.; Yoshimori, T.; Cossart, P.; Higgins, D.E.; Brumell, J.H. Listeria monocytogenesEvades Killing by Autophagy During Colonization of Host Cells. Autophagy 2007, 3, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tattoli, I.; Sorbara, M.T.; Yang, C.; Tooze, S.A.; Philpott, D.J.; Girardin, S.E. Listeriaphospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures. EMBO J. 2013, 32, 3066–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, Y.; Ogawa, M.; Hain, T.; Yoshida, M.; Fukumatsu, M.; Kim, M.; Mimuro, H.; Nakagawa, I.; Yanagawa, T.; Ishii, T.; et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 2009, 11, 1233–1240. [Google Scholar] [CrossRef]
- Mitchell, G.; Cheng, M.I.; Chen, C.; Nguyen, B.N.; Whiteley, A.T.; Kianian, S.; Cox, J.S.; Green, D.R.; McDonald, K.L.; Portnoy, D.A. Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E210–E217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluschko, A.; Farid, A.; Herb, M.; Grumme, D.; Krönke, M.; Schramm, M. Macrophages target Listeria monocytogenes by two discrete non-canonical autophagy pathways. Autophagy 2022, 18, 1090–1107. [Google Scholar] [CrossRef]
- Fratti, R.A.; Chua, J.; Vergne, I.; Deretic, V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 2003, 100, 5437–5442. [Google Scholar] [CrossRef] [Green Version]
- Deretic, V.; Via, L.E.; Fratti, R.A.; Deretic, D. Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking. Electrophoresis 1997, 18, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell. Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.H.; Liu, H.; Ge, B. Innate immunity in tuberculosis: Host defense vs pathogen evasion. Cell. Mol. Immunol. 2017, 14, 963–975. [Google Scholar] [CrossRef]
- Chandra, P.; Ghanwat, S.; Matta, S.K.; Yadav, S.S.; Mehta, M.; Siddiqui, Z.; Singh, A.; Kumar, D. Mycobacterium tuberculosis Inhibits RAB7 Recruitment to Selectively Modulate Autophagy Flux in Macrophages. Sci. Rep. 2015, 5, 16320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, N.; Lacas-Gervais, S.; Bertout, J.; Paz, I.; Freche, B.; Van Nhieu, G.T.; van der Goot, F.G.; Sansonetti, P.J.; Lafont, F. Shigella Phagocytic Vacuolar Membrane Remnants Participate in the Cellular Response to Pathogen Invasion and Are Regulated by Autophagy. Cell Host Microbe 2009, 6, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, K.; Bobard, A.; Danckaert, A.; Paz-Haftel, I.; Clair, C.; Ehsani, S.; Tang, C.; Sansonetti, P.; Tran, G.V.; Enninga, J. Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell. Microbiol. 2010, 12, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Allaoui, A.; Mounier, J.; Prévost, M.-C.; Sansonetti, P.J.; Parsot, C. icsB: A Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol. Microbiol. 1992, 6, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Suzuki, T.; Tatsuno, I.; Abe, H.; Sasakawa, C. IcsB, secreted via the type III secretion system, is chaperoned by IpgA and required at the post-invasion stage of Shigella pathogenicity. Mol. Microbiol. 2003, 48, 913–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Heijden, J.; Finlay, B.B. Type III effector-mediated processes in Salmonella infection. Futur. Microbiol. 2012, 7, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, J.A.; Steele-Mortimer, O. Salmonella—The ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell. Microbiol. 2009, 11, 1579–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, F.S.; Thomas, M.; Sachse, M.; Santos, A.J.; Figueira, R.; Holden, D.W. The Salmonella Deubiquitinase SseL Inhibits Selective Autophagy of Cytosolic Aggregates. PLoS Pathog. 2012, 8, e1002743. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Noda, T.; Yoshimori, T.; Tanaka, Y.; Ishii, T.; George, M.D.; Klionsky, D.J.; Ohsumi, M.; Ohsumi, Y. A protein conjugation system essential for autophagy. Nature 1998, 395, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Omori, H.; Saitoh, T.; Sone, T.; Guan, J.-L.; Akira, S.; Imamoto, F.; Noda, T.; Yoshimori, T. The LC3 recruitment mechanism is separate from Atg9L1-dependent membrane formation in the autophagic response againstSalmonella. Mol. Biol. Cell 2011, 22, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.R. The type III secretion injectisome. Nat. Rev. Genet. Microbiol. 2006, 4, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Mesquita, F.S.; Holden, D.W. The DUB-ious lack of ALIS in Salmonella infection: A Salmonella deubiquitinase regulates the autophagy of protein aggregates. Autophagy 2012, 8, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Bartholomew, C.R.; Zhou, W.; Klionsky, D.J. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy 2014, 5, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, Y.; Bruns, S.A.; Rohde, M.; Prajsnar, T.K.; Foster, S.J.; Schmitz, I. Intracellular Staphylococcus aureus eludes selective autophagy by activating a host cell kinase. Autophagy 2016, 12, 2069–2084. [Google Scholar] [CrossRef] [Green Version]
- Gibson, J.F.; Prajsnar, T.K.; Hill, C.J.; Tooke, A.K.; Serba, J.J.; Tonge, R.D.; Foster, S.J.; Grierson, A.J.; Ingham, P.W.; Renshaw, S.A.; et al. Neutrophils use selective autophagy receptor Sqstm1/p62 to target Staphylococcus aureus for degradation in vivo in zebrafish. Autophagy 2020, 17, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Gresham, H.D.; Lowrance, J.H.; Caver, T.E.; Wilson, B.S.; Cheung, A.L.; Lindberg, F.P. Survival ofStaphylococcus aureusInside Neutrophils Contributes to Infection. J. Immunol. 2000, 164, 3713–3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thwaites, G.E.; Gant, V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat. Rev. Genet. Microbiol. 2011, 9, 215–222. [Google Scholar] [CrossRef]
- Prajsnar, T.K.; Hamilton, R.; Garcia-Lara, J.; McVicker, G.; Williams, A.; Boots, M.; Foster, S.J.; Renshaw, S.A. A privileged intraphagocyte niche is responsible for disseminated infection of S taphylococcus aureus in a zebrafish model. Cell. Microbiol. 2012, 14, 1600–1619. [Google Scholar] [CrossRef] [Green Version]
- Stamm, C.E.; Collins, A.C.; Shiloh, M.U. Sensing ofMycobacterium tuberculosisand consequences to both host and bacillus. Immunol. Rev. 2015, 264, 204–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiersinga, W.J.; Wieland, C.W.; Dessing, M.C.; Chantratita, N.; Cheng, A.C.; Limmathurotsakul, D.; Chierakul, W.; Leendertse, M.; Florquin, S.; De Vos, A.F.; et al. Toll-Like Receptor 2 Impairs Host Defense in Gram-Negative Sepsis Caused by Burkholderia pseudomallei (Melioidosis). PLoS Med. 2007, 4, e248. [Google Scholar] [CrossRef] [PubMed]



| Bacteria | Virulence Factor | LAP-Specific Evasion Mechanisms | References |
|---|---|---|---|
| Legionella dumoffii | RavZ | Evasion of LAP by the T4SS effector protein RavZ, which inhibits LC3 lipidation and phagosome-lysosome fusion | [21,29] |
| Legionella pneumophila | RavZ | Evasion of LAP by the T4SS effector protein RavZ, which inhibits LC3 lipidation and phagosome–lysosome fusion | [29,30] |
| Burkholderia pseudomallei | BopA, BipD | Escapes from LAPosome and inhibits the recruitment of LC3 via BopA and BipD | [31,32,33] |
| Listeria monocytogenes | LLO | Upregulation of mitochondrial calcium signaling leads to the acetylation of Rubicon, interfering with LAP formation and the recruitment of NADPH oxidase | [26,27,34,35,36,37,38] |
| Streptococcus pneumoniae | PLY | Unknown | [39,40,41,42,43] |
| Mycobacterium tuberculosis | CpsA | Evasion of LAP by secreting CpsA protein to prevent NOX2 from assembling on phagosomes and inhibiting ROS production | [27,44,45,46,47,48,49] |
| Mycobacterium marinum | Type VII secretion systemESX1, CpsA | Evasion of LAP via non-acidifying LC3-positive vesicle, which is established through the ESX-1 secretion system | [50,51,52,53,54] |
| Shigella flexneri | IcsB, VirA | Evasion of LAPosome by inhibition of LC3 recruitment through interaction between TOCA-1 and IcsB | [55,56,57] |
| Yersinia pseudotuberculosis | Unknown | VAMP3 and VAMP7 co-localize with YCVs resulted in inhibiting LC3 recruitment | [58,59,60] |
| Salmonella enterica Typhimurium | PhoP, PurA, FlhD | Reduce TLR activation, inhibit LC3 recruitment, and inhibit phagolysosomal fusion | [61,62,63,64] |
| Staphylococcus aureus | Unknown | Forming spacious GFP-LC3-positive vacuoles that do not acidify | [48,65,66,67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Zhang, Q.; Chen, S.; Yan, M.; Yue, L. LC3-Associated Phagocytosis in Bacterial Infection. Pathogens 2022, 11, 863. https://doi.org/10.3390/pathogens11080863
Yuan J, Zhang Q, Chen S, Yan M, Yue L. LC3-Associated Phagocytosis in Bacterial Infection. Pathogens. 2022; 11(8):863. https://doi.org/10.3390/pathogens11080863
Chicago/Turabian StyleYuan, Jin, Qiuyu Zhang, Shihua Chen, Min Yan, and Lei Yue. 2022. "LC3-Associated Phagocytosis in Bacterial Infection" Pathogens 11, no. 8: 863. https://doi.org/10.3390/pathogens11080863
APA StyleYuan, J., Zhang, Q., Chen, S., Yan, M., & Yue, L. (2022). LC3-Associated Phagocytosis in Bacterial Infection. Pathogens, 11(8), 863. https://doi.org/10.3390/pathogens11080863






