Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism
Abstract
:1. Introduction
2. Results
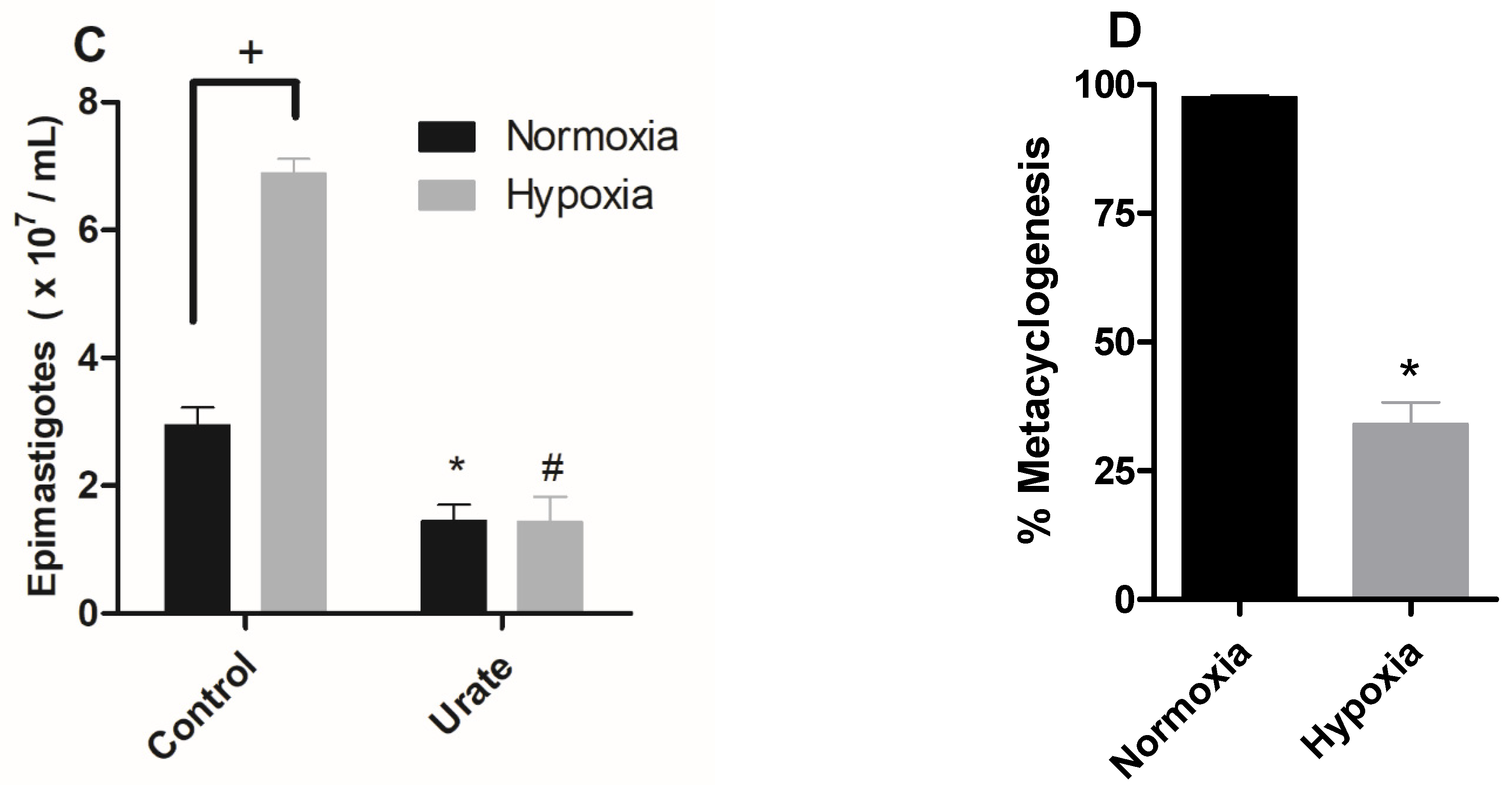
2.1. Hypoxia Induced Proliferation of Epimastigotes, ROS Production, and Inhibition of Metacyclogenesis
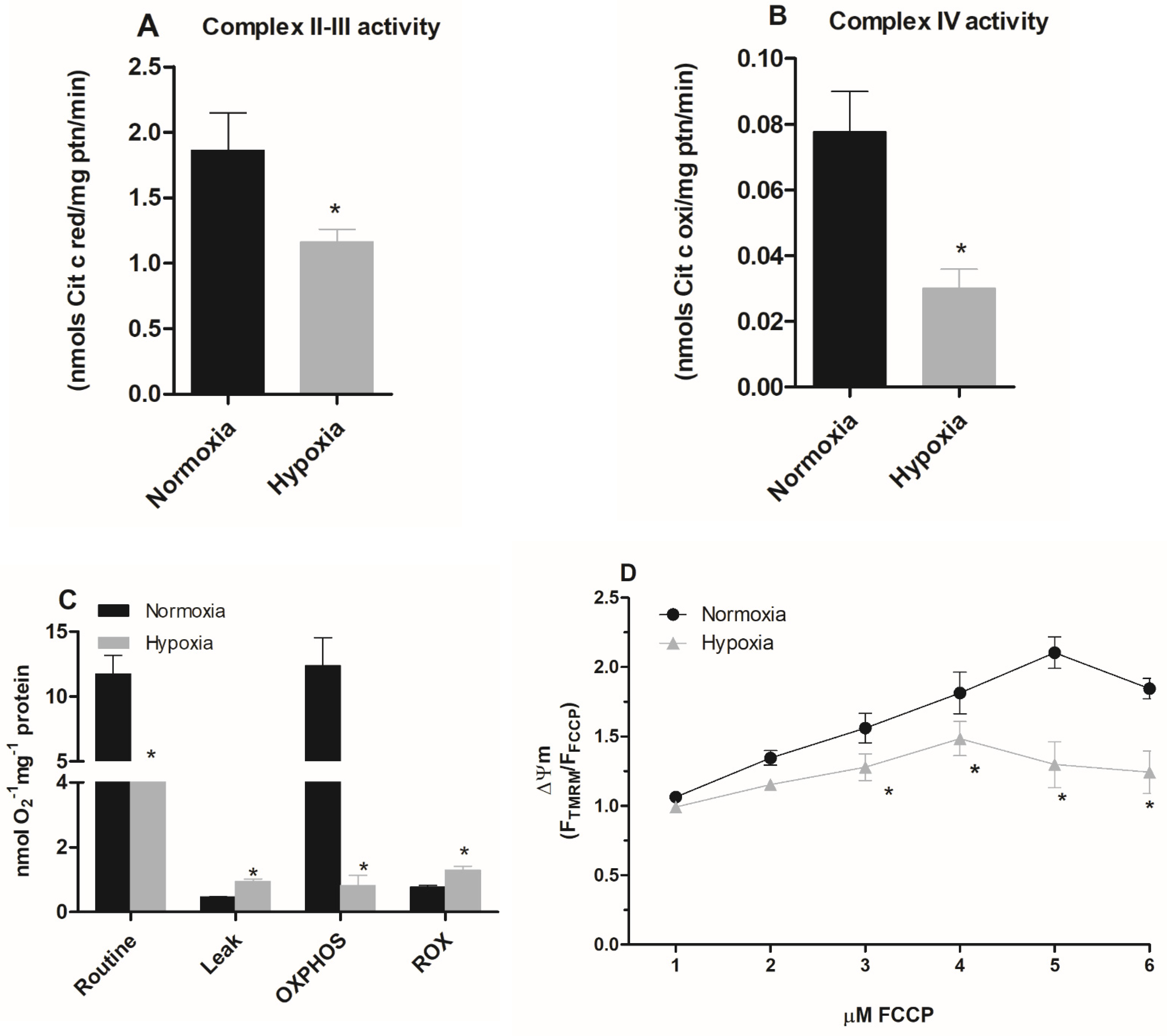
2.2. A Hypoxic Challenge Induces a Remodeling of the Activities of the Mitochondrial Complexes
2.3. Parasites Maintained under Hypoxia Show a Reduction in the Mitochondrial Respiration
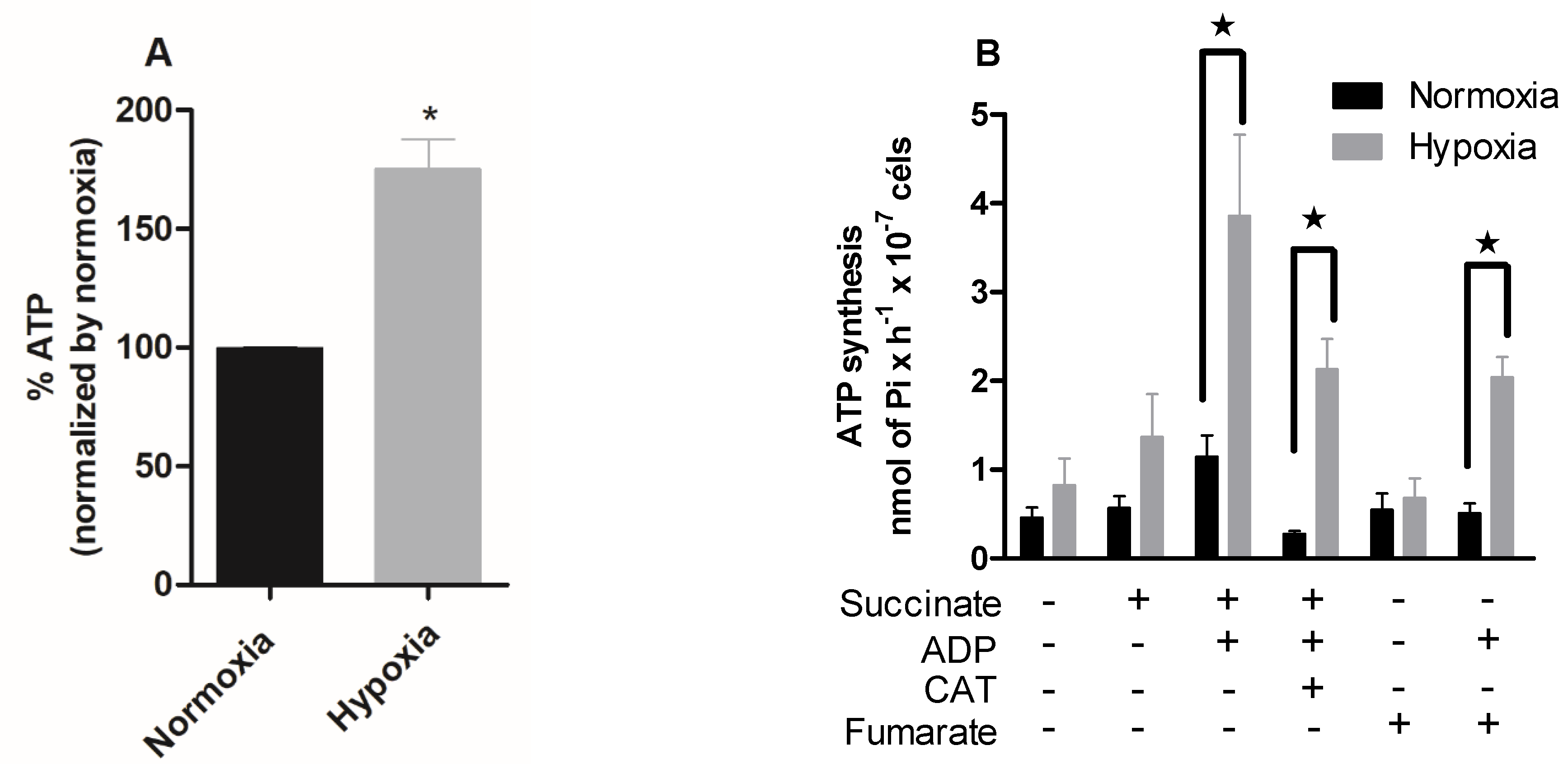
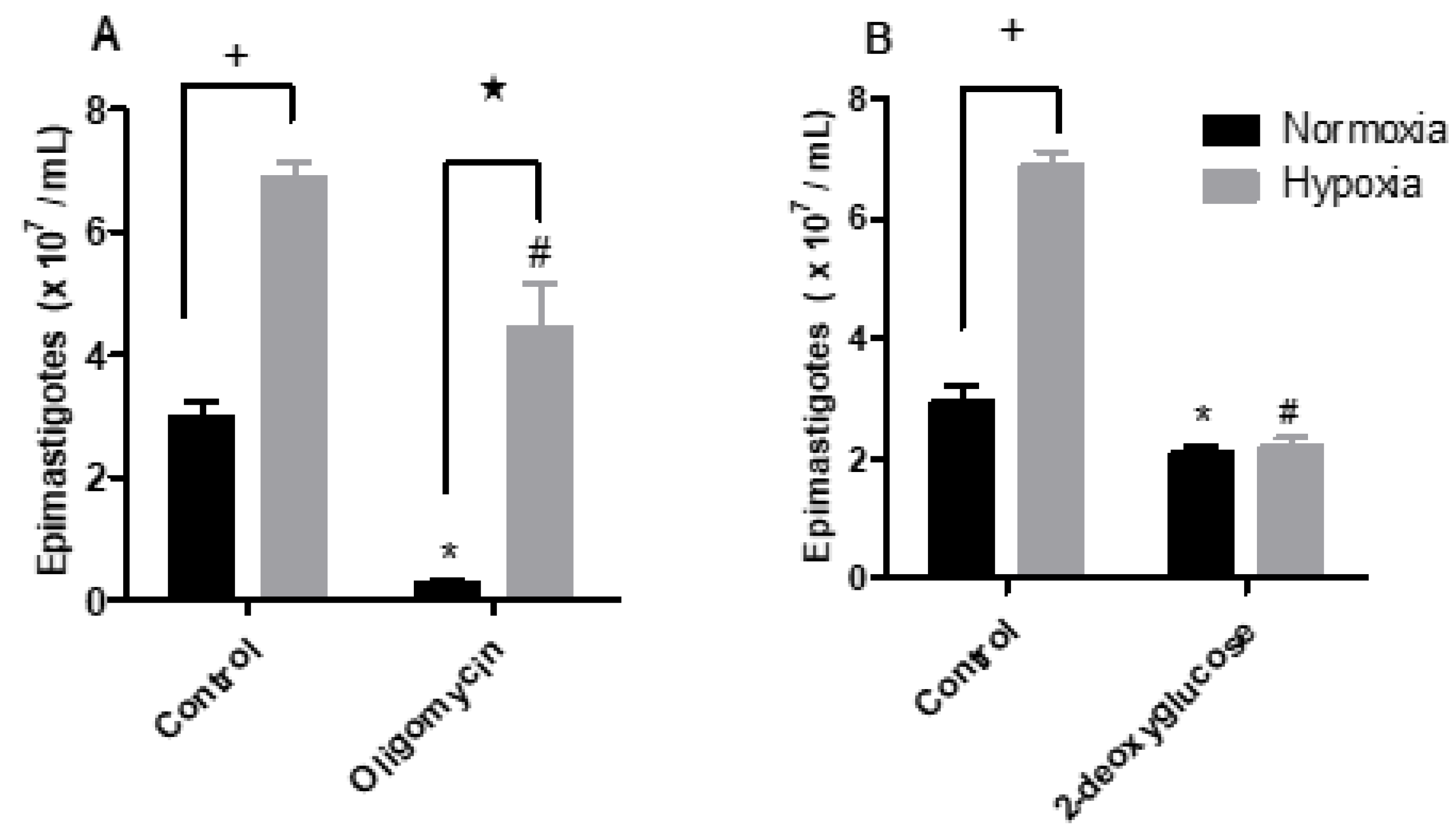
2.4. Hypoxia Induces a Metabolic Shift in Epimastigotes
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Parasites
5.2. Hypoxic Condition
5.3. Susceptibility of T. cruzi to Metabolic Modulators
5.4. Oxidant Species Production
5.5. In Vitro Metacyclogenesis
5.6. Mitochondrial Complex Activities
5.7. Oxygen Consumption Rates
5.8. Mitochondrial Membrane Potential
5.9. ATP Quantification
5.10. Differential Expression of Hexokinase and NADH Fumarate Reductase Enzymes
5.11. Quantification of Intracellular NAD(P)H Levels
5.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowne, A.J.; Guerra, C.A.; Alves, R.V.; Costa, V.M.; Wilson, A.L.; Pigott, D.M.; Hay, S.I.; Lindsay, S.W.; Golding, N.; Moyes, C.L. The contemporary distribution of Trypanosoma cruzi infection in humans, alternative hosts and vectors. Sci. Data 2017, 4, 170050. [Google Scholar]
- Coura, J.R.; Vĩas, P.A. Chagas disease: A new worldwide challenge. Nature 2010, 465, S6–S7. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. J. Phys. Oceanogr. 2019, 7, 166. [Google Scholar] [CrossRef]
- WHO Chagas Disease-Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 17 April 2019).
- Melo, R.F.P.; Guarneri, A.A.; Silber, A.M. The Influence of Environmental Cues on the Development of Trypanosoma cruzi in Triatominae Vector. Front. Cell. Infect. Microbiol. 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Mazliah, D.; Ward, A.I.; Lewis, M.D. Host-parasite dynamics in Chagas disease from systemic to hyper-local cales. Parasite Immunol. 2021, 43, e12786. [Google Scholar] [CrossRef] [PubMed]
- Barisón, M.J.; Rapado, L.N.; Merino, E.F.; Pral, E.M.F.; Mantilla, B.S.; Marchese, L.; Nowicki, C.; Silber, A.M.; Cassera, M.B. Metabolomic profiling reveals a finely tuned, starvationinduced metabolic switch in Trypanosoma cruzi epimastigotes. J. Biol. Chem. 2017, 292, 8964–8977. [Google Scholar] [CrossRef] [Green Version]
- De-Simone, S.G.; Bourguignon, S.C.; Gonçalves, P.S.; Lechuga, G.C.; Provance, D.W., Jr. Metabolic Alteration of Trypanosoma cruzi during differentiation of Epimastigote to Trypomastigote Forms. Pathogens 2022, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.L.S.; Barreto, R.F.S.M.; Polycarpo, C.R.; Gadelha, F.R.; Castro, S.L.; Oliveira, M.F. A comparative assessment of mitochondrial function in epimastigotes and bloodstream trypomastigotes of Trypanosoma cruzi. J. Bioenerg. Biomembr. 2011, 43, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Atwood, J.A.; Weatherly, D.B.; Minning, T.A.; Bundy, B.; Cavola, C.; Opperdoes, F.R.; Orlando, R.; Tarleton, R.L. The Trypanosoma cruzi proteome. Science 2005, 309, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, D.A.; Cannata, J.J.B.; Cazzulo, J.J. Glucose metabolism in Trypanosoma cruzi. Essays Biochem. 2011, 51, 15–30. [Google Scholar]
- Shah-Simpson, S.; Lentini, G.; Dumoulin, P.C.; Burleigh, B.A. Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog. 2017, 13, e1006747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wong, H.S. Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol. 2021, 224, jeb221606. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.U.; Flashman, E.; Mohlin, S.; Licausi, F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science 2020, 370, eaba3512. [Google Scholar] [CrossRef]
- Jantsch, J.; Schödel, J. Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology 2015, 220, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Arena, E.T.; Tinevez, J.Y.; Nigro, G.; Sansonetti, P.J.; Marteyn, B.S. The infectious hypoxia: Occurrence and causes during Shigella infection. Microbes. Infect. 2017, 19, 157–165. [Google Scholar] [CrossRef]
- Colhone, C.; Arrais-Silva, W.W.; Picoli, C.; Giorgio, S. Effect of Hypoxia on Macrophage Infection by Leishmania amazonensis. J. Parasitol. 2004, 90, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Degrossoli, A.; Bosetto, M.C.; Lima, C.B.; Giorgio, S. Expression of hypoxia-inducible factor 1alpha in mononuclear phagocytes infected with Leishmania amazonensis. Immunol. Lett. 2007, 114, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lerchner, J.; Sartori, M.R.; Volpe, P.O.L.; Lander, N.; Mertens, F.; Vercesi, A.E. Direct determination of anaerobe contributions to the energy metabolism of Trypanosoma cruzi by chip calorimetry. Anal. Bioanal. Chem. 2019, 411, 3763–3768. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, N.P.; Saraiva, F.M.S.; Sultano, P.E.; Cunha, P.R.B.B.; Laranja, G.A.T.; Justo, G.A.; Sabino, K.C.C.; Coelho, M.G.P.; Rossini, A.; Atella, G.C.; et al. Proliferation and differentiation of Trypanosoma cruzi inside its vector have a new trigger: Redox status. PLoS ONE 2015, 10, e0116712. [Google Scholar] [CrossRef] [Green Version]
- De Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ouali, R.; de Brito, K.C.V.; Salmon, D.; Bousbata, S. High-throughput identification of the Rhodnius prolixus midgut proteome unravels a sophisticated hematophagic machinery. Proteomes 2020, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Clanton, T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 2007, 102, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef]
- He, A.; Dean, J.M.; Lodhi, I.J. Peroxisomes as Cellular Adaptors to Metabolic and Environmental Stress. Trends Cell Biol. 2021, 8, 656–670. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Gluenz, E.; Taylor, M.C.; Kelly, J.M. The Trypanosoma cruzi metacyclic-specific protein Met-III associates with the nucleolus and contains independent amino and carboxyl terminal targeting elements. Int. J. Parasitol. 2007, 37, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Carranza, C.J.; Kowaltowski, A.J.; Mendonça, M.A.G.; De Oliveira, T.C.; Gadelha, F.R.; Zingales, B. Mitochondrial bioenergetics and redox state are unaltered in Trypanosoma cruzi isolates with compromised mitochondrial complex I subunit genes. J. Bioenerg. Biomembr. 2009, 41, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Sendoel, A.; Hengartner, M.O. Apoptotic Cell Death Under Hypoxia. Physiology 2014, 29, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, B.; Abergel, Z.; Zelmanovich, V.; Romero, L.; Abergel, R.; Livshits, L.; Smith, Y.; Gross, E. Characterization of gene expression associated with the adaptation of the nematode C. elegans to hypoxia and reoxygenation stress reveals an unexpected function of the neuroglobin GLB-5 in innate immunity. Free Radic. Biol. Med. 2017, 108, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Wanandi, S.I.; Ningsih, S.S.; Asikin, H.; Hosea, R.; Neolaka, G.M.G. Metabolic interplay between tumour cells and cancer-associated fibroblasts (CAFs) under hypoxia versus normoxia. Malays. J. Med. Sci. 2018, 25, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, E.; Bradley, T. Temperature-dependent variation in gas exchange patterns and spiracular control in Rhodnius prolixus. J. Exp. Biol. 2014, 217, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coon, K.L.; Valzania, L.; McKinney, D.A.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl Acad Sci. USA 2017, 114, E5362–E5369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valzania, L.; Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito Aedes aegypti. Proc. Natl. Acad Sci. USA 2018, 115, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Mota, F.F.; Marinho, L.P.; Moreira, C.J.C.; Lima, M.M.; Mello, C.B.; Garcia, E.S.; Carels, N.; Azambuja, P. Cultivation-independent methods reveal differences among bacterial gut microbiota in triatomine vectors of Chagas disease. PLoS Negl. Trop. Dis. 2012, 6, e1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumiel, M.; da Mota, F.F.; Rizzo, V.S.; Sarquis, O.; de Castro, D.P.; Lima, M.M.; Garcia, E.S.; Carels, N.; Azambuja, P. Characterization of the microbiota in the guts of Triatoma brasiliensis and Triatoma pseudomaculata infected by Trypanosoma cruzi in natural conditions using culture independent methods. Parasites Vectors 2015, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Carels, N.; Gumiel, M.; da Mota, F.F.; de Carvalho Moreira, C.J.; Azambuja, P. A Metagenomic Analysis of Bacterial Microbiota in the Digestive Tract of Triatomines. Bioinform. Biol. Insights 2017, 11, 1177932217733422. [Google Scholar] [CrossRef] [PubMed]
- Lages, Y.M.; Nascimento, J.M.; Lemos, G.A.; Galina, A.; Castilho, L.R.; Rehen, S.K. Low oxygen alters mitochondrial function and response to oxidative stress in human neural progenitor cells. PeerJ 2015, 3, e1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hals, I.K.; Bruerberg, S.G.; Ma, Z.; Scholz, H.; Björklund, A.; Grill, V. Mitochondrial Respiration in Insulin-Producing β-Cells: General Characteristics and Adaptive Effects of Hypoxia. PLoS ONE 2015, 10, e0138558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, G.; Venditti, P.; Fasciolo, G.; Esposito, D.; Uliano, E.; Agnisola, C. Acute hypoxia/reoxygenation affects muscle mitochondrial respiration and redox state as well as swimming endurance in zebrafish. J. Comp. Physiol. B 2019, 189, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Kream, R.M. Glycolytic Coupling to Mitochondrial Energy Production Ensures Survival in an Oxygen Rich Environment. Med. Sci. Monit. 2016, 22, 2571–2575. [Google Scholar] [CrossRef] [Green Version]
- Hillmann, F.; Shekhova, E.; Kniemeyer, O. Insights into the cellular responses to hypoxia in filamentous fungi. Curr. Genet. 2015, 61, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Metheni, M.; Lombès, A.; Bouillaud, F.; Batteux, F.; Langsley, G. HIF-1α induction, proliferation and glycolysis of T heileria -infected leukocytes. Cell. Microbiol. 2017, 17, 467–472. [Google Scholar] [CrossRef]
- Qualls, J.E.; Murray, P.J. Immunometabolism within the tuberculosis granuloma: Amino acids, hypoxia, and cellular respiration. Semin. Immunopathol. 2016, 38, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilaki, N.; Frakolaki, E. Virus–Host interactions under hypoxia. Microbes Infect. 2017, 19, 193–203. [Google Scholar] [CrossRef] [PubMed]
- de Graef, M.R.; Alexeeva, S.; Snoep, J.L.; Teixeira de Mattos, M.J. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J. Bacteriol. 1999, 181, 2351–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Metabolic Impact of Redox Cofactor Perturbations on the Formation of Aroma Compounds in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2016, 82, 174–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzulo, J.J. Aerobic fermentation of glucose by trypanosomatids. FASEB J. 1992, 6, 3153–3161. [Google Scholar] [CrossRef]
- Inaoka, D.K.; Shiba, T.; Sato, D.; Balogun, E.O.; Sasaki, T.; Nagahama, M.M.; Matsuoka, S.; Ohmori, J.; Honma, T.; Inoue, M.; et al. Structural Insights into the Molecular Design of Flutolanil Derivatives Targeted for Fumarate Respiration of Parasite Mitochondria. Int. J. Mol. Sci. 2015, 16, 15287–15308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Daly, L.A.; Mennerich, D.; Kietzmann, T.; Sée, V. The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity. Int. J. Mol. Sci. 2021, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, T.; Fandrey, J.; Prost-Fingerle, K. Ways into Understanding HIF Inhibition. Cancers 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, E.C.; Haas, B.; et al. The genome of the African trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, N.M.; Myler, P.J.; Bartholomeu, D.C.; Nilsson, D.; Aggarwal, G.; Tran, A.N.; Ghedin, E.; Worthey, E.A.; Delcher, A.L.; Blandin, G.; et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 2005, 309, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downing, T.; Imamura, H.; Decuypere, S.; Clark, T.G.; Coombs, G.H.; Cotton, J.A.; Hilley, J.D.; de Doncker, S.; Maes, I.; Mottram, J.C.; et al. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res. 2011, 21, 2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, I.; Ferreira, C.; Moreira, D.; Kluck, G.E.G.; Barbosa, A.M.; Torrado, E.; Dinis-Oliveira, R.J.; Gonçalves, L.G.; Beauparlant, C.-J.; Torrado, E.; et al. The Absence of HIF-1α Increases Susceptibility to Leishmania donovani Infection via Activation of BNIP3/mTOR/SREBP-1c Axis. Cell Rep. 2020, 30, 4052–4064.e7. [Google Scholar] [CrossRef]
- Schatz, V.; Strüssmann, Y.; Mahnke, A.; Schley, G.; Waldner, M.; Ritter, U.; Wild, J.; Willam, C.; Dehne, N.; Brüne, B.; et al. Myeloid Cell-Derived HIF-1α Promotes Control of Leishmania major. J. Immunol. 2016, 197, 4034–4041. [Google Scholar] [CrossRef] [Green Version]
- McGettrick, A.F.; Corcoran, S.E.; Barry, P.J.; McFarland, J.; Crès, C.; Curtis, A.M.; Franklin, E.; Corr, S.C.; Mok, K.H.; Cummins, E.P.; et al. Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion. Proc. Natl. Acad. Sci. USA 2016, 113, E7778–E7787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Contreras, V.T.; Araujo-Jorge, T.C.; Bonaldo, M.C.; Thomaz, N.; Barbosa, H.S. Biological aspects of the Dm 28c clone of Trypanosoma cruzi after metacyclogenesis in chemically defined media. Mem. Inst. Oswaldo Cruz 1988, 83, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J. Biol. Chem. 1955, 217, 395–407. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Vieyra, A.; Caruso-Neves, C.; Meyer-Fernandes, J.R. ATP in equilibrium with 32Pi exchange catalyzed by plasma membrane Ca(2+)-ATPase from kidney proximal tubules. J. Biol. Chem. 1991, 266, 10324–10330. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Hermes-Lima, M.; Meyer-Fernandes, J.R.; Vieyra, A. calcium inhibition of the ATP in equilibrium with [32P]Pi exchange and of net ATP synthesis catalyzed by bovine submitochondrial particles. Biochim. Biophys. Acta 1990, 1020, 101–106. [Google Scholar] [CrossRef]
- Cooney, D.A.; Jayaram, H.N.; Homan, E.R.; Motley, C.F. A facile radiometric technique for measuring ATP. Anal. Biochem. 1974, 62, 157–165. [Google Scholar] [CrossRef]
- Martins, C.; Baptista, C.S.; Iennea, S.; Cerqueira, G.C.; Bartholomeu, D.C.; Zingales, B. Genomic organization and transcription analysis of the 195-bp satellite DNA in Trypanosoma cruzi. Mol. Bioch. Parasitol. 2008, 160, 60–64. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Salmon, J.M.; Kohen, E.; Viallet, P.; Hirschberg, J.G.; Wouters, A.W.; Kohen, C.; Thorell, B. Microspectrofluorometric approach to the study of free/bound nad(p)h ratio as metabolic indicator in various cell types. Photochem. Photobiol. 1982, 36, 585–593. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, F.M.S.; Cosentino-Gomes, D.; Inacio, J.D.F.; Almeida-Amaral, E.E.; Louzada-Neto, O.; Rossini, A.; Nogueira, N.P.; Meyer-Fernandes, J.R.; Paes, M.C. Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism. Pathogens 2022, 11, 897. https://doi.org/10.3390/pathogens11080897
Saraiva FMS, Cosentino-Gomes D, Inacio JDF, Almeida-Amaral EE, Louzada-Neto O, Rossini A, Nogueira NP, Meyer-Fernandes JR, Paes MC. Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism. Pathogens. 2022; 11(8):897. https://doi.org/10.3390/pathogens11080897
Chicago/Turabian StyleSaraiva, Francis M. S., Daniela Cosentino-Gomes, Job D. F. Inacio, Elmo E. Almeida-Amaral, Orlando Louzada-Neto, Ana Rossini, Natália P. Nogueira, José R. Meyer-Fernandes, and Marcia C. Paes. 2022. "Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism" Pathogens 11, no. 8: 897. https://doi.org/10.3390/pathogens11080897
APA StyleSaraiva, F. M. S., Cosentino-Gomes, D., Inacio, J. D. F., Almeida-Amaral, E. E., Louzada-Neto, O., Rossini, A., Nogueira, N. P., Meyer-Fernandes, J. R., & Paes, M. C. (2022). Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism. Pathogens, 11(8), 897. https://doi.org/10.3390/pathogens11080897





