Zoonotic Giardia duodenalis Genotypes and Other Gastrointestinal Parasites in a Badger Population Living in an Anthropized Area of Central Italy
Abstract
1. Introduction
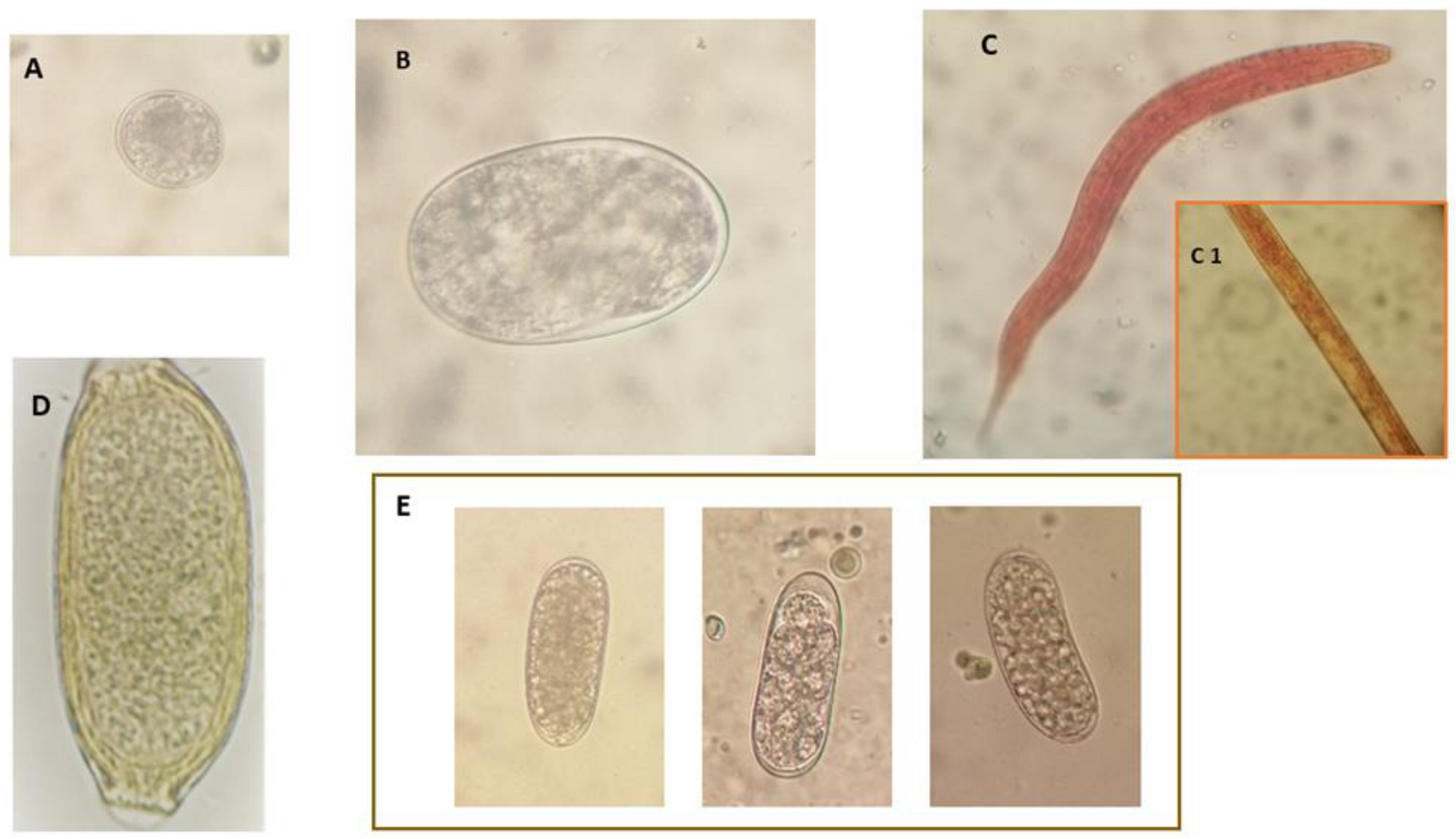
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area and Sampling
4.2. Parasitological Analysis
4.3. Molecular Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melis, C.; Cagnacci, F.; Bargagli, L. Food habits of the Eurasian badger in a rural Mediterranean area. Z. Jagdwiss 2002, 48, 236–246. [Google Scholar] [CrossRef]
- Balestrieri, A.; Remonti, L.; Prigioni, C. Diet of the Eurasian badger (Meles meles) in an agricultural riverine habitat (North-West Italy). Hystrix It. J. Mamm. 2004, 15. [Google Scholar] [CrossRef]
- Roper, T.J. Badger, Book 114; Collins New Naturalist Library, Collins: London, UK, 2010. [Google Scholar]
- Kruuk, H. Spatial organization and territorial behaviour of the European badger Meles meles. J. Zool. Lond. 1978, 184, 1–19. [Google Scholar] [CrossRef]
- Roper, T.J. The structure and function of badger setts. Zool. Lond. 1992, 227, 691–698. [Google Scholar] [CrossRef]
- Roper, T.J.; Ostler, J.R.; Schmid, T.K.; Christian, S.F. Sett use in European badgers Meles meles. Behaviour 2001, 138, 173–187. [Google Scholar] [CrossRef]
- Coppola, F.; Dari, C.; Vecchio, G.; Scarselli, D.; Felicioli, A. Cohabitation of settlements among crested porcupine (Hystrix cristata), red fox (Vulpes vulpes) and European badger (Meles meles). Curr. Sci. 2020, 119, 817–822. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Loureiro, F.; Macdonald, D.W.; Santon-Reis, M. Dietary shifts of the badger (Meles meles) in Mediterranean woodlands: An opportunistic forager with seasonal specialisms. Mamm. Biol. 2005, 70, 12–23. [Google Scholar] [CrossRef]
- Prigioni, C.; Deflorian, M.C. Sett-site selection by the Eurasian badger (Meles meles) in an Italian Alpine area. Ital. J. Zool. 2005, 72, 43–48. [Google Scholar] [CrossRef]
- Remonti, L.; Balestrieri, A.; Prigioni, C. Range of the Eurasian badger (Meles meles) in an agricultural area of northern Italy. Ethol. Ecol. Evol. 2006, 18, 61–67. [Google Scholar] [CrossRef]
- Fabrizio, M.; Di Febbraro, M.; D’Amico, M.; Frate, L.; Roscioni, F.; Loy, A. Habitat suitability vs. landscape connectivity determining roadkill risk at a regional scale: A case study on European badger (Meles meles). Eur. J. Wildl. Res. 2019, 65, 7. [Google Scholar] [CrossRef]
- Ferroglio, E.; Ragagli, C.; Trisciuoglio, A. Physaloptera sibirica in foxes and badgers from the Western Alps (Italy). Vet. Parasitol. 2009, 163, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Magi, M.; Banchi, C.; Barchetti, A.; Guberti, V. The parasites of the badger (Meles meles) in the north of Mugello (Florence, Italy). Parassitologia 1999, 41, 533–536. [Google Scholar] [PubMed]
- Di Cerbo, A.R.; Manfredi, M.T.; Bregoli, M.; Ferro Milone, N.; Cova, M. Wild carnivores as source of zoonotic helminths in north-eastern Italy. Helminthologia 2008, 45, 13–19. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Torres, J.; Santos-Reis, M.A. Survey of helminth infection in Eurasian badgers (Meles meles) in relation to their foraging behaviour in a Mediterranean environment in southwest Portugal. Eur. J. Wildl. Res. 2006, 52, 202–206. [Google Scholar] [CrossRef]
- Akdesir, E.; Origgi, F.C.; Wimmershoff, J.; Frey, J.; Frey, C.F.; Ryser-Degiorgis, M.P. Causes of mortality and morbidity in free-ranging mustelids in Switzerland: Necropsy data from over 50 years of general health surveillance. BMC Vet. Res. 2018, 14, 195. [Google Scholar] [CrossRef]
- Byrne, R.L.; Fogarty, U.; Mooney, A.; Harris, E.; Good, M.; Marples, N.M.; Holland, C.V. The helminth parasite community of European badgers (Meles meles) in Ireland. J. Helminthol. 2019, 94, e37. [Google Scholar] [CrossRef]
- Mateo, M.; de Mingo, M.H.; de Lucio, A.; Morales, L.; Balseiro, A.; Espí, A.; Barral, M.; Lima Barbero, J.F.; Habela, M.Á.; Fernández-García, J.L.; et al. Occurrence and molecular genotyping of Giardia duodenalis and Cryptosporidium spp. in wild mesocarnivores in Spain. Vet. Parasitol. 2017, 235, 86–93. [Google Scholar] [CrossRef]
- Barlow, A.M.; Mullineaux, E.; Wood, R.; Taweenan, W.; Wastling, J.M. Giardiosis in Eurasian badgers (Meles meles). Vet. Rec. 2010, 167, 1017. [Google Scholar] [CrossRef]
- Millán, J.; Sevilla, I.; Gerrikagoitia, X.; García-Pérez, A.L.; Barral, M. Helminth parasites of the Eurasian badger (Meles meles L.) in the Basque Country (Spain). Eur J. Wildl. Res. 2004, 50, 37–40. [Google Scholar] [CrossRef]
- Anwar, M.A.; Newman, C.; MacDonald, D.W.; Woolhouse, M.E.; Kelly, D.W. Coccidiosis in the European badger (Meles meles) from England, an epidemiological study. Parasitology 2000, 120, 255–260. [Google Scholar] [CrossRef]
- Sapp, S.G.H.; Gupta, P.; Martin, M.K.; Murray, M.H.; Niedringhaus, K.D.; Pfaff, M.A.; Yabsley, M.J. Beyond the raccoon roundworm: The natural history of non-raccoon Baylisascaris species in the New World. Int. J. Parasitol. Parasites Wildl. 2017, 6, 85–99. [Google Scholar] [CrossRef]
- De Liberato, C.; Berrilli, F.; Marangi, M.; Santoro, M.; Trogu, T.; Putignani, L.; Lanfranchi, P.; Ferretti, F.; D’Amelio, S.; Giangaspero, A. Giardia duodenalis in Alpine (Rupicapra rupicapra rupicapra) and Apennine (Rupicapra pyrenaica ornata) chamois. Parasit. Vectors. 2015, 8, 650. [Google Scholar] [CrossRef]
- Di Francesco, C.E.; Smoglica, C.; Paoletti, B.; Angelucci, S.; Innocenti, M.; Antonucci, A.; Di Domenico, G.; Marsilio, F. Detection of selected pathogens in Apennine wolf (Canis lupus italicus) by a non-invasive GPS-based telemetry sampling of two packs from Majella National Park, Italy. Eur. J. Wildl. Res. 2019, 65, 84. [Google Scholar] [CrossRef]
- Guadano Procesi, I.; Montalbano Di Filippo, M.; De Liberato, C.; Lombardo, A.; Brocherel, G.; Perrucci, S.; Di Cave, D.; Berrilli, F. Giardia duodenalis in Wildlife: Exploring Genotype Diversity in Italy and Across Europe. Pathogens 2022, 11, 105. [Google Scholar] [CrossRef]
- Coppola, F.; Maestrini, M.; Berrilli, F.; Procesi, I.G.; Felicioli, A.; Perrucci, S. First report of Giardia duodenalis infection in the crested porcupine (Hystrix cristata L., 1758). Int. J. Parasitol. Parasites Wildl. 2020, 11, 108–113. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M.; Tokarska, M.; Zalewska, H.; Popiołek, M.; Zalewski, A. Digestive tract nematode infections in non-native invasive American mink with the first molecular identification of Molineus patens. Int. J. Parasitol. Parasites Wildl. 2020, 14, 48–52. [Google Scholar] [CrossRef]
- Zalewski, A.; Kołodziej-Sobocińska, M.; Bartoń, K.A. A tale of two nematodes: Climate mediates mustelid infection by nematodes across the geographical range. Int. J. Parasitol. Parasites. Wildl. 2022, 17, 218–224. [Google Scholar] [CrossRef]
- Ramírez-Pizarro, F.; Silva-de la Fuente, C.; Hernández-Orellana, C.; López, J.; Madrid, V.; Fernández, Í.; Martín, N.; González-Acuña, D.; Sandoval, D.; Ortega, R.; et al. Zoonotic Pathogens in the American mink in its southernmost distribution. Vector Borne Zoonotic Dis. 2019, 19, 908–914. [Google Scholar] [CrossRef]
- Popiołek, M.; Szczęsna-Staśkiewicz, J.; Bartoszewicz, M.; Okarma, H.; Smalec, B.; Zalewski, A. Helminth parasites of an introduced invasive carnivore species, the raccoon (Procyon lotor L.), from the Warta Mouth National Park (Poland). J. Parasitol. 2011, 97, 357–360. [Google Scholar] [CrossRef]
- Bowman, D.D.; Hendrix, C.M.; Lindsay, D.S.; Barr, S.C. Feline Clinical Parasitology; Iowa State University Press: Ames, IW, USA, 2002. [Google Scholar] [CrossRef]
- Bauer, C. Baylisascariosis-infections of animals and humans with ‘unusual’ roundworms. Vet. Parasitol. 2013, 193, 404–412. [Google Scholar] [CrossRef]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Stojecki, K.; Sroka, J.; Cacciò, S.M.; Cencek, T.; Dutkiewicz, J.; Kusyk, P. Prevalence and molecular typing of Giardia duodenalis in wildlife from eastern Poland. Folia Parasitol. 2015, 62, 042. [Google Scholar] [CrossRef]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef]
- Cai, W.; Ryan, U.; Xiao, L.; Feng, Y. Zoonotic giardiasis: An update. Parasitol. Res. 2021, 120, 4199–4218. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Šoba, B.; Islamovi’c, S.; Skvarˇc, M.; Cacciò, S.M. Multilocus genotyping of Giardia duodenalis (Lambl, 1859) from symptomatic human infections in Slovenia. Folia Parasitol. 2015, 62, 10–14411. [Google Scholar] [CrossRef]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef]
- La Repubblica. Available online: https://www.repubblica.it/argomenti/maltempo (accessed on 19 July 2022).
- La Nazione. Available online: https://www.lanazione.it/cronaca/maltempo-toscana-1.6848480 (accessed on 19 July 2022).
- Hamnes, I.S.; Gjerde, B.K.; Forberg, T.; Robertson, L.J. Occurrence of Giardia and Cryptosporidium in Norwegian red foxes (Vulpes vulpes). Vet. Parasitol. 2007, 143, 347–353. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Pagh, S.; Stensgaard, A.S.; Chriel, M.; Petersen, H.H. Prevalence of Toxoplasma gondii and Cryptosporidium in Feral and Farmed American Mink (Neovison vison) in Denmark. Acta Parasitol. 2021, 66, 1285–1291. [Google Scholar] [CrossRef]
- Newman, C.; Macdonald, D.W.; Anwar, M.A. Coccidiosis in the European badger, Meles meles in Wytham Woods: Infection and consequences for growth and survival. Parasitology 2001, 123, 133–142. [Google Scholar] [CrossRef]
- Górski, P.; Zalewski, A.; Lakomy, M. Parasites of carnivorous mammals in Białowieza Primeval Forest. Wiad Parazytol. 2006, 52, 49–53. [Google Scholar]
- Seguel, M.; Gottdenker, N. The diversity and impact of hookworm infections in wildlife. Int J. Parasitol. Parasites Wildl. 2017, 6, 177–194. [Google Scholar] [CrossRef]
- Torres, J.; Miquel, J.; Motjé, M. Helminth parasites of the Eurasian badger (Meles meles L.) in Spain: A biogeographic approach. Parasitol Res. 2001, 87, 259–263. [Google Scholar] [CrossRef]
- Sharifdini, M.; Heckmann, R.A.; Mikaeili, F. The morphological and molecular characterization of Baylisascaris devosi Sprent, 1952 (Ascaridoidea, Nematoda), collected from Pine marten (Martes martes) in Iran. Parasit Vectors. 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabi, M.N.S.; Chriél, M.; Hansen, M.S.; Enemark, H.L. Baylisascaris procyonis in wild raccoons (Procyon lotor) in Denmark. Vet. Parasitol. Reg. Stud. Rep. 2015, 1, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Solís, Z.; Birka, S.; Schmäschke, R.; Król, N.; Obiegala, A. First detection of Baylisascaris procyonis in wild raccoons (Procyon lotor) from Leipzig, Saxony, Eastern Germany. Parasitol. Res. 2018, 117, 3289–3292. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Solís, Z.; Meyer-Kayser, E.; Obiegala, A.; Ackermann, F.; Król, N.; Birka, S. Cryptosporidium sp. skunk genotype in wild raccoons (Procyon lotor) naturally infected with Baylisascaris procyonis from Central Germany. Parasitol. Int. 2020, 79, 102159. [Google Scholar] [CrossRef]
- Heddergott, M.; Steinbach, P.; Schwarz, S.; Anheyer-Behmenburg, H.E.; Sutor, A.; Schliephake, A.; Jeschke, D.; Striese, M.; Müller, F.; Meyer-Kayser, E.; et al. Geographic Distribution of Raccoon Roundworm, Baylisascaris procyonis, Germany and Luxembourg. Emerg Infect. Dis. 2020, 26, 821–823. [Google Scholar] [CrossRef]
- Maas, M.; Tatem-Dokter, R.; Rijks, J.M.; Dam-Deisz, C.; Franssen, F.; van Bolhuis, H.; Heddergott, M.; Schleimer, A.; Schockert, V.; Lambinet, C.; et al. Population genetics, invasion pathways and public health risks of the raccoon and its roundworm Baylisascaris procyonis in northwestern Europe. Transbound Emerg. Dis. 2021, 69, 2191–2200. [Google Scholar] [CrossRef]
- Duscher, G.G.; Frantz, A.C.; Kuebber-Heiss, A.; Fuehrer, H.P.; Heddergott, M. A potential zoonotic threat: First detection of Baylisascaris procyonis in a wild raccoon from Austria. Transbound Emerg. Dis. 2021, 68, 3034–3037. [Google Scholar] [CrossRef]
- Lombardo, A.; Brocherel, G.; Donnini, C.; Fichi, G.; Mariacher, A.; Diaconu, E.L.; Carfora, V.; Battisti, A.; Cappai, N.; Mattioli, L.; et al. First report of the zoonotic nematode Baylisascaris procyonis in non-native raccoons (Procyon lotor) from Italy. Parasit. Vectors 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Romeo, C.; Cafiso, A.; Fesce, E.; Martínez-Rondán, F.J.; Panzeri, M.; Martinoli, A.; Cappai, N.; Defilippis, G.; Ferrari, N. Lost and found: Helminths infecting invasive raccoons introduced to Italy. Parasitol. Int. 2021, 83, 102354. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Feliu, C.; Fernández-Morán, J.; Ruíz-Olmo, J.; Rosoux, R.; Santos-Reis, M.; Miquel, J.; Fons, R. Helminth parasites of the Eurasian otter Lutra lutra in southwest Europe. J. Helminthol. 2004, 78, 353–359. [Google Scholar] [CrossRef]
- Torres, J.; Miquel, J.; Fournier, P.; Fournier-Chambrillon, C.; Liberge, M.; Fons, R.; Feliu, C. Helminth communities of the autochthonous mustelids Mustela lutreola and M. putorius and the introduced Mustela vison in south-western France. J. Helminthol. 2008, 82, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, M.C.; Kaloustian, L.L.; Gerhold, R.W. Endoparasites of American marten (Martes americana): Review of the literature and parasite survey of reintroduced American marten in Michigan. Int J. Parasitol. Parasites Wildl. 2016, 5, 240–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ko, P.P.; Suzuki, K.; Canales-Ramos, M.; Aung, M.P.P.T.H.H.; Htike, W.W.; Yoshida, A.; Montes, M.; Morishita, K.; Gotuzzo, E.; Maruyama, H.; et al. Phylogenetic relationships of Strongyloides species in carnivore hosts. Parasitol. Int. 2020, 78, 102151. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, F.; Coppola, F.; Furzi, F.; Gabrielli, S.; Baldanti, S.; Boni, C.B.; Felicioli, A. Taeniid cestodes in a wolf pack living in a highly anthropic hilly agro-ecosystem. Parasite 2021, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Lang, A. Tracce di Animali. Impronte, Escrementi, Tracce di Pasti, Borre, Tane e Nidi; Zanichelli: Bologna, Italy, 1989; p. 127. [Google Scholar]
- Chame, M. Terrestrial mammal feces: A morphometric summary and description. Mem. Inst. Oswaldo Cruz. 2003, 98 (Suppl. 1), 71–94. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Bowman, D.D. Georgis’ Parasitology for Veterinarians, 6th ed.; W. B. Saunders Company: Philadelphia, PA, USA, 1995; pp. 295–296. [Google Scholar]
- Read, C.; Walters, J.; Robertson, I.D.; Thompson, R.C.A. Correlation between genotype of Giardia duodenalis and diarrhoea. Int. J. Parasitol. 2002, 32, 229–231. [Google Scholar] [CrossRef]
- Cacciò, S.M.; De Giacomo, M.; Pozio, E. Sequence analysis of the ß-giardin gene and development of a polymerase chain reac-tion-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 2002, 32, 1023–1030. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Fayer, R.; Bern, C.; Gilman, R.H.; Trout, J.M.; Schantz, P.M.; Das, P.; Lal, A.A.; Xiao, L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003, 9, 1444–1452. [Google Scholar] [CrossRef] [PubMed]

| Parasites | No. Positive Samples (%) | EPG/OPG * (Range) |
|---|---|---|
| Helminths | ||
| Uncinaria criniformis eggs a | 33/43 (76.7%) | 84.8 (10–800) |
| Unidentified gastrointestinal Strongyle-type eggs a | 3/43 (7%) | 10 EPG |
| Capillariid eggs a | 1/43 (2.3%) | 10 EPG |
| Ascarid eggs a | 1/43 (2.3%) | 10 EPG |
| Strongyloides sp. b | 3/43 (7%) | - |
| Protozoa | ||
| Giardia duodenalisc | 21/43 (48.8%) | - |
| Cryptosporidium spp. c | 10/43 (23.2%) | - |
| Coccidian oocysts a | 3/43 (7%) | 10–55 OPG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrini, M.; Berrilli, F.; Di Rosso, A.; Coppola, F.; Guadano Procesi, I.; Mariacher, A.; Felicioli, A.; Perrucci, S. Zoonotic Giardia duodenalis Genotypes and Other Gastrointestinal Parasites in a Badger Population Living in an Anthropized Area of Central Italy. Pathogens 2022, 11, 906. https://doi.org/10.3390/pathogens11080906
Maestrini M, Berrilli F, Di Rosso A, Coppola F, Guadano Procesi I, Mariacher A, Felicioli A, Perrucci S. Zoonotic Giardia duodenalis Genotypes and Other Gastrointestinal Parasites in a Badger Population Living in an Anthropized Area of Central Italy. Pathogens. 2022; 11(8):906. https://doi.org/10.3390/pathogens11080906
Chicago/Turabian StyleMaestrini, Michela, Federica Berrilli, Alessia Di Rosso, Francesca Coppola, Isabel Guadano Procesi, Alessia Mariacher, Antonio Felicioli, and Stefania Perrucci. 2022. "Zoonotic Giardia duodenalis Genotypes and Other Gastrointestinal Parasites in a Badger Population Living in an Anthropized Area of Central Italy" Pathogens 11, no. 8: 906. https://doi.org/10.3390/pathogens11080906
APA StyleMaestrini, M., Berrilli, F., Di Rosso, A., Coppola, F., Guadano Procesi, I., Mariacher, A., Felicioli, A., & Perrucci, S. (2022). Zoonotic Giardia duodenalis Genotypes and Other Gastrointestinal Parasites in a Badger Population Living in an Anthropized Area of Central Italy. Pathogens, 11(8), 906. https://doi.org/10.3390/pathogens11080906








