Abstract
Toxoplasma gondii constitutes a major zoonotic agent but also has been frequently identified as an important cause of clinical disease (e.g., abortion, pneumonia, encephalitis) in wildlife; specifically, T. gondii has been associated with neurological disease in cetaceans. This study investigated the genetic diversity of T. gondii strains involved in infections in dolphins found stranded in the Mediterranean coastlines of Italy. Tissue samples from 16 dolphins (Stenella coeruleoalba and Tursiops truncatus species) positive for T. gondii-DNA presence by PCR were examined by histology and subjected to further genetic characterization of strains detected by PCR-RFLP and multilocus PCR-sequencing assays. According to fully genotyped samples, the genotypes ToxoDB#3 (67%) and #2 (22%) were detected, the latter being reported for the first time in cetaceans, along with a mixed infection (11%). Subtyping by PCR-seq procedures provided evidence of common point mutations in strains from southwestern Europe. Despite evidence of T. gondii as a cause of neurological disease in dolphins, sources of infections are difficult to identify since they are long-living animals and some species have vast migration areas with multiple chances of infection. Finally, the genetic diversity of T. gondii found in the dolphins studied in the Mediterranean coastlines of Italy reflects the main genotypes circulating inland in the European continent.
1. Introduction
Toxoplasma gondii (Apicomplexa) constitutes one of the most successful protozoan parasites worldwide and can cause harmful effects in the host, especially mammals, in which abortions and fetal malformations in pregnant people, and severe pneumonia and encephalitis in immunocompromised individuals, may be observed [1].
Toxoplasma gondii has a wide range of susceptible intermediate hosts, including cetaceans, in which clinical infections mostly associated to encephalitis have been frequently reported [2,3,4,5]. One of the most intriguing facts in toxoplasmosis research has to do with linking the clinical outcomes with the genotype of the strain causing the infection [6]; until date, such an association has only been observed for a few genotypes (e.g., ToxoDB#65 causing ocular disease in humans). Other aspects, such as the physiological status of the host and its genetic background, may be necessarily involved in the virulence of the T. gondii strains [7].
To date, the T. gondii genetic population is known to be structured in 16 well-defined haplogroups assorted into six major clades (clade A–F) worldwide. Three clonal types dominate the northern hemisphere, and a fourth clonal lineage (HG12) is largely confined to wild animals in North America. In contrast, much greater genetic diversity is observed in South America, where there seems to be no predominance of any genetic type [6]. Data on the genetic diversity of the T. gondii strains involved in infections of cetaceans are scarce [1]. Some genotypes predominate in certain geographical areas, but dolphins have wide-ranging areas, and their migration routes may constitute a key factor in the potential diversity of the parasite. In addition, mixed infections can be expected due to their long-life expectancy. Recently, molecular data of T. gondii strains infecting dolphins and other cetaceans in Europe have been summarized [8,9,10], but still a notable gap of information remains. The interest in further research on T. gondii in cetaceans is undeniable as an important issue in their conservation, and as potential sentinels for environmental contamination by T. gondii oocysts in a highly anthropized area such as the Mediterranean Sea.
The present paper aimed at providing detailed molecular characterization (genotyping) of the T. gondii organisms infecting dolphins found stranded along the Italian coasts, covering new cases and others previously reported that have been revisited.
2. Results
2.1. Parasite Detection
All sixteen cases resulted positive for T. gondii presence by conventional nested PCR performed during routine procedures at time of necropsy; twenty-eight target organs from the aforementioned dolphins were subjected to further analyses, based on tissue availability (Table 1).

Table 1.
Histological and parasite load findings in tissue samples from dolphins stranded along the Italian coastlines that tested positive to Toxoplasma gondii DNA presence.
2.2. Histological and Parasite Burden Findings
Histopathological data were available for all the tissues submitted to the molecular/genotyping characterization, with the spleen of case #15 being the only exception due to logistical issues during necropsy procedures.
Eighteen tissues from 13 cetaceans showed microscopic lesions suggestive of or, at least, compatible with T. gondii infection (Figure 1).
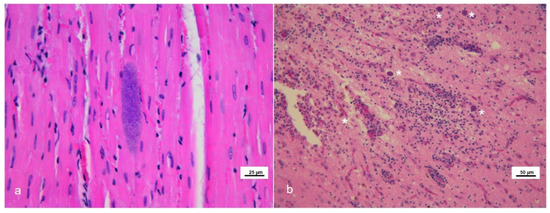
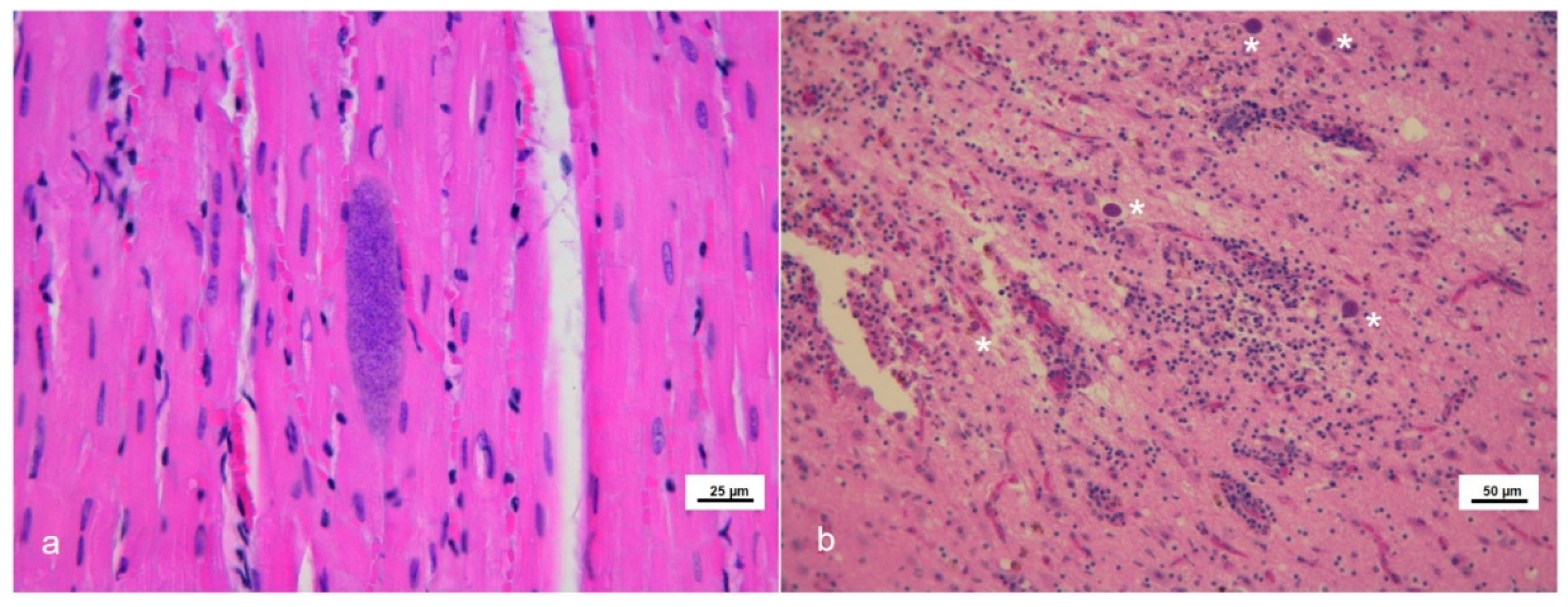
Figure 1.
Microscopic lesions in stranded cetaceans with Toxoplasma gondii infection. Hematoxylin and eosin (HE) staining. (a) Skeletal muscle (case #6). Toxoplasma-like tissue cyst. (b) Brain frontal cortex (case #7). Severe non-suppurative necrotizing encephalitis in the presence of several Toxoplasma-like tissue cysts (asterisks).
Thirteen out of the above eighteen specimens presented with mature T. gondii-like tissue cyst structures (12/13 brains and 1/13 skeletal muscle) (Table 1). Parasite load estimation by qPCR ranged from 0.44 to 5507.32 zoites/mg of analyzed tissues; as expected, most of the higher parasite burdens were observed in CNS specimens belonging to animals showing an apparently active T. gondii neurological infection (severe encephalitis) (Table 1). Some samples that tested positive by the initial nested PCR resulted negative by the subsequent quantitative PCR assay (n = 4) and it was not possible to quantify the parasite load, demonstrating the greater sensitivity of the former protocol. Association of the T. gondii infection with the hypothetical causa mortis or the cause of stranding was not among the aims of present paper.
2.3. Genotyping Results
All tissues that tested positive for T. gondii-DNA presence by qPCR, some belonging to the same animal, were subjected to PCR-RFLP and PCR-sequencing procedures. Finally, typing was possible only for tissues from 13 different dolphins (Table 2), but a full genotyping profile was obtained only for nine animals. Genotypes ToxoDB #3 (67%, (6/9)) and ToxoDB #2 (22%, (2/9)) were observed. In addition, a mixed infection (11%, (1/9)) predictably involving type II and III strains (liver from case #4, Table 2) was observed.

Table 2.
Results of PCR-RFLP genotyping analyses carried out on Toxoplasma gondii strains identified in the dolphins stranded along the Italian coastlines.
The CS3 marker, proposed to have a highly predictive value on virulence in mice [11], presented type II alleles in all strains with the ToxoDB #3 genotype, whereas type III alleles were detected in all isolates with the ToxoDB #2 genotype (Table 2).
Regarding subtyping by multilocus PCR-sequencing procedures, we conducted PCR sequencing of three polymorphic genes, GRA6, GRA7 and SAG3. First, all GRA6 sequences corresponding to type III alleles (dolphin cases #13 and #16; ON814572) presented a 100% homology with MT370490 (sheep, Spain), MK055338 (cattle, Iran), MG587985 (wild boar, Italy), and many other sequences deposited in GenBank. On the other hand, all GRA6 sequences corresponding to type II alleles (rest of dolphin cases; ON814571) showed 100% homology with MT370491 (sheep, Spain), MG587975 (pig, Italy), MG587959 (pig, Italy), and many other sequences deposited in GenBank. Concerning the GRA7 marker, all sequences corresponding to type III alleles (dolphin cases #13 and #16; ON982169) presented a 100% homology with MT361129 (sheep, Spain), LN714496 (VEG reference strain), HQ852155 (goat, USA) and many other sequences deposited in GenBank. Moreover, all GRA7 sequences corresponding to type II alleles (rest of dolphin cases; ON982166) showed 100% homology with MT361127 (sheep Spain), DQ459445 (PRU strain reference), JX045585 (sheep, USA) and other deposited sequences. Furthermore, the alignment of all SAG3 sequences from samples that showed a type II allele identified a single nucleotide polymorphism (SNP), G1691T, which splits our type II and type II-like samples into two groups. The first group (dolphin cases #1, 2, 6, 9, 10 and 15; IIa SAG3 allele, ON814568) had 100% homology with MT361125 (sheep, Spain), KU599489 (cat, Turkey), KU599478 (chicken, Portugal), ON814566 (Me49 reference strain), and others deposited in GenBank. The other group (G1691T, cases #4, 7, 11 and 14; IIb SAG3 allele, ON814569) showed 100% identity with MT361126 (sheep, Spain), KU599488 (cat, Turkey), KU599479 (pig, Portugal), and KU599412 (sheep, France), among many other sequences deposited. The SNP leads to an amino acid change at codon 368 from Met to Ile, and this had been previously described in a large collection of samples collected from sheep abortion cases in Spain [12]. Finally, all SAG3 sequences corresponding to type III alleles (dolphin cases #13 and #16; ON814570) presented a 100% homology with MT361130 (sheep, Spain), MK801823 (sheep, Iraq), LC414534 (rat, Iran), KU599490 (human, Turkey), and many other sequences deposited in GenBank. It should be noted the complete lack of sequences from some marine mammals infections deposited in the NCBI database.
In the case #4 (1267/15), an attempt to confirm the mixed infection by sequencing the PCR products obtained resulted in only type II allele amplification.
2.4. Phylogenetic Analyses
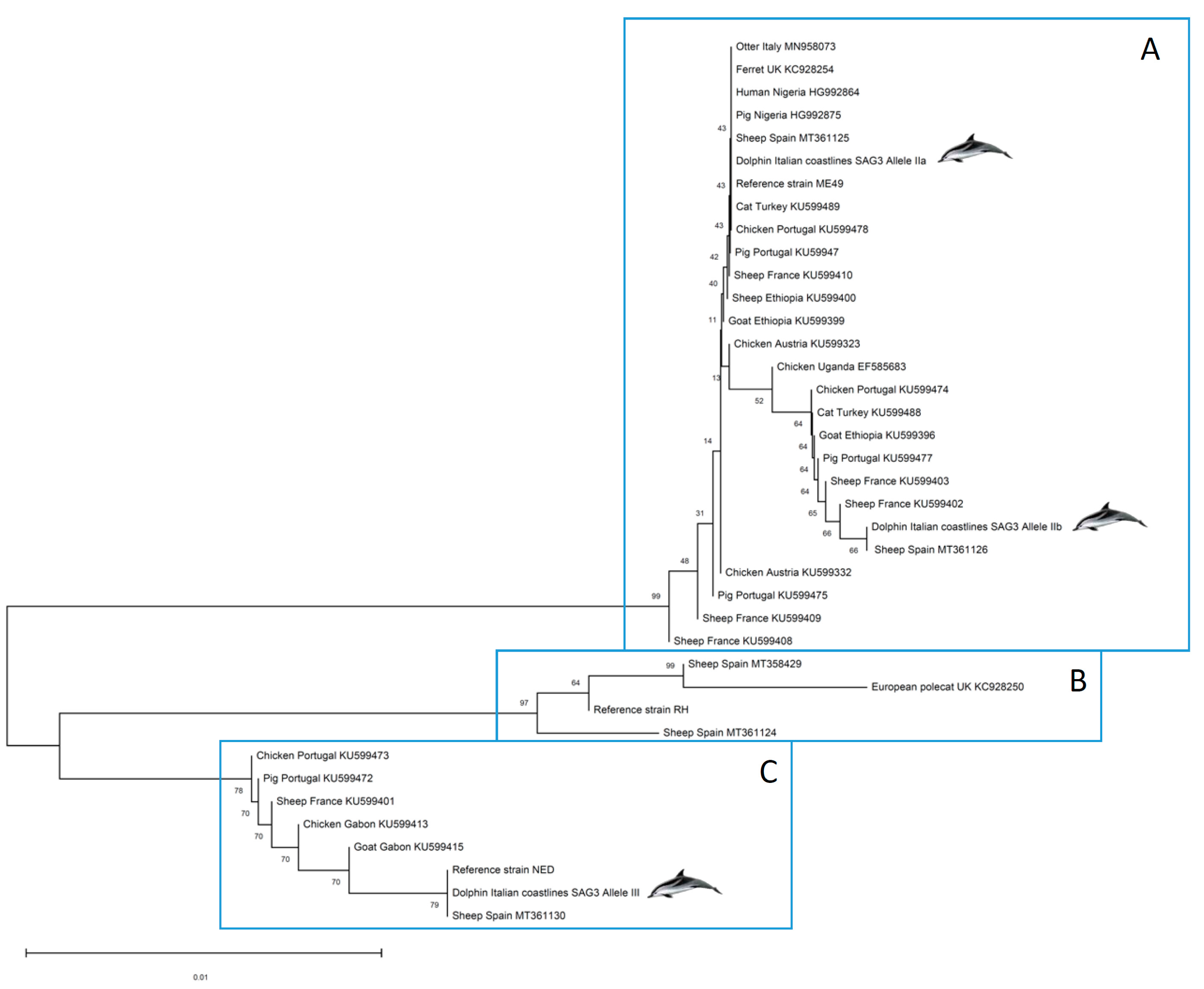
Phylogenetic analyses were carried out aiming at positioning the strains infecting the dolphins in a One Health approach. Thus, SAG3 marker sequences deposited in GenBank and belonging to T. gondii strains isolated from inland African and European hosts (representing the Mediterranean context) were included in the construction of a phylogenetic tree along with those obtained here from dolphin clinical samples and the clonal reference strains used (TgRH, TgMe49 and TgNED) (Figure 2). A separation of the selected sequences into three well-differentiated groups is evident. High bootstrap (BP) values at each node supported the clustering into A, B and C clusters (BP = 76–99%). Cluster A includes sequences with type II alleles, cluster B involves sequences with type I alleles, and finally, cluster C groups sequences with type III alleles. It should be noted that, within cluster A, although IIa and IIb along with other variants are grouped, low BP values (BP = 9–40%) indicate that the phylogenetic position of the different Toxoplasma strains included is not conclusive based on SAG3 sequences used, probably due to a low diversity at the nucleotide sequence level and the short length of the SAG3 sequences obtained.
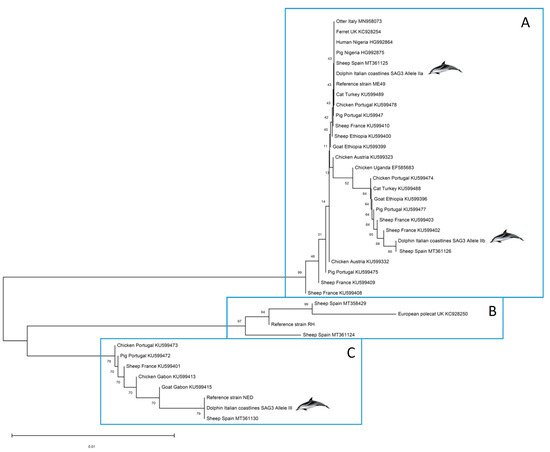
Figure 2.
Phylogenetic positioning of the Toxoplasma gondii organism found in stranded dolphins tissues based on the SAG3 gene. This analysis involved 39 nucleotide sequences from T. gondii strains/isolates infecting human, domestic and wild hosts located in Europe and Africa.
3. Discussion
The study of the diseases that commonly affect cetaceans, and in particular the dolphins, is a challenging issue due to the difficult access to samples from these animals, which in most cases are derived from dead animals found stranded several hours or days after death.
Some previously investigated cases were revisited and molecularly characterized in depth here, aiming to provide reliable and comparable information of the cases and final phylogenetic analyses. In areas such as North America, clinical toxoplasmosis is a common finding in marine mammals (e.g., sea otters) [8]; in the Mediterranean basin, although there are many uncovered areas, protozoal meningoencephalitis has been linked to T. gondii subacute to chronic infections in dolphins [2,3,13,14,15], including through congenital transmission [16,17]. In the present study a noticeable proportion of cases (13/16–81%) presented with encephalitis and other lesions compatible with active toxoplasmosis. There is still a clear debate on the role of T. gondii as the cause of death in cetaceans and a predisposing factor for stranding, although in recent decades different authors have proposed its role as a primary pathogen in various stranding events [2,18,19].
In the present paper, despite the limited genetic diversity in T. gondii strains infecting dolphins studied, it is suggested that infections may have occurred near the coastlines of Europe where only four different genotypes (ToxoDB#1, 2, 3, 10) have been identified, along with a few recombinant and non-canonical strains [10]. This is the first report of RFLP genotype #2 in cetaceans. In addition, dolphins may be exposed to strains circulating in North Africa where a noticeable proportion of type III strains (ToxoDB#2) is present, along with a number of African genotypes, the latter being not identified in the present collection, [20]. Indeed, striped dolphins, that in our study accounted for almost the totality of the individuals (15/16–94%), consist of a species for which vast migrations within the Mediterranean basin have been hypothesized [21,22], differently for what reported for bottlenose dolphins, characterized by a residential attitude [23].
Noteworthy also is the marked susceptibility to the infection of the striped dolphin, regarded as a pelagic species; the severe disease patterns described in this study and by several other authors [2,3,14], could be related to the lack of a mutual host–parasite coevolution that exists for coastal species, such as the bottlenose dolphin, whose members are more frequently exposed to the protozoan [24].
It is well known that T. gondii genotypes are somehow restricted to specific regions [25]. As commented before, there are a few reports regarding the T. gondii genetic diversity in cetaceans and specifically in dolphins [8], and this appears to be low or limited, with most of the cases related to clonal type II lines, such as ToxoDB#1 in South Carolina, USA [26], Costa Rica [27] and Italy [28], and variants of the type II (ToxoDB#3) in Canada [29], New Zealand [30] and Italy [13]. Two reports deserve attention, the one reported by [31] described a case of fatal disseminated T. gondii infection by a ToxoDB#3 strain in a captive harbor porpoise (Phocoena phocoena) that lived in an open sea basin in Denmark, and the report by [32] that identified, by three microsatellite markers, a type II T. gondii strain infecting a stranded Mediterranean fin whale (Balaenoptera physalus) from Italy. The study of T. gondii strains present in other cetaceans different than dolphins will enrich the current knowledge of “marine” life cycles of T. gondii.
Unfortunately, no information on the prevalence of the infection can be drawn through this study, due to the criterion of selection of the cases investigated. Future studies are warranted to obtain precious data on the epidemiology of the parasite in the Italian waters.
The transmission pathways and the pathogenesis of the parasite in cetaceans still remain to be clarified. For those species living the offshore waters, such as striped dolphins, oocyst-contaminated wastewaters discharged from ships have been suggested as a likely source of infection [2]. However, aquatic mammals can also become infected by feeding on mussels or fish contaminated with the protozoan. A novel marine transmission pathway comprising suspended bioparticles, bio-films, small invertebrates and gastropods has been in fact recently proposed [33].
In conclusion, findings in aquatic animals of the present study are somehow a reflection of the data observed in continental (“inland”) Europe [10] and North African countries [20,34].
Unlike in the Americas [26,27,29], there are no T. gondii isolates from cetaceans available in Europe; such information will add important knowledge regarding intra-genotype genetic diversity and will allow us to evaluate to phenotypic traits, and allow the implementation of unified procedures [7] adding essential data for the virulence degree of such isolates.
Future investigations are warranted because of the interest of unraveling the potential (pathogenic) role of T. gondii in the stranding of cetaceans, and the nature of their sources and vias of infections; furthermore, parasite (T. gondii) isolation from cetaceans will provide valuable information both at the genomic (Whole Genome Sequencing, WGS) and phenotypic level if virulence evaluation is addressed.
4. Materials and Methods
4.1. Materials
All cases were stranded cetaceans diagnosed during routine pathological and cause-of-death assessment by the Italian stranding network of Istituti Zooprofilattici Sperimentali, veterinary public health institutions placed under the Italian Health Ministry supervision. The animals were examined and submitted to a complete post mortem examination, according to standard protocols [35].
Epidemiological (location and date of stranding) and biological data (species, sex, age class, nutritional and decomposition status) were systematically recorded (Supplementary Table S1). The animals were divided into 3 age categories (newborn–calf, juvenile–subadult and adult) based on the total body length [35,36]. The decomposition condition of the carcasses (DCC) was classified as code 1 (extremely fresh carcass, just dead), code 2 (fresh), code 3 (moderate decomposition), code 4 (advanced decomposition), or code 5 (mummified or skeletal remains) [37]. The nutritional condition state (NCC) was classified as good, moderate or poor based morphologically on anatomical parameters such as the convexity of the dorsal profile, the rib prominence and the amount of body fat.
During necropsy, tissue samples from all the major organs were collected and split into 3 aliquots for subsequent analyses: one was kept frozen at −20 °C for microbiological investigations, one at −80 °C for biomolecular analyses, and the other was preserved in neutral buffered formalin for histological investigations [9]. According to [5], when available, ten different areas from the central nervous system (CNS) were sampled and examined, including basal nuclei, thalamus, mesencephalon, pons, obex, spinal cord and frontal, parietal, occipital and cerebellar cortex. After being fixed in 10% neutral buffered formalin, tissues were embedded in paraffin, sectioned at 4 ± 2 μm, stained with haematoxylin and eosin (H&E) and examined through a light microscope.
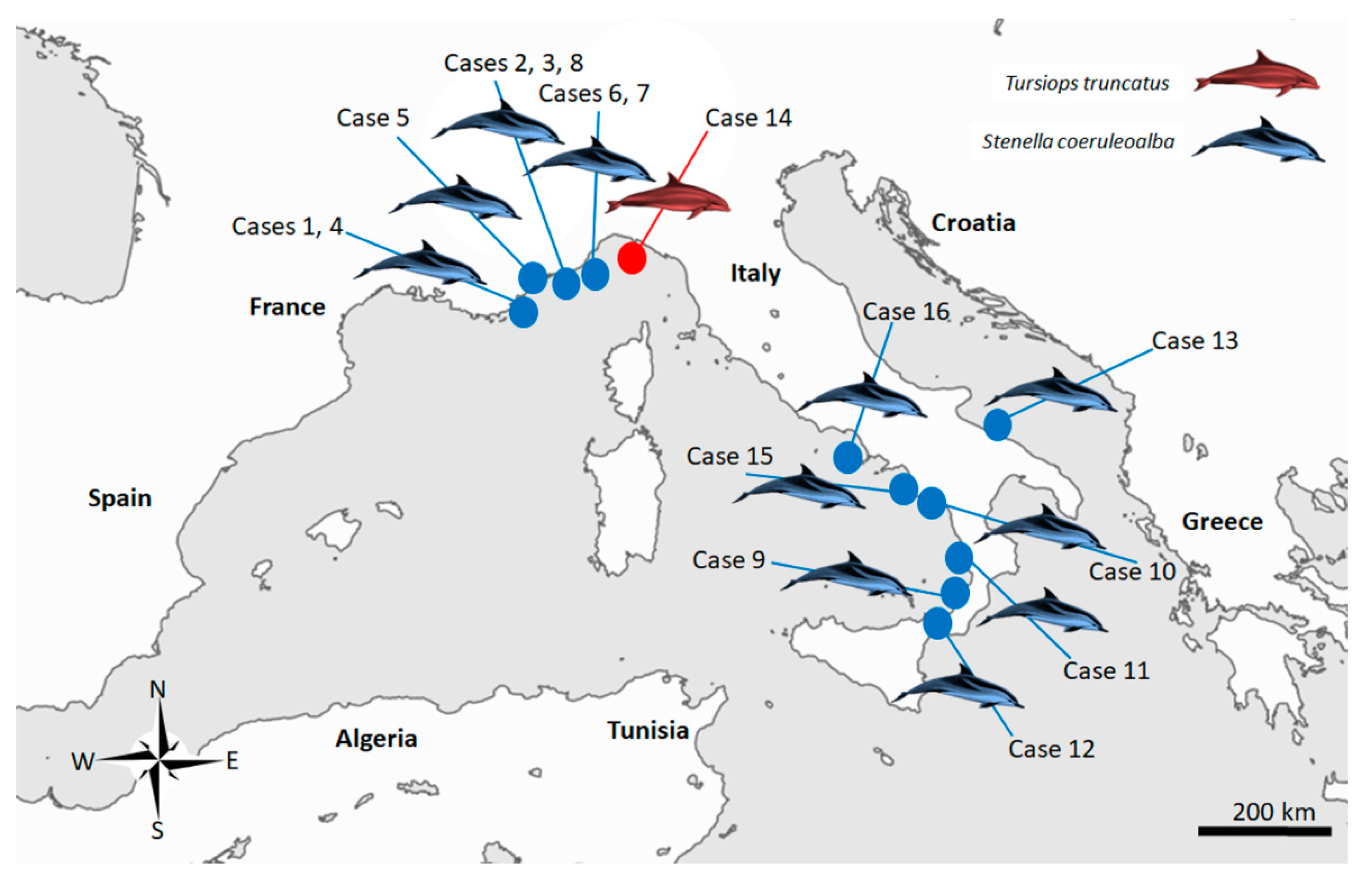
Sixteen cases (Figure 3), resulting in being molecularly positive for T. gondii through a nested PCR targeting the ITS-1 fragment [38] during routine procedures at least in one of the target organ examined (brain, lung, lymph nodes, liver, spleen, heart and muscle) (Table 1) whose tissues were easily and quickly available at the time of the present study, were retrieved and selected for the molecular analysis performed for this investigation.
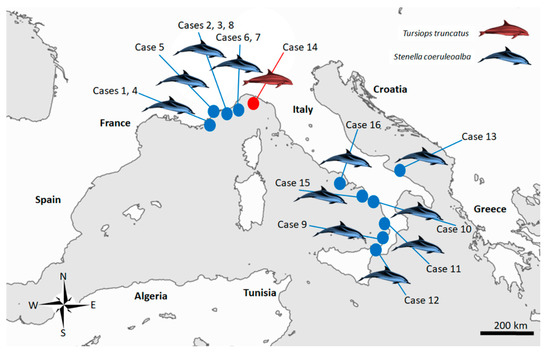
Figure 3.
Map of the study area in the Italian Mediterranean coastline, displaying the stranding locations of the 16 cetaceans infected with Toxoplasma gondii selected for present study.
Histopathological diagnostic reports of the selected tissues were retrieved and further analyzed with the focus on the microscopic changes compatible with T. gondii infection [4,5,30,39].
A few cases had been previously published and revisited here (Supplementary Table S1).
4.2. DNA Extraction from Tissues
DNA was purified from 25 mg of frozen tissue specimens, based on tissue availability and previous positive detection to the protozoon; samples were first homogenized using the TissueLyser II (QIAGEN, Hilden, Germany) by high-speed shaking in Eppendorf tubes with stainless steel beads (5 mm diameter, QIAGEN). Homogenates were centrifuged at 14,000 rpm for 3 min to remove the suspended solids, without removing beads. Supernatants were submitted to DNA extraction using the ReliaPrep TM gDNA Tissue Miniprep System kit (Promega Corporation, Madison, WI, USA) as described by the manufacturer (Standard Protocol for Animal Tissue). The genomic DNA was placed at −20 °C for long-term storage after quantification with VivaSpec Spectrophotometer (Sartorius Stedim Biotech, Aubagne, France).
4.3. Parasite Quantification in Tissues
Trying to link the severity of histological lesions with the parasite burden, T. gondii DNA quantification was performed using a duplex qPCR assay adapted from [40]. It included the amplification of the species-specific 529RE locus and an internal amplification control (IAC) to aid the identification of false negative results [41]. The qPCR reactions were performed in a final volume of 25 μL using the SensiFAST Probe Lo-ROX Kit (Bioline, Memphis, TN, USA; BIO-84020); each primer was at a final concentration of 0.25 μM, HEX (529RE locus) and Cy5 (IAC) probes were at a final concentration of 0.15 μM, as well as 5 μL of DNA. Amplification and fluorescence detection were performed on an Applied Biosystems 7500 FAST Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using 96-well PCR plates under the following conditions: initial denaturation at 95 °C for 5 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 40 s.
Quantification (number of T. gondii parasites) was calculated by interpolating the average Ct values on a standard curve equivalent to 1 × 105 − 1 × 10−1 tachyzoites generated by tenfold serial dilutions of parasite DNA. Standard curves for T. gondii showed an average slope always close to −3.3 and an R2 > 0.98. Parasite load in tissues was expressed as the zoites/mg of tissue.
4.4. Molecular Characterization-Genotyping of T. gondii Strains (Organisms)
All qPCR-positive samples were subjected to further genotyping analysis. DNA extracts were subjected to the widely used Mn-PCR restriction fragment length polymorphism (RFLP) method, with the markers SAG1, SAG2 (5′–3′ SAG2, and alt. SAG2), SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, and Apico [42].
Aiming for a deeper genetic characterization of the T. gondii population detected in dolphins, other cases of infection reported earlier (Supplementary Table S1), have been revisited. ToxoDB RFLP genotype was identified according to http://toxodb.org/toxo/ accessed on 25 April 2022.
In addition, alleles for the CS3 marker [11], which are suggested to have highly predictive value for the virulence degree in mice, and those from the well-known virulence gene GRA7 [43] were also studied. In these cases, the methodology was based on nested PCR-DNA sequencing and in silico digestion of each locus sequences obtained. All details are described in [44]. The sequencing procedures were carried out at the Center for Genomic Technologies of the Complutense University of Madrid (Spain) using the BigDye® Terminator kit v 3.1 (Applied Biosystems, Foster City, CA, USA) and analyzed on an ABI 3130 Genetic Analyzer (Applied Biosystems). The resulting sequences were imported, read, edited manually if necessary, and analyzed using BioEdit software (version 7.0.5.3; [https://www.bioedit.software.informer.com], accessed on 10 August 2022) [45]. Necessary alignments were performed using Clustal Omega software [https://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 1 June 2022]. Finally, in silico digestion was conducted by the NEBCutter 2.0 program [46].
4.5. Phylogenetic Analyses
A phylogenetic tree was constructed based on SAG3 sequences obtained from cetacean samples included in the present study in addition to those from a set of sequences deposited in GenBank selected based on their geographical origin (Europe and Africa) in order to represent the Western Mediterranean context. Sequences from the clonal reference strains TgRH (type I), TgMe49 (type II) and TgNED (type III) were also included. The evolutionary history was inferred using the Neighbor-Joining method [47]. A bootstrap test (10,000 replicates) to evaluate the percentage of replicate trees in which the associated taxa clustered together was conducted [48]. The evolutionary distances were computed using the Maximum Composite Likelihood method [49]. Evolutionary analyses were conducted in MEGA11 [50].
5. Conclusions
Toxoplasma gondii is frequently found infecting stranded wild dolphins, and such infections are frequently associated to neuropathy; in addition, despite the limited genetic diversity found in the dolphins studied in the Mediterranean coastlines of Italy, findings reflect the main genotypes circulating inland in the European continent. Indeed, the first detection of ToxoDB genotype #2 in a cetacean in this study can be highlighted.
There are still many gaps that should be covered, such as: (i) how frequent are dolphin infections in nature, and (ii) what are the main sources and vias of infection for cetaceans. Therefore, it is expected that T. gondii isolation from free-ranging dolphins (and other cetaceans), and the use of tools with higher resolution power (WGS and other next generation sequencing-based methods) will provide evidence of the sources of infections and the similarity of T. gondii strains in dolphins with those strains found inland.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11080909/s1, Table S1: Anamnestic and stranding data of the sixteen dolphins selected in the present study. References [5,9,14,51,52] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, C.C., L.M.O.-M. and R.C.-B.; formal analysis, M.F.-E., F.G. and R.C.-B.; funding acquisition, C.C. and L.M.O.-M.; investigation, M.F.-E., F.G., V.M., T.A., K.V., C.G., F.D.N., G.L. and R.C.-B.; methodology, M.F.-E., F.G., V.M., T.A., K.V., C.G., F.D.N., G.L. and R.C.-B.; supervision, C.C. and R.C.-B.; writing—original draft, M.F.-E., F.G. and R.C.-B.; writing—review and editing, L.M.O.-M., C.C. and R.C.-B. All authors have read and agreed to the published version of the manuscript.
Funding
M.F.-E. is funded by UCM-POP 2021 post-doctoral grants. This research was funded by the Italian Ministry of Health [Ricerca Corrente 2019 IZS PLV 05/19 and Ricerca Corrente 2021 IZS PLV 06/21]. Also, this study was part of TOXOSOURCES project, supported by funding from the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No. 773830: One Health European Joint Programme.
Institutional Review Board Statement
Not applicable. Ethical review and approval were waived for this study, as neither animals were sacrificed nor experiments were performed with live animals. The permission for the management of dead stranded cetaceans was issued by the Italian Ministry of Health.
Informed Consent Statement
Not applicable.
Data Availability Statement
DNA sequences of the markers SAG3 (ON814566-70), GRA6 (ON814571, ON814572), and GRA7 (ON982166-70) have been deposited in GenBank.
Acknowledgments
The authors are grateful to Milena Monnier and Goria Maria for biomolecular, analyses. Particular thanks are due to Walter Mignone and Enrica Berio for necropsying some of the animals for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubey, J.P. Toxoplasmosis Of Animals And Humans, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–542. [Google Scholar]
- Di Guardo, G.; Proietto, U.; Di Francesco, C.E.; Marsilio, F.; Zaccaroni, A.; Scaravelli, D.; Mignone, W.; Garibaldi, F.; Kennedy, S.; Forster, F.; et al. Cerebral Toxoplasmosis in Striped Dolphins (Stenella coeruleoalba) Stranded along the Ligurian Sea Coast of Italy. Vet. Pathol. 2010, 47, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Pintore, M.D.; Mignone, W.; Di Guardo, G.; Mazzariol, S.; Ballardini, M.; Florio, C.L.; Goria, M.; Romano, A.; Caracappa, S.; Giorda, F.; et al. Neuropathologic findings in cetaceans stranded in Italy (2002–2014). J. Wildl. Dis. 2018, 54, 295–303. [Google Scholar] [CrossRef]
- Sierra, E.; Fernández, A.; Felipe-Jiménez, I.; Zucca, D.; Díaz-Delgado, J.; Puig-Lozano, R.; Câmara, N.; Consoli, F.; Díaz-Santana, P.; Suárez-Santana, C.; et al. Histopathological Differential Diagnosis of Meningoencephalitis in Cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Front. Vet. Sci. 2020, 7, 650. [Google Scholar] [CrossRef]
- Giorda, F.; Crociara, P.; Iulini, B.; Gazzuola, P.; Favole, A.; Goria, M.; Serracca, L.; Dondo, A.; Crescio, M.I.; Audino, T.; et al. Neuropathological Characterization of Dolphin Morbillivirus Infection in Cetaceans Stranded in Italy. Animals 2022, 12, 452. [Google Scholar] [CrossRef]
- Dardé, M.L.; Mercier, A.; Su, C.; Khan, A.; Grigg, M.E. Chapter 3: Molecular Epidemiology and Population Structure of Toxoplasma gondii. In Toxoplasma gondii: The Model Apicomplexan—Perspectives and Methods, 3rd ed.; Weiss, L.M., Kim, K., Eds.; Academic Press: New York, NY, USA, 2020; pp. 1–1222. [Google Scholar]
- Calero-Bernal, R.; Fernández-Escobar, M.; Katzer, F.; Su, C.; Ortega-Mora, L.M. Unifying Virulence Evaluation in Toxoplasma gondii: A Timely Task. Front. Cell. Infect. Microbiol. 2022, 12, 868727. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Grigg, M.E. Recent epidemiologic and clinical importance of Toxoplasma gondii infections in marine mammals: 2009–2020. Vet. Parasitol. 2020, 288, 109296. [Google Scholar] [CrossRef] [PubMed]
- Giorda, F.; Romani-Cremaschi, U.; Marsh, A.E.; Grattarola, C.; Iulini, B.; Pautasso, A.; Varello, K.; Berio, E.; Gazzuola, P.; Marsili, L.; et al. Evidence for Unknown Sarcocystis-Like Infection in Stranded Striped Dolphins (Stenella coeruleoalba) from the Ligurian Sea, Italy. Animals 2021, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, M.; Schares, G.; Maksimov, P.; Joeres, M.; Ortega-Mora, L.M.; Calero-Bernal, R. Toxoplasma gondii Genotyping: A Closer Look Into Europe. Front. Cell. Infect. Microbiol. 2022, 12, 842595. [Google Scholar] [CrossRef] [PubMed]
- Pena, H.F.; Gennari, S.M.; Dubey, J.P.; Su, C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol. 2008, 38, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, M.; Calero-Bernal, R.; Benavides, J.; Regidor-Cerrillo, J.; Guerrero-Molina, M.C.; Gutiérrez-Expósito, D.; Collantes-Fernández, E.; Ortega-Mora, L.M. Isolation and genetic characterization of Toxoplasma gondii in Spanish sheep flocks. Parasit Vectors 2020, 13, 396. [Google Scholar] [CrossRef]
- Di Guardo, G.; Di Cesare, A.; Otranto, D.; Casalone, C.; Iulini, B.; Mignone, W.; Tittarelli, C.; Meloni, S.; Castagna, G.; Forster, F.; et al. Genotyping of Toxoplasma gondii isolates in meningo-encephalitis affected striped dolphins (Stenella coeruleoalba) from Italy. Vet. Parasitol. 2011, 183, 31–36. [Google Scholar] [CrossRef]
- Grattarola, C.; Giorda, F.; Iulini, B.; Pintore, M.D.; Pautasso, A.; Zoppi, S.; Goria, M.; Romano, A.; Peletto, S.; Varello, K.; et al. Meningoencephalitis and Listeria monocytogenes, Toxoplasma gondii and Brucella spp. coinfection in a dolphin in Italy. Dis. Aquat. Organ. 2016, 118, 169–174. [Google Scholar] [CrossRef]
- Bigal, E.; Morick, D.; Scheinin, A.P.; Salant, H.; Berkowitz, A.; King, R.; Levy, Y.; Melero, M.; Sánchez-Vizcaíno, J.M.; Goffman, O.; et al. Detection of Toxoplasma gondii in three common bottlenose dolphins (Tursiops truncatus); A first description from the Eastern Mediterranean Sea. Vet Parasitol. 2018, 258, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Jardine, J.E.; Dubey, J.P. Congenital toxoplasmosis in a Indo-Pacific bottlenose dolphin (Tursiops aduncus). J. Parasitol. 2002, 88, 197–199. [Google Scholar] [CrossRef]
- Resendes, A.R.; Almería, S.; Dubey, J.P.; Obon, E.; Juan-Salles, C.; Degollada, E.; Alegre, F.; Cabezon, O.; Pont, S.; Domingo, M. Disseminated toxoplasmosis in a Mediterranean pregnant Risso’s dolphin (Grampus griseus) with transplacental fetal infection. J. Parasitol. 2002, 88, 1029–1032. [Google Scholar] [CrossRef]
- Miller, M.A.; Sverlow, K.; Crosbie, P.R.; Barr, B.C.; Lowenstine, L.J.; Gulland, F.M.; Packham, A.; Conrad, P.A. Isolation and characterization of two parasitic protozoa from a Pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. J. Parasitol. 2001, 87, 816–822. [Google Scholar] [CrossRef]
- Gonzales-Viera, O.; Marigo, J.; Ruoppolo, V.; Rosas, F.C.W.; Kanamura, C.T.; Takakura, C.; Fernández, A.; Catão-Dias, J.L. Toxoplasmosis in a Guiana dolphin (Sotalia guianensis) from Parana, Brazil. Vet. Parasitol. 2013, 191, 358–362. [Google Scholar] [CrossRef]
- Galal, L.; Ajzenberg, D.; Hamidović, A.; Durieux, M.F.; Dardé, M.L.; Mercier, A. Toxoplasma and Africa: One Parasite, Two Opposite Population Structures. Trends Parasitol. 2018, 34, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Laran, S.; Dulau, V. Seasonal variation of striped dolphins, fin- and sperm whales’ abundance in the Ligurian Sea (Mediterranean Sea). J. Mar. Biol. Assoc. U. K. 2007, 87, 345–352. [Google Scholar] [CrossRef]
- Meissner, A.M.; Macleod, C.D.; Richard, P.; Ridoux, V.; Pierce, G. Feeding ecology of striped dolphins, Stenella coeruleoalba, in the north-western Mediterranean Sea based on stable isotope analyses. J. Mar. Biol. Assoc. U. K. 2012, 8, 1677–1687. [Google Scholar] [CrossRef]
- Marini, C.; Fossa, F.; Paoli, C.; Bellingeri, M.; Gnone, G.; Vassallo, P. Predicting bottlenose dolphin distribution along Liguria coast (northwestern Mediterranean Sea) through different modeling techniques and indirect predictors. J. Environ. Manag. 2015, 150, 9–20. [Google Scholar] [CrossRef]
- Di Guardo, G.; Falconi, A.; Di Francesco, A.; Mazzariol, S.; Centelleghe, C.; Casalone, C.; Pautasso, A.; Cocumelli, C.; Eleni, C.; Petrella, A.; et al. Western blot expression of 5-lipoxygenase in the brain from striped dolphins (Stenella coeruleoalba) and bottlenose dolphins (Tursiops truncatus) with or without encephalitis/meningo-encephalitis of infectious nature. J. Biol. Regul. Homeost. Agents. 2015, 29, 245–250. [Google Scholar]
- Shwab, E.K.; Zhu, X.Q.; Majumdar, D.; Pena, H.F.; Gennari, S.M.; Dubey, J.P.; Su, C. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 2014, 141, 453–461. [Google Scholar] [CrossRef]
- Dubey, J.P.; Fair, P.A.; Sundar, N.; Velmurugan, G.; Kwok, O.C.H.H.; McFee, W.E.; Majumdar, D.; Su, C. Isolation of Toxoplasma gondii From Bottlenose Dolphins (Tursiops truncatus). J. Parasitol. 2008, 94, 821–823. [Google Scholar] [CrossRef]
- Dubey, J.P.; Morales, J.A.; Sundar, N.; Velmurugan, G.V.; González-Barrientos, C.R.; Hernández-Mora, G.; Su, C. Isolation and genetic characterization of Toxoplasma gondii from striped dolphin (Stenella coeruleoalba) from Costa Rica. J. Parasitol. 2007, 93, 710–711. [Google Scholar] [CrossRef]
- Terracciano, G.; Fichi, G.; Comentale, A.; Ricci, E.; Mancusi, C.; Perrucci, S. Dolphins Stranded along the Tuscan Coastline (Central Italy) of the Pelagos Sanctuary: A Parasitological Investigation. Pathogens 2020, 9, 612. [Google Scholar] [CrossRef]
- Dubey, J.P.; Mergi, J.; Gehring, E.; Sundar, N.; Velmurugan, G.V.; Kwok, O.C.H.; Grigg, M.E.; Su, C.; Martineau, D. Toxoplasmosis in captive dolphins (Tursiops truncatus) and Walrus (Odobenus rosmarus). J. Parasitol. 2009, 95, 82–85. [Google Scholar] [CrossRef]
- Roe, W.D.; Howe, L.; Baker, E.J.; Burrows, L.; Hunter, S.A. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’ dolphins (Cephalorhynchus hectori). Vet. Parasitol. 2013, 192, 67–74. [Google Scholar] [CrossRef]
- Herder, V.; van de Velde, N.; Højer Kristensen, J.; van Elk, C.; Peters, M.; Kilwinski, J.; Schares, G.; Siebert, U.; Wohlsein, P. Fatal Disseminated Toxoplasma gondii Infection in a Captive Harbour Porpoise (Phocoena phocoena). J. Comp. Pathol. 2015, 153, 357–362. [Google Scholar] [CrossRef]
- Marcer, F.; Marchiori, E.; Centelleghe, C.; Ajzenberg, D.; Gustinelli, A.; Meroni, V.; Mazzariol, S. Parasitological and pathological findings in fin whales Balaenoptera physalus stranded along Italian coastlines. Dis. Aquat. Organ. 2019, 133, 25–37. [Google Scholar] [CrossRef]
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health 2019, 66, 826–834. [Google Scholar] [CrossRef]
- Lachkhem, A.; Galal, L.; Lahmar, I.; Passebosc, K.; Riahi, H.; Plault, N.; Dardé, M.L.; Mercier, A.; Babba, H. First isolation and genotyping of Toxoplasma gondii strains from domestic animals in Tunisia. Sci. Rep. 2021, 11, 9328. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.J.; Texas A & M University. Sea Grant College Program. In Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; Volume 486, ISBN 9780977460908. [Google Scholar]
- Carlini, R.; de Francesco, M.C.; Libera, S. Della Biometric measures indicating sexual dimorphism in Stenella coeruleoalba (Meyen, 1833) (Delphinidae) in the north-central Tyrrhenian sea. Aquat. Mamm. 2014, 40, 59–68. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling. In Proceedings of the 25th Meeting of the Advisory Committee, Stralsund, Germany, 17–19 September 2019; p. 73. [Google Scholar]
- Vitale, M.; Galluzzo, P.; Currò, V.; Gozdzik, K.; Schillaci, D.; Di Marco Lo Presti, V. A high sensitive nested PCR for Toxoplasma gondii detection in animal and food samples. J. Microb. Biochem. Technol. 2013, 5, 39–41. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Groch, K.R.; Hernani, H.G.C.; Castaldo Colosio, A.; Farias Alves, B.; Pena, H.; Catão-Dias, J. Fatal Systemic Toxoplasmosis by a Novel Non-archetypal Toxoplasma gondii in a Bryde’s Whale (Balaenoptera edeni). Front. Mar. Sci. 2020, 7, 336. [Google Scholar] [CrossRef]
- Slany, M.; Dziedzinska, R.; Babak, V.; Kralik, P.; Moravkova, M.; Slana, I. Toxoplasma gondii in vegetables from fields and farm storage facilities in the Czech Republic. FEMS Microbiol. Lett. 2019, 366, fnz170. [Google Scholar] [CrossRef]
- Slana, I.; Kralik, P.; Kralova, A.; Pavlik, I. On-farm spread of Mycobacterium avium subsp. paratuberculosis in raw milk studied by IS900 and F57 competitive real time quantitative PCR and culture examination. Int. J. Food Microbiol. 2008, 128, 250–257. [Google Scholar] [CrossRef]
- Su, C.; Shwab, E.K.; Zhou, P.; Zhu, X.Q.; Dubey, J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 2010, 137, 1–11. [Google Scholar] [CrossRef]
- Bottós, J.; Miller, R.H.; Belfort, R.N.; Macedo, A.C.; UNIFESP Toxoplasmosis Group; Belfort, R., Jr.; Grigg, M.E. Bilateral retinochoroiditis caused by an atypical strain of Toxoplasma gondii. Br. J. Ophthalmol. 2009, 93, 1546–1550. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Calero-Bernal, R.; Regidor-Cerrillo, J.; Vallejo, R.; Benavides, J.; Collantes-Fernández, E.; Ortega-Mora, L.M. In vivo and in vitro models show unexpected degrees of virulence among Toxoplasma gondii type II and III isolates from sheep. Vet. Res. 2021, 52, 82. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Vincze, T.; Posfai, J.; Roberts, R.J. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003, 31, 3688–3691. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence Limits On Phylogenies: An Approach Using The Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, F.; Giorda, F.; Grattarola, C.; Mignone, W.; Beltramo, C.; Keck, N.; Lorusso, A.; Di Francesco, G.; Di Renzo, L.; Di Guardo, G.; et al. Specific capture and whole-genome phylogeography of Dolphin morbillivirus. Sci Rep. 2020, 10, 20831. [Google Scholar] [CrossRef]
- Garofolo, G.; Petrella, A.; Lucifora, G.; Di Francesco, G.; Di Guardo, G.; Pautasso, A.; Iulini, B.; Varello, K.; Giorda, F.; Goria, M.; et al. Occurrence of Brucella ceti in striped dolphins from Italian Seas. PLoS ONE 2020, 15, e0240178. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).