Ovarian Filariasis in a Wild Southern Tamandua (Tamanduatetradactyla; Mammalia: Myrmecophagidae)
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection and Tissue Processing
2.2. DNA Extraction and PCR
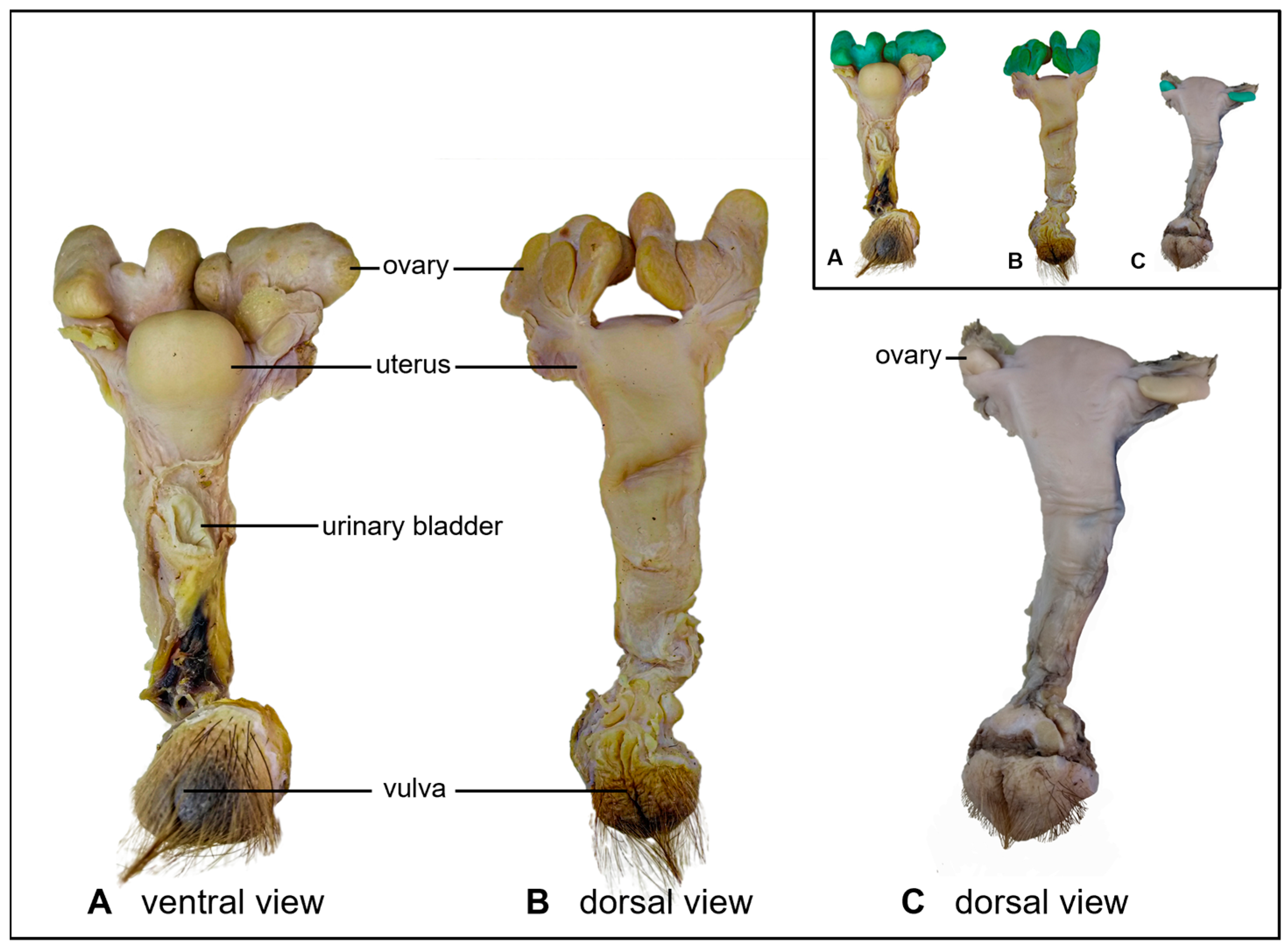
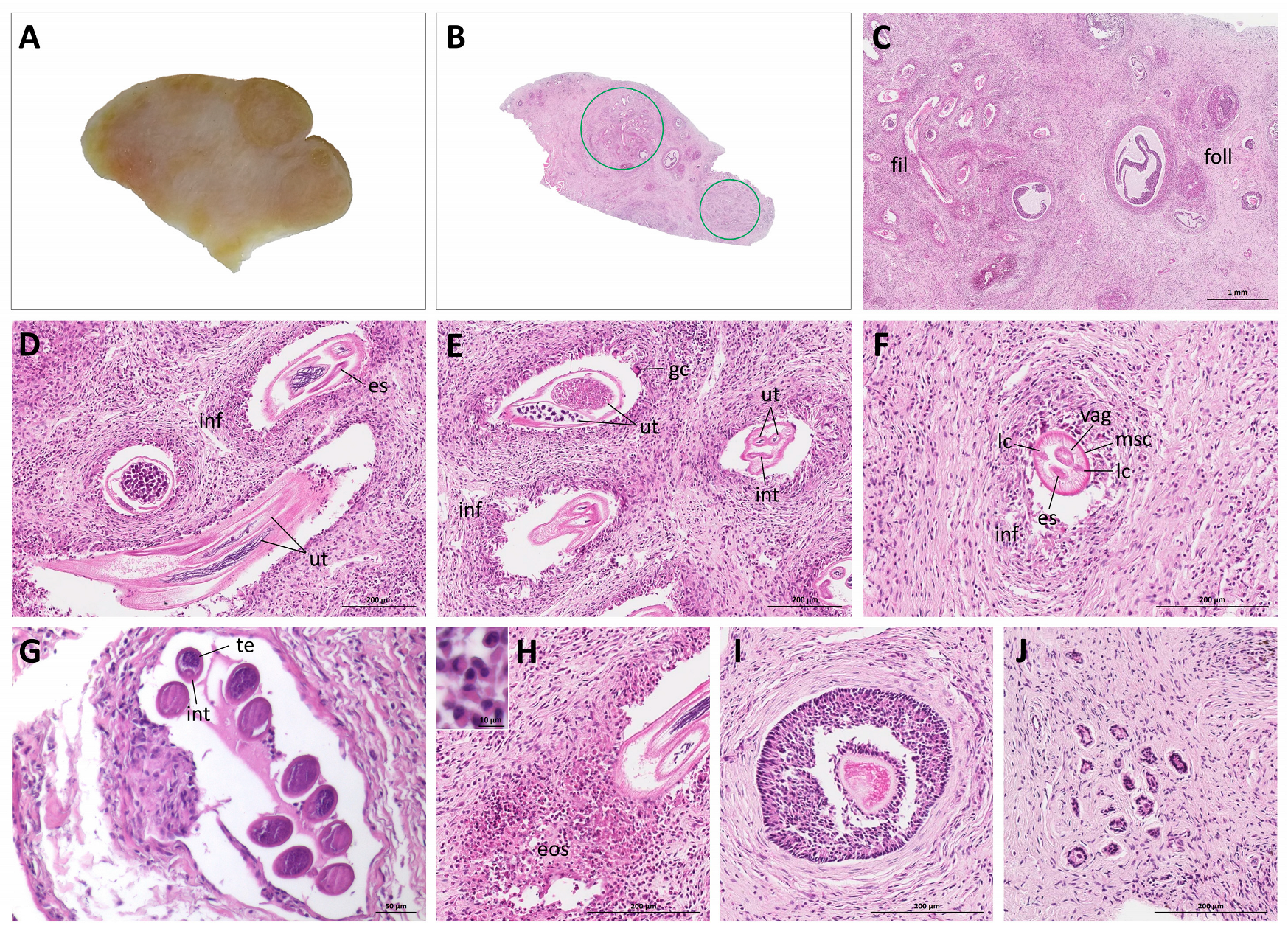
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, A.L. Magnorder Xenarthra. In Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews, and Bats; Gardner, A.L., Ed.; University of Chicago Press: Chicago, IL, USA, 2008; Volume 1, pp. 127–176. [Google Scholar]
- Delsuc, F.; Douzery, E.J.P. Armadillos, Anteaters, and Sloths (Xenarthra). In The Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 475–478. [Google Scholar]
- Gaudin, T.J.; Croft, D.A. Paleogene Xenarthra and the Evolution of South American Mammals. J. Mammal. 2015, 96, 622–634. [Google Scholar] [CrossRef]
- Novacek, M.J. Mammalian Phylogeny: Shaking the Tree. Nature 1992, 356, 121–125. [Google Scholar] [CrossRef]
- Aguilar, R.F.; Superina, M. Xenarthra. In Fowler’s Zoo and Wild Animal Medicine; Miller, R.E., Fowler, M.E., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2014; Volume 8, pp. 355–369. [Google Scholar]
- Kaudern, W. Studien über die Männlichen Geschlechtsorgane von Edentaten. Ark. Zool. 1914, 9, 1–53. [Google Scholar]
- Rossi, L.F.; Luaces, J.P.; Aldana Marcos, H.J.; Cetica, P.D.; Perez Jimeno, G.; Merani, M.S. Anatomy and Histology of the Male Reproductive Tract and Spermatogenesis Fine Structure in the Lesser Anteater (Tamandua tetradactyla, Myrmecophagidae, Xenarthra): Morphological Evidences of Reproductive Functions. Anat. Histol. Embryol. 2012, 42, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Cetica, P.D.; Aldana Marcos, H.J.; Merani, M.S. Morphology of Female Genital Tracts in Dasypodidae (Xenarthra, Mammalia): A Comparative Survey. Zoomorphology 2005, 124, 57–65. [Google Scholar] [CrossRef]
- Rossi, L.F.; Luaces, J.P.; Aldana Marcos, H.J.; Cetica, P.D.; Gachen, G.; Pérez Jimeno, G.; Merani, M.S. Female Reproductive Tract of the Lesser Anteater (Tamandua tetradactyla, Myrmecophagidae, Xenarthra). Anatomy and Histology. J. Morphol. 2011, 272, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Superina, M.; Loughry, W.J. Why Do Xenarthrans Matter? J. Mammal. 2015, 96, 617–621. [Google Scholar] [CrossRef]
- Hossotani, C.M.d.S.; Silva e Luna, H. Reproductive Patterns of the Lesser Anteater (Tamandua tetradactyla Linnaeus, 1758). Rev. Bras. Reprodução Anim. 2016, 4, 95–98. [Google Scholar]
- Rezende, L.C.; Galdos-Riveros, A.C.; Miglino, M.A.; Ferreira, J.R. Aspectos da Biologia Reprodutiva em Preguiça e Tamanduá: Uma Revisão. Rev. Bras. Reprodução Anim. 2013, 37, 354–359. [Google Scholar]
- Arenales, A.; Gardiner, C.H.; Miranda, F.R.; Dutra, K.S.; Oliveira, A.R.; Mol, J.P.; Texeira da Costa, M.E.; Tinoco, H.P.; Coelho, C.M.; Silva, R.O.; et al. Pathology of Free-Ranging and Captive Brazilian Anteaters. J. Comp. Pathol. 2020, 180, 55–68. [Google Scholar] [CrossRef]
- Macêdo, A.A.; Silva, A.P.C.; Pessanha, Â.T.; Soave, S.A.; Paixão, T.A.; Santos, R.L. Endometrite Purulenta em Tamanduá-mirim (Tamandua tetradactyla) e Tamanduá-bandeira (Myrmecophaga tridactyla). Arch. Vet. Sci. 2013, 18, 435–437. [Google Scholar]
- Wislocki, G.B. Nematode Parasites in the Ovaries of the Anteater (Tamandua tetradactyla). J. Mammal. 1928, 9, 318–319. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: London, UK, 2019; pp. 40–138. [Google Scholar]
- Hayssen, V. Tamandua tetradactyla (Pilosa: Myrmecophagidae). Mamm. Species 2011, 43, 64–74. [Google Scholar] [CrossRef]
- Chitwood, M.; Lichtenfels, J.R. Parasitological Review: Identification of Parasitic Metazoa in Tissue Sections. Exp. Parasitol. 1972, 32, 407–519. [Google Scholar] [CrossRef]
- Gardiner, C.H.; Poynton, S.L. An Atlas of Metazoan Parasites in Animal Tissues. In Armed Forces Institute of Pathology; American Registry of Pathology: Washington, DC, USA, 2006. [Google Scholar]
- Suster, D.; Liu, M.Z.; Lin, D.I. Benign Diseases of the Ovary. In Gynecologic and Obstetric Pathology; Springer: Singapore, 2019; pp. 79–120. [Google Scholar]
- Weidner, N.; Dabbs, D.J.; Peterson, M. Ovaries. In Modern Surgical Pathology; Weidner, N., Cote, R.J., Suster, S., Weiss, L.M., Eds.; Saunders, Elsevier: Philadelphia, PA, USA, 2009; Volume 1, pp. 1356–1409. [Google Scholar]
- Foster, R.A. Female Reproductive System and Mammary Gland. In Pathologic Basis of Veterinary Disease; Zachary, J.F., McGavin, M.D., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2012; pp. 1085–1126. [Google Scholar]
- Hong, S.-T.; Choi, M.-H.; Chai, J.-Y.; Kim, Y.T.; Kim, M.K.; Kim, K.R. A Case of Ovarian Enterobiasis. Korean J. Parasitol. 2002, 40, 149–151. [Google Scholar] [CrossRef]
- McCabe, K.; Nahn, P.A.K.; Sahin, A.A.; Mitchell, M.F. Enterobiasis of the Ovary in a Patient with Cervical Carcinoma in Situ. Infect. Dis. Obstet. Gynecol. 1995, 2, 231–234. [Google Scholar] [CrossRef]
- Russell, P.; Robboy, S.J. Chapter 21 Normal Ovaries, Inflammatory and Non-Neoplastic Conditions. In Robboy’s Pathology of the Female Reproductive Tract; Robboy, S.J., Mutter, G.L., Prat, J., Bentley, R.C., Russell, P., Anderson, M.C., Eds.; Elsevier Health Sciences: London, UK, 2009. [Google Scholar]
- Smolyakov, R.; Talalay, B.; Yanai-Inbar, I.; Pak, I.; Alkan, M. Enterobius vermicularis Infection of Female Genital Tract: A Report of Three Cases and Review of Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 220–222. [Google Scholar] [CrossRef]
- Goel, P.; Tandon, R.; Saha, P.K.; Prabhakar, S.; Goel, B.; Kaur, R.; Kaur, N.; Singhal, N. A Rare Case of Ovarian and Pelvic Filariasis. Trop. Dr. 2013, 43, 108–109. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, A.; Chakraborty, R.N.; Mandal, S. Ovarian Filariasis Presenting as Tubo-Ovarian Mass: Report of a Rare Case. J. Health Res. 2017, 4, 136. [Google Scholar]
- Sethi, S.; Misra, K.; Singh, U.R.; Kumar, D. Lymphatic Filariasis of the Ovary and Mesosalpinx. J. Obstet. Gynaecol. Res. 2001, 27, 285–292. [Google Scholar] [CrossRef]
- Vasantham, V.; Yadav, S.K.; Sarin, N.; Singh, S.; Pruthi, S.K. Incidental Detection of Microfilaria in Cyst Fluid of Mucinous Cystadenocarcinoma of Ovary: A Rare Case Report. Int. J. Surg. Case Rep. 2020, 70, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N.; Raoot, A. An Unusual Case of Adult Filarial Oophoritis. Int. J. Gynecol. Pathol. 2011, 30, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, G.; Di Caro, G.; Capucchio, M.; Gaglio, G.; Reina, V.; Lo Giudice, C.; Zanet, S.; Marino, F. Mesocestoidosis and Multivisceral Tetrathyridiosis in a European Cat. Vet. Med. 2017, 62, 356–362. [Google Scholar] [CrossRef]
- Webbe, G.; James, C.; Nelson, G.S. Schistosoma haematobium in the Baboon (Papio anubis). Ann. Trop. Med. Parasitol. 1974, 68, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Bain, O.; Dedet, J.P. Une nouvelle filaire, Chabfilaria jonathani n. gen., n. sp., Onchocercidae parasite de Xénarthre. Ann. Parasitol. Hum. Comparée 1983, 58, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.-S. On a Filarial Parasite, Deraiophoronema freitaslerai n.sp., from the Giant Anteater, Myrmecophaga tridactyla from British Guiana, and a Proposed Reclassification of Dipetalonema and Related Genera. Parasitology 1957, 47, 196–205. [Google Scholar]
- Deem, S.L.; Noss, A.J.; Fiorello, C.V.; Manharth, A.L.; Robbins, R.G.; Karesh, W.B. Health Assessment of Free-Ranging Three-Banded (Tolypeutes matacus) and Nine-Banded (Dasypus novemcinctus) Armadillos in the Gran Chaco, Bolivia. J. Zoo Wildl. Med. 2009, 40, 245–256. [Google Scholar] [CrossRef]
- Eberhard, M.L. Dipetalonema (Dasypafilaria) averyi subgen. et sp. n. (Nematoda: Filarioidea) from the Nine-Banded Armadillo, Dasypus novemcinctus in Louisiana. J. Parasitol. 1982, 68, 325–328. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Campo-Aasen, I. Acanthocheilonema sabanicolae n. sp. (Filarioidea: Onchocercidae) from the Savanna Armadillo (Dasypus sabanicola) in Venezuela, with Comments on the Genus Acanthocheilonema. J. Parasitol. 1986, 72, 245–248. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Orihel, T.C.; Campo-Aasen, I. Strianema venezuelensis gen. et sp. n. (Filarioidea: Onchocercidae) from Venezuelan armadillos (Dasypus spp.). Ann. Parasitol. Hum. Comp. 1993, 68, 234–238. [Google Scholar] [CrossRef]
- Ezquiaga, M.C.; Abba, A.M.; Navone, G.T. Helminth Fauna of the Screaming Hairy Armadillo Chaetophractus vellerosus from Argentina: The Consequence of Host Isolation on Parasite Diversity. Heliyon 2019, 5, e01605. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, J.; Navone, G.T. Systematic and distribution of Orihelia anticlava (Molin, 1858) (Nematoda, Onchocercidae) from dasypodids of South America. Acta Parasitol. 2003, 48, 103–110. [Google Scholar]
- Eberhard, M.L. Dirofilaria macrodemos and D. panamensis spp. n. (Nematoda: Filarioidea) from Central and South American Sloths. J. Parasitol. 1978, 64, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 4th ed.; John Wiley & Sons Ltd.: Oxford, UK, 2016. [Google Scholar]
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species, Version 2021-3; International Union for Conservation of Nature: Gland, Switzerland, 2021; Available online: https://www.iucnredlist.org (accessed on 5 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fromme, L.; Yogui, D.R.; Alves, M.H.; Díaz-Delgado, J.; Desbiez, A.L.J.; Santos, A.L.Q.; Guerra, J.M.; Langeheine, M.; Siebert, U.; Brehm, R.; et al. Ovarian Filariasis in a Wild Southern Tamandua (Tamanduatetradactyla; Mammalia: Myrmecophagidae). Pathogens 2022, 11, 918. https://doi.org/10.3390/pathogens11080918
Fromme L, Yogui DR, Alves MH, Díaz-Delgado J, Desbiez ALJ, Santos ALQ, Guerra JM, Langeheine M, Siebert U, Brehm R, et al. Ovarian Filariasis in a Wild Southern Tamandua (Tamanduatetradactyla; Mammalia: Myrmecophagidae). Pathogens. 2022; 11(8):918. https://doi.org/10.3390/pathogens11080918
Chicago/Turabian StyleFromme, Lilja, Débora Regina Yogui, Mario Henrique Alves, Josué Díaz-Delgado, Arnaud Leonard Jean Desbiez, André Luis Quagliatto Santos, Juliana Mariotti Guerra, Marion Langeheine, Ursula Siebert, Ralph Brehm, and et al. 2022. "Ovarian Filariasis in a Wild Southern Tamandua (Tamanduatetradactyla; Mammalia: Myrmecophagidae)" Pathogens 11, no. 8: 918. https://doi.org/10.3390/pathogens11080918






