Identification and Evolutionary Relationship of Corynebacterium striatum Clinical Isolates
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of the Isolated Specimens
2.2. Antibiotic Resistance Profiles of Isolates
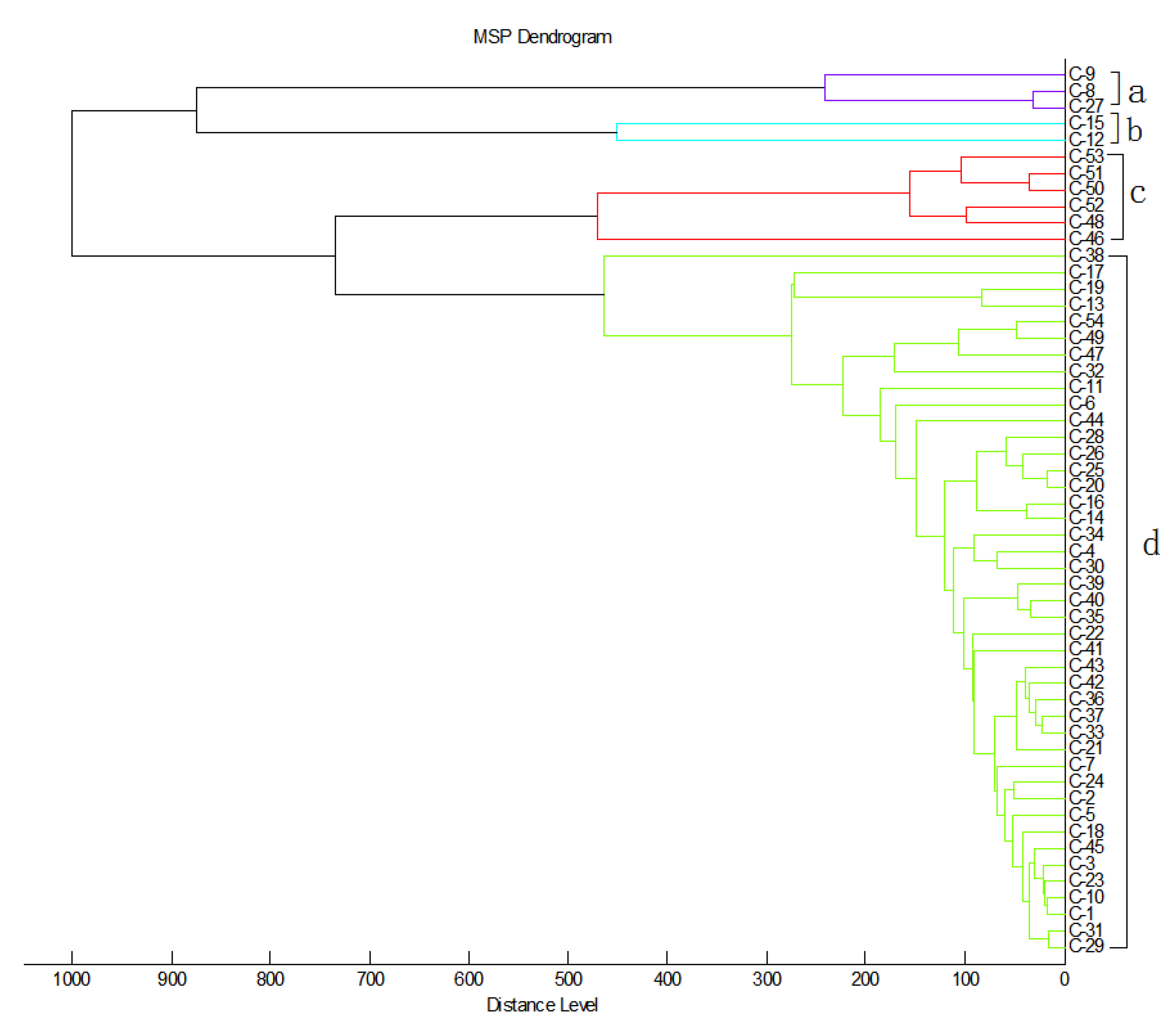
2.3. Cluster Analysis of MDR C. striatum
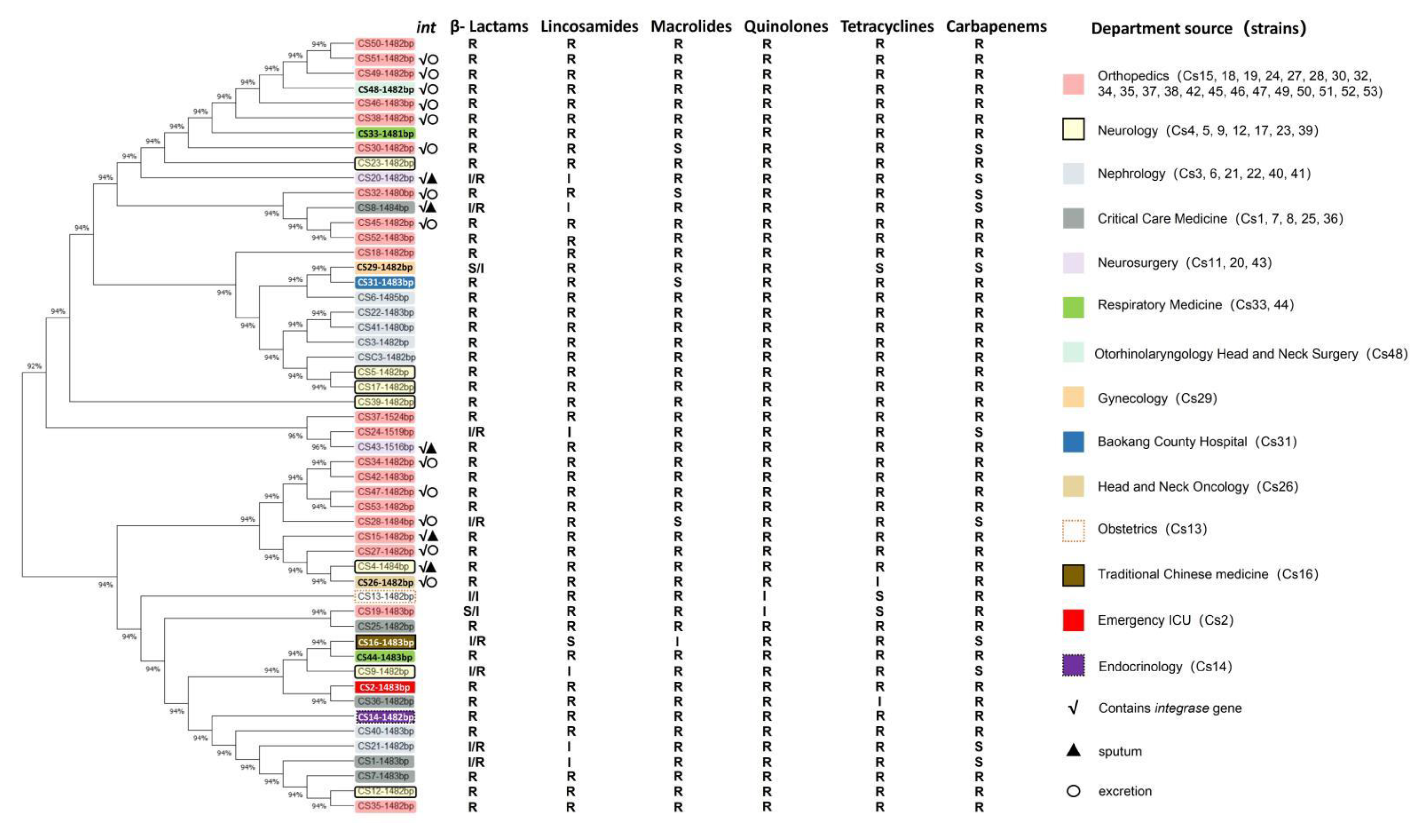
2.4. Phylogenetic Evolution Analysis of MDR-C. striatum Microbiota
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Isolation and Identification of C. striatum Strains with MALDI-TOF MS
4.3. Cloning and Identification of 16s rRNA/Integrase Gene of C. striatum
4.4. Bioinformatics Analysis and Phylogenetic Tree Analysis of MDR C. striatum
4.5. Antibiotic Susceptibility Testing
4.6. Ethical Approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alibi, S.; Ramos-Vivas, J.; Ben Selma, W.; Ben Mansour, H.; Boukadida, J.; Navas, J. Virulence of clinically relevant multidrug resistant Corynebacterium striatum strains and their ability to adhere to human epithelial cells and inert surfaces. Microb. Pathog. 2021, 155, 104887. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Chen, D.; Du, P.; Lan, R.; Qiu, X.; Hou, X.; Liu, Z.; Sun, L.; Xu, S.; et al. Whole-Genome Sequencing Reveals a Prolonged and Persistent Intrahospital Transmission of Corynebacterium striatum, an Emerging Multidrug-Resistant Pathogen. J. Clin. Microbiol. 2019, 57, e00683-19. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santana, G.; Silva, C.M.F.; Olivella, J.G.B.; Silva, I.F.; Fernandes, L.M.O.; Sued-Karam, B.R.; Santos, C.S.; Souza, C.; Mattos-Guaraldi, A.L. Worldwide survey of Corynebacterium striatum increasingly associated with human invasive infections, nosocomial outbreak, and antimicrobial multidrug-resistance, 1976–2020. Arch. Microbiol. 2021, 203, 1863–1880. [Google Scholar] [CrossRef]
- Simpson-Lourêdo, L.; Silva, C.M.F.; Hacker, E.; Souza, N.F.; Santana, M.M.; Antunes, C.A.; Nagao, P.E.; Hirata, R., Jr.; Burkovski, A.; Villas Bôas, M.H.S.; et al. Detection and virulence potential of a phospholipase D-negative Corynebacterium ulcerans from a concurrent diphtheria and infectious mononucleosis case. Antonie Van Leeuwenhoek 2019, 112, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Leyton, B.; Ramos, J.N.; Baio, P.V.P.; Veras, J.F.C.; Souza, C.; Burkovski, A.; Mattos-Guaraldi, A.L.; Vieira, V.V.; Abanto Marin, M. Treat Me Well or Will Resist. Uptake of Mobile Genetic Elements Determine the Resistome of Corynebacterium striatum. Int. J. Mol. Sci. 2021, 22, 7499. [Google Scholar] [CrossRef]
- Orosz, L.; Sóki, J.; Kókai, D.; Burián, K. Corynebacterium striatum-Got Worse by a Pandemic? Pathogens 2022, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Campanile, F.; Carretto, E.; Barbarini, D.; Grigis, A.; Falcone, M.; Goglio, A.; Venditti, M.; Stefani, S. Clonal multidrug-resistant Corynebacterium striatum strains, Italy. Emerg. Infect. Dis. 2009, 15, 75–78. [Google Scholar] [CrossRef]
- Nøhr-Meldgaard, K.; Struve, C.; Ingmer, H.; Agersø, Y. The Tetracycline Resistance Gene, tet(W) in Bifidobacterium animalis subsp. lactis Follows Phylogeny and Differs from tet(W) in Other Species. Front. Microbiol. 2021, 12, 658943. [Google Scholar] [CrossRef]
- Alibi, S.; Ferjani, A.; Boukadida, J.; Cano, M.E.; Fernández-Martínez, M.; Martínez-Martínez, L.; Navas, J. Occurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital. Sci. Rep. 2017, 7, 9704. [Google Scholar] [CrossRef]
- Galimand, M.; Fishovitz, J.; Lambert, T.; Barbe, V.; Zajicek, J.; Mobashery, S.; Courvalin, P. AAC(3)-XI, a new aminoglycoside 3-N-acetyltransferase from Corynebacterium striatum. Antimicrob. Agents Chemother. 2015, 59, 5647–5653. [Google Scholar] [CrossRef] [Green Version]
- Alina, O. Mechanisms of Antibiotic Resistance in Corynebacterium spp. Causing Infections in People. In Antibiotic Resistant Bacteria; Rijeka, M.P., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Reddy, B.S.; Chaudhury, A.; Kalawat, U.; Jayaprada, R.; Reddy, G.; Ramana, B.V. Isolation, speciation, and antibiogram of clinically relevant non-diphtherial Corynebacteria (Diphtheroids). Indian J. Med. Microbiol. 2012, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yam, E.L.Y. COVID-19 will further exacerbate global antimicrobial resistance. J. Travel Med. 2020, 27, taaa098. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. WHO 2020, 98, 442–442A. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance. mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Tauch, A.; Krieft, S.; Kalinowski, J.; Pühler, A. The 51,409-bp R-plasmid pTP10 from the multiresistant clinical isolate Corynebacterium striatum M82B is composed of DNA segments initially identified in soil bacteria and in plant, animal, and human pathogens. Mol. Gen. Genet. 2000, 263, 1–11. [Google Scholar] [CrossRef]
- Zhao, F.; Ding, T.; Li, M.; Wang, Y.; Zhang, X.; Ren, H.; Tong, Y. Complete genome analysis of a novel temperate bacteriophage induced from Corynebacterium striatum. Arch. Virol. 2019, 164, 2877–2880. [Google Scholar] [CrossRef]
- Nesvera, J.; Hochmannová, J.; Pátek, M. An integron of class 1 is present on the plasmid pCG4 from gram-positive bacterium Corynebacterium glutamicum. FEMS Microbiol. Lett. 1998, 169, 391–395. [Google Scholar] [CrossRef]
- Hennart, M.; Panunzi, L.G.; Rodrigues, C.; Gaday, Q.; Baines, S.L.; Barros-Pinkelnig, M.; Carmi-Leroy, A.; Dazas, M.; Wehenkel, A.M.; Didelot, X.; et al. Population genomics and antimicrobial resistance in Corynebacterium diphtheriae. Genome Med. 2020, 12, 107. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, Y.; Du, K.; Xu, S.; Wang, Y.; Krupovic, M.; Chen, X. A novel family of tyrosine integrases encoded by the temperate pleolipovirus SNJ2. Nucleic Acids Res. 2018, 46, 2521–2536. [Google Scholar] [CrossRef]
- Farrugia, D.N.; Elbourne, L.D.; Mabbutt, B.C.; Paulsen, I.T. A novel family of integrases associated with prophages and genomic islands integrated within the tRNA-dihydrouridine synthase A (dusA) gene. Nucleic Acids Res. 2015, 43, 4547–4557. [Google Scholar] [CrossRef] [Green Version]
- Kiga, K.; Tan, X.E.; Ibarra-Chávez, R.; Watanabe, S.; Aiba, Y.; Sato’o, Y.; Li, F.Y.; Sasahara, T.; Cui, B.; Kawauchi, M.; et al. Development of CRISPR-Cas13a-based antimicrobials capable of sequence-specific killing of target bacteria. Nat. Commun. 2020, 11, 2934. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; San Millán, Á.; Toll-Riera, M.; Connolly, J.; Flor-Duro, A.; Chen, J.; Ubeda, C.; MacLean, R.C.; Penadés, J.R. Staphylococcal phages and pathogenicity islands drive plasmid evolution. Nat. Commun. 2021, 12, 5845. [Google Scholar] [CrossRef] [PubMed]
- Asgin, N.; Otlu, B. Antimicrobial Resistance and Molecular Epidemiology of Corynebacterium striatum Isolated in a Tertiary Hospital in Turkey. Pathogens 2020, 9, 136. [Google Scholar] [CrossRef]
- Renom, F.; Gomila, M.; Garau, M.; Gallegos, M.D.; Guerrero, D.; Lalucat, J.; Soriano, J.B. Respiratory infection by Corynebacterium striatum: Epidemiological and clinical determinants. New Microbes New Infect. 2014, 2, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.W.; Ju, Y.; Lee, C.K.; Sohn, J.W.; Kim, M.J.; Yoon, Y.K. Molecular epidemiology and clinical significance of Corynebacterium striatum isolated from clinical specimens. Infect. Drug Resist. 2019, 12, 161–171. [Google Scholar] [CrossRef]
- Tauch, A.; Zheng, Z.; Pühler, A.; Kalinowski, J. Corynebacterium striatum chloramphenicol resistance transposon Tn5564: Genetic organization and transposition in Corynebacterium glutamicum. Plasmid 1998, 40, 126–139. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Pierneef, R.; Bezuidt, O.K.I.; Van De Peer, Y.; Cowan, D.A.; Makhalanyane, T.P. A reservoir of ‘historical’ antibiotic resistance genes in remote pristine Antarctic soils. Microbiome 2018, 6, 40. [Google Scholar] [CrossRef]
- Hwengwere, K.; Paramel Nair, H.; Hughes, K.A.; Peck, L.S.; Clark, M.S.; Walker, C.A. Antimicrobial resistance in Antarctica: Is it still a pristine environment? Microbiome 2022, 10, 71. [Google Scholar] [CrossRef]
- Jara, D.; Bello-Toledo, H.; Domínguez, M.; Cigarroa, C.; Fernández, P.; Vergara, L.; Quezada-Aguiluz, M.; Opazo-Capurro, A.; Lima, C.A.; González-Rocha, G. Antibiotic resistance in bacterial isolates from freshwater samples in Fildes Peninsula, King George Island, Antarctica. Sci. Rep. 2020, 10, 3145. [Google Scholar] [CrossRef] [Green Version]
- Antelo, V.; Giménez, M.; Azziz, G.; Valdespino-Castillo, P.; Falcón, L.I.; Ruberto, L.A.M.; Mac Cormack, W.P.; Mazel, D.; Batista, S. Metagenomic strategies identify diverse integron-integrase and antibiotic resistance genes in the Antarctic environment. MicrobiologyOpen 2021, 10, e1219. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.; Deschênes, F.; Burrus, V. Genomic islands targeting dusA in Vibrio species are distantly related to Salmonella Genomic Island 1 and mobilizable by IncC conjugative plasmids. PLoS Genet. 2021, 17, e1009669. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.J.; Webb, H.K.; Crawford, R.J.; Malherbe, F.; Butt, H.; Knight, R.; Mikhailov, V.V.; Ivanova, E.P. Updating the taxonomic toolbox: Classification of Alteromonas spp. using multilocus phylogenetic analysis and MALDI-TOF mass spectrometry. Antonie Van Leeuwenhoek 2013, 103, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI guideline M45; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016; pp. 18–19. [Google Scholar]


| Departments | Specimen Type (Number, n) | Number (Ratio %) |
|---|---|---|
| Orthopedics | sputum (1); secretion (21) | 22 (40.7) |
| Neurology | sputum (6); secretion (1); blood (1) | 8 (14.8) |
| Nephrology | urine (2); secretion (1); blood (3) | 6 (11.1) |
| Critical Care Medicine | sputum (4); secretion (1); | 5 (9.3) |
| Neurosurgery | sputum (3) | 3 (5.6) |
| Respiratory medicine | sputum (1); secretion (1) | 2 (3.7) |
| Others * | urine (1); secretion (7) | 8 (14.8) |
| Total | 54 (100) |
| Antibiotic Name | Drug Resistance (%) | Class of Antibiotics |
|---|---|---|
| Penicillin/Ceftriaxone | 77.8/94.4 | β-lactamases |
| Clindamycin | 85.2 | Lincosamides |
| Erythromycin | 90.7 | Macrolides |
| Ciprofloxacin | 96.3 | Quinolones |
| Tetracycline | 90.7 | Tetracyclines |
| Meropenem | 75.9 | Carbapenems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Pei, J.; Liu, M.; Huang, R.; Li, J.; Liao, S.; Liang, J. Identification and Evolutionary Relationship of Corynebacterium striatum Clinical Isolates. Pathogens 2022, 11, 1012. https://doi.org/10.3390/pathogens11091012
Wang J, Pei J, Liu M, Huang R, Li J, Liao S, Liang J. Identification and Evolutionary Relationship of Corynebacterium striatum Clinical Isolates. Pathogens. 2022; 11(9):1012. https://doi.org/10.3390/pathogens11091012
Chicago/Turabian StyleWang, Jiao, Jiao Pei, Mingming Liu, Rui Huang, Jiqiang Li, Shiying Liao, and Jian Liang. 2022. "Identification and Evolutionary Relationship of Corynebacterium striatum Clinical Isolates" Pathogens 11, no. 9: 1012. https://doi.org/10.3390/pathogens11091012
APA StyleWang, J., Pei, J., Liu, M., Huang, R., Li, J., Liao, S., & Liang, J. (2022). Identification and Evolutionary Relationship of Corynebacterium striatum Clinical Isolates. Pathogens, 11(9), 1012. https://doi.org/10.3390/pathogens11091012






