Epidemiological and Phylogeographic Study of Equid Herpesviruses in Tunisia
Abstract
1. Introduction
2. Results
2.1. Virus Detection by PCR
2.2. Association of EHVs Infection and Presence of Clinical Signs
2.3. Molecular Characterization and Phylogenetic Analysis
2.3.1. EHV1 and EHV4
2.3.2. EHV2
2.3.3. EHV5
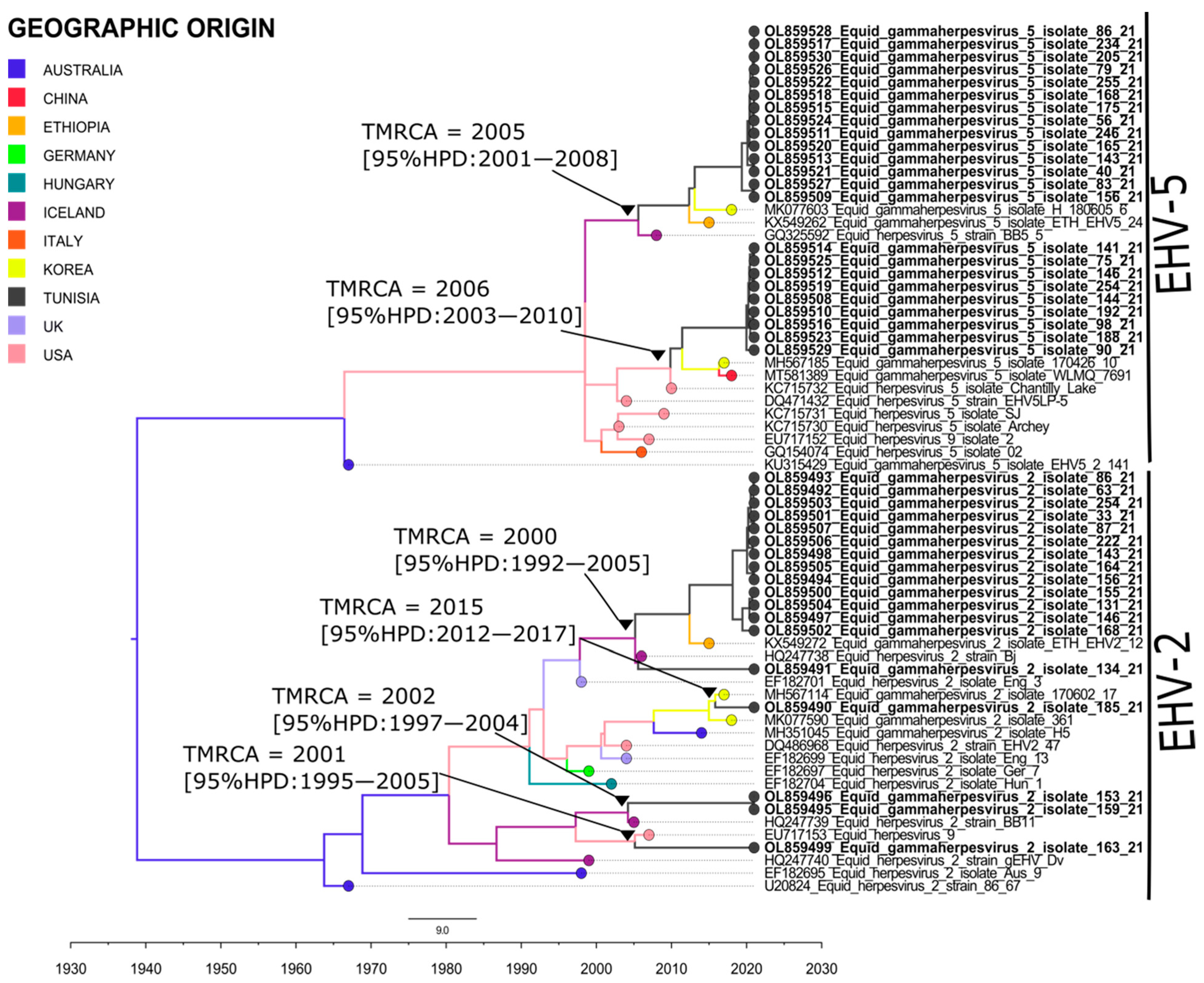
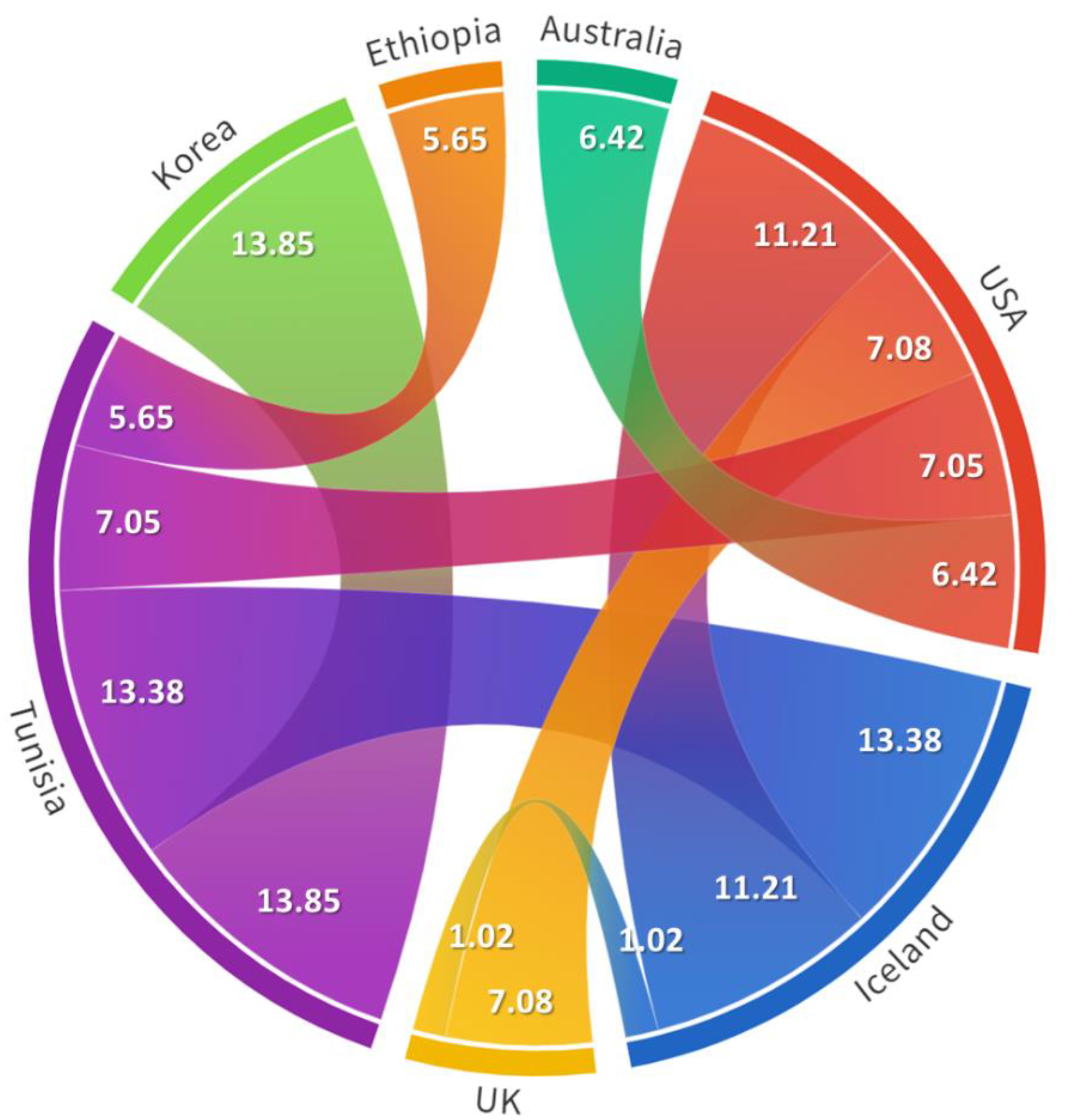
2.4. Spatiotemporal Dynamics of Herpesvirus Isolates
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. DNA Extraction and qPCR Amplification
4.3. Conventional PCR and Sequencing
4.4. Statistical Analyses
4.5. Phylogeographic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef]
- O’Callaghan, D.J.; Osterrieder, N. Herpesviruses of Horses. In Encyclopedia of Virology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 411–420. ISBN 9780123744104. [Google Scholar] [CrossRef]
- Patel, J.R.; Heldens, J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)—Epidemiology, disease and immunoprophylaxis: A brief review. Vet. J. 2005, 170, 14–23. [Google Scholar] [CrossRef]
- Pavulraj, S.; Eschke, K.; Theisen, J.; Westhoff, S.; Reimers, G.; Andreotti, S.; Osterrieder, N.; Azab, W. Equine herpesvirus type 4 (EHV-4) outbreak in Germany: Virological, serological, and molecular investigations. Pathogens 2021, 10, 810. [Google Scholar] [CrossRef]
- Mureşan, A.; Mureşan, C.; Siteavu, M.; Avram, E.; Bochynska, D.; Taulescu, M. An outbreak of equine herpesvirus-4 in an ecological donkey milk farm in Romania. Vaccines 2022, 10, 468. [Google Scholar] [CrossRef]
- Xie, J.; Tong, P.; Zhang, L.; Ren, M.; Song, X.; Jia, C.; Palidan, N.; Zhang, L.; Kuang, L. First detection and genetic characterization of equid herpesvirus 2, 4, and 5 in China. Arch. Virol. 2021, 166, 1421–1426. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar, C.; Herskin, M.; et al. Scientific opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Infection with Equine Herpesvirus-1. EFSA J. 2022, 20, 7036–7106. [Google Scholar] [CrossRef]
- Easton-Jones, C. Recent advancements in our understanding of equid gammaherpesvirus infections. Equine Vet. J. 2021, 54, 11–23. [Google Scholar] [CrossRef]
- Wong, D.M.; Belgrave, R.L.; Williams, K.J.; Del Piero, F.; Alcott, C.J.; Bolin, S.R.; Marr, C.M.; Nolen-Walston, R.; Myers, R.K.; Wilkins, P.A. Multinodular pulmonary fibrosis in five horses. J. Am. Vet. Med. Assoc. 2008, 232, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Vander Werf, K.A.; Davis, E.G.; Janardhan, K.; Bawa, B.; Bolin, S.; Almes, K. Identification of equine herpesvirus 5 in horses with lymphoma. J. Equine Vet. Sci. 2014, 34, 738–741. [Google Scholar] [CrossRef][Green Version]
- Herder, V.; Barsnick, R.; Walliser, U.; Teifke, J.P.; König, P.; Czerwinski, G.; Hansmann, F.; Baumgärtner, W.; Hewicker-Trautwein, M. Equid herpesvirus 5-associated dermatitis in a horse—Resembling herpes-associated erythema multiform. Vet. Microbiol. 2012, 155, 420–424. [Google Scholar] [CrossRef]
- Kutasi, O.; Moravszki, L.; Sardi, S.; Bohak, Z.; Biksi, I.; Basaka, F. Systemic granulomatous disease in a Hungarian Warmblood gelding. J. Equine Vet. Sci. 2014, 34, 810–815. [Google Scholar] [CrossRef]
- Rushton, J.O.; Kolodziejek, J.; Tichy, A.; Nell, B.; Nowotny, N. Detection of equid herpesviruses 2 and 5 in a herd of 266 Lipizzaners in association with ocular findings. Vet. Microbiol. 2013, 164, 139–144. [Google Scholar] [CrossRef]
- Ruszczyk, A.; Cywinska, A.; Banbura, M.W. Equine herpes virus 2 infection in horse population in Poland. Acta. Virol. 2004, 48, 189–192. [Google Scholar]
- Zarski, L.M.; High, E.A.; Nelli, R.K.; Bolin, S.R.; Williams, K.J.; Hussey, G. Development and application of a quantitative PCR assay to study equine herpesvirus 5 invasion and replication in equine tissues in vitro and in vivo. J. Virol. Methods 2017, 248, 44–53. [Google Scholar] [CrossRef]
- Nordengrahn, A.; Rusvai, M.; Merza, M.; Ekstrom, J.; Morein, B.; Belak, S. Equine herpesvirus type 2 (EHV-2) as a predisposing factor for Rhodococcus equi pneumonia in foals: Prevention of the bifactorial disease with EHV-2 immunostimulating complexes. Vet. Microbiol. 1996, 51, 55–68. [Google Scholar] [CrossRef]
- Blakeslee, J.R.; Olsen, R.G.; McAllister, E.S.; Fassbender, J.; Dennis, R. Evidence of respiratory tract infection induced by equine herpesvirus type 2, in the horse. Can. J. Microbiol. 1975, 21, 1940–1946. [Google Scholar] [CrossRef]
- Kershaw, O.; von Oppen, T.; Glitz, F.; Deegen, E.; Ludwig, H.; Borchers, K. Detection of equine herpesvirus type 2 (EHV-2) in horses with keratoconjunctivitis. Virus Res. 2001, 80, 93–99. [Google Scholar] [CrossRef]
- Krisova, S.; Tothova, K.; Molinkova, D.; Makra, Z.; Zisopoulou, A.M. Prevalence of equine herpesvirus 2 (EHV-2) in equine ocular disease. Acta. Vet. 2020, 89, 115–123. [Google Scholar] [CrossRef]
- Mumford, J.A.; Rossdale, P.D. Virus and its relationship to the ‘poor performance’ syndrome. Equine Vet. J. 1980, 12, 3–9. [Google Scholar] [CrossRef]
- Nolte, L.C.; Rosiak, M.; Baechlein, C.; Baumgärtner, W.; Allnoch, L. Equine Idiopathic Systemic Granulomatous disease with manifestation in the cerebellum associated with Equid Gammaherpesvirus 2. J. Equine Vet. Sci. 2020, 94, 103225. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, I. The molecular detection of equine herpesviruses 2 and 5 in genital swabs from clinically normal thoroughbred mares in South Korea. J. Equine Vet. Sci. 2019, 79, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Marenzoni, M.L.; Bietta, A.; Lepri, E.; Casagrande Proietti, P.; Cordioli, P.; Canelli, E.; Stefanetti, V.; Coletti, M.; Timoney, P.J.; Passamonti, F. Role of equine herpesviruses as co-infecting agents in cases of abortion, placental disease and neonatal foal mortality. Vet. Res. Commun. 2013, 37, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Horohov, D.W.; Chambers, T.M. EHV-1: A constant threat to the horse industry. Front. Microbiol. 2019, 10, 2668. [Google Scholar] [CrossRef]
- FNARC. Rapport d’Activité Annuel; Fondation Nationale d’Amélioration de la Race Chevaline: Sidi Thabet, Tunisie, 2015. [Google Scholar]
- Toumi, M. Serological Survey on Equine Rhinopneumonia in National Stud Farms in Tunisia; Veterinary Thesis; ENMV: Sidi-Thabet, Tunisia, 1985. [Google Scholar]
- Ghram, A.; Chabchoub, A.; Boussetta, M.; Baazaoui, S.; Ibn Amor, H.; Landolsi, F. Enquête séroépidémiologique de la rhinopneumonie des équidés en Tunisie. J. Animal Husb. Vet. Med. Trop. Count. 1997, 50, 273–276. [Google Scholar] [CrossRef]
- Chabchoub, A.; Pronost, S.; Abidi, N.; Legrand, L.; Fortier, G. Frequency of the equine herpes virus type 2 in equines with chronic respiratory diseases in Tunisia. Rev. Med. Vet. 2010, 161, 564–569. [Google Scholar]
- El-Hage, C.; Mekuria, Z.; Dynon, K.; Hartley, C.; McBride, K.; Gilkerson, J. Association of equine herpesvirus 5 with mild respiratory disease in a survey of EHV-1, -2, -4 and -5 in 407 Australian horses. Animals 2021, 11, 3418. [Google Scholar] [CrossRef]
- Wang, L.; Raidal, S.L.; Pizzirani, A.; Wilcox, G.E. Detection of respiratory herpesviruses in foals and adult horses determined by nested multiplex PCR. Vet. Microbiol. 2007, 121, 18–28. [Google Scholar] [CrossRef]
- Laabassi, F.; Hue, E.; Fortier, C.; Morilland, E.; Legrand, L.; Hans, A.; Pronost, S. Epidemiology and molecular detection of equine herpesviruses in western Algeria in 2011. Vet. Microbiol. 2017, 207, 205–209. [Google Scholar] [CrossRef]
- Negussie, H.; Gizaw, D.; Tesfaw, L.; Li, Y.; Oguma, K.; Sentsui, H.; Tessema, T.S.; Nauwynck, H.J. Detection of equine herpesvirus (EHV) −1, −2, −4 and −5 in Ethiopian equids with and without respiratory problems and genetic characterization of EHV-2 and EHV-5 strains. Transbound. Emerg. Dis. 2017, 64, 1970–1978. [Google Scholar] [CrossRef]
- Craig, M.I.; Barrandeguy, M.E.; Fernandez, F.M. Equine herpesvirus 2 (EHV-2) infection in thoroughbred horses in Argentina. BMC Vet. Res. 2005, 1, 9. [Google Scholar] [CrossRef]
- Thorsteinsdottir, L.; Torfason, E.G.; Torsteinsdottir, S.; Svansson, V. Genetic diversity of equine gammaherpesviruses (γ-EHV) and isolation of a syncytium forming EHV-2 strain from a horse in Iceland. Res. Vet. Sci. 2013, 94, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, V.S.; Bilge-Dagalp, S.; Oguzoglu, T.C.; Karapinar, Z.; Güzel, M.; Tan, M.T. Detection and sequence analysis of equine Gammaherpesviruses from horses with respiratory tract disease in Turkey. Transbound. Emerg. Dis. 2010, 57, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-G.; Ouh, I.-O.; Lee, S.K.; Lee, J.-S.; Kwon, O.-D.; Kwak, D. molecular detection and genetic characteristics of equine herpesvirus in Korea. Pathogens 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, K.; Dunowska, M.; Rola, J. Prevalence and sequence analysis of equid herpesviruses from the respiratory tract of Polish horses. Virol. J. 2018, 15, 106. [Google Scholar] [CrossRef]
- Nordengrahn, A.; Merza, M.; Ros, C.; Lindholm, A.; Hannant, D.; Belak, S. Prevalence of equine herpesvirus types 2 and 5 in horse population by using type-specific PCR assays. Vet. Res. 2002, 33, 251–259. [Google Scholar] [CrossRef]
- Dunowska, M.; Wilks, C.R.; Studdert, M.J.; Meers, J. Viruses associated with outbreaks of equine respiratory disease in New Zealand. N. Z. Vet. J. 2002, 50, 132–139. [Google Scholar] [CrossRef]
- Torfason, E.G.; Thorsteinsdóttir, L.; Torsteinsdóttir, S.; Svansson, V. Study of equid herpesviruses 2 and 5 in Iceland with a type-specific polymerase chain reaction. Res. Vet. Sci. 2008, 85, 605–611. [Google Scholar] [CrossRef]
- Pusterla, N.; Mapes, S.; Madigan, J.E.; MacLachlan, N.J.; Ferraro, G.L.; Watson, J.L.; Spier, S.J.; Wilson, W.D. Prevalence of EHV-1 in adult horses transported over long distances. Vet. Rec. 2009, 165, 473–475. [Google Scholar] [CrossRef]
- Bell, S.A.; Balasuriya, U.B.; Gardner, I.A.; Barry, P.A.; Wilson, W.D.; Ferraro, G.L.; MacLachlan, N.J. Temporal detection of equine herpesvirus infections of a cohort of mares and their foals. Vet. Microbiol. 2006, 116, 249–257. [Google Scholar] [CrossRef]
- Back, H.; Kendall, A.; Grandón, R.; Ullman, K.; Treiberg-Berndtsson, L.; Ståhl, K.; Pringle, J. Equine Multinodular Pulmonary Fibrosis in association with asinine herpesvirus type 5 and equine herpesvirus type 5: A case report. Acta. Vet. Scand. 2012, 54, 57. [Google Scholar] [CrossRef]
- Temesgen, T.; Getachew, Y.; Negussie, H. Molecular identification of equine herpesvirus 1, 2, and 5 in equids with signs of respiratory disease in central Ethiopia. Vet. Med. 2021, 12, 337–345. [Google Scholar] [CrossRef]
- Back, H.; Ullman, K.; Treiberg Berndtsson, L.; Riihimäki, M.; Penell, J.; Ståhl, K.; Valarcher, J.F.; Pringle, J. Viral load of equine herpesviruses 2 and 5 in nasal swabs of actively racing Standardbred trotters: Temporal relationship of shedding to clinical findings and poor performance. Vet. Microbiol. 2015, 179, 142–148. [Google Scholar] [CrossRef]
- Carlson, J.K.; Traub-Dargatz, J.L.; Lunn, D.P.; Morley, P.S.; Kohler, A.; Kasper, K.; Landolt, G.A.; Barnett, D.C.; Lunn, K.F. Equine viral respiratory pathogen surveillance at horse shows and sales. J. Equine Vet. Sci. 2013, 33, 229–237. [Google Scholar] [CrossRef]
- Hue, E.S.; Fortier, G.D.; Fortier, C.I.; Leon, A.M.; Richard, E.A.; Legrand, L.J.; Pronost, S.L. Detection and quantitation of equid gammaherpesviruses (EHV-2, EHV-5) in nasal swabs using an accredited standardised quantitative PCR method. J. Virol. Methods 2014, 198, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gilkerson, J.R.; Bailey, K.E.; Diaz-Mendez, A.; Hartley, C.A. Update on viral diseases of the equine respiratory tract. Vet. Clin. N. Am. Equine Pract. 2015, 31, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, K.; Dunowska, M.; Rola, J. Kinetics of the equid herpesvirus 2 and 5 infections among mares and foals from three Polish national studs. Viruses 2022, 14, 713. [Google Scholar] [CrossRef]
- El Brini, Z.; Fassi Fehri, O.; Paillot, R.; Lotfi, C.; Amraoui, F.; El Ouadi, H.; Dehhaoui, M.; Colitti, B.; Alyakine, H.; Piro, M. Seroprevalence of equine herpesvirus 1 (EHV-1) and equine herpesvirus 4 (EHV-4) in the Northern Moroccan horse populations. Animals 2021, 11, 2851. [Google Scholar] [CrossRef]
- Zientra, S.; Plateau, E. Vaccin et vaccination chez le cheval. Point Vét. 1993, 24, 601–610. [Google Scholar]
- Delannoy, I.; Dubourget, P.; Fayet, G. Le point sur la rhinopneuomnie. Prat. Vét. Equine 1995, 27, 31–47. [Google Scholar]
- Paillot, R. Special issue “Equine Viruses”: Old “friends” and new foes? Viruses 2020, 12, 153. [Google Scholar] [CrossRef]
- Muscat, K.E.; Padalino, B.; Hartley, C.A.; Ficorilli, N.; Celi, P.; Knight, P.; Raidal, S.; Gilkerson, J.R.; Muscatello, G. Equine transport and changes in equid herpesvirus’ status. Front. Vet. Sci. 2018, 5, 224. [Google Scholar] [CrossRef] [PubMed]
- Crabb, B.S.; Studdert, M.J. Equine herpesviruses 4 (equine rhinopneumonitis virus) and 1 (equine abortion virus). Adv. Virus Res. 1995, 45, 153–190. [Google Scholar] [CrossRef] [PubMed]
- Edington, N.; Welch, H.M.; Griffiths, L. The prevalence of latent equid herpesviruses in the tissues of 40 abattoir horses. Equine Vet. J. 1994, 26, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.P.; Bryans, J.T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog. Vet. Microbiol. Immunol. 1986, 2, 78–144. [Google Scholar] [PubMed]
- Bannai, H.; Takahashi, Y.; Ohmura, H.; Ebisuda, Y.; Mukai, K.; Kambayashi, Y.; Nemoto, M.; Tsujimura, K.; Ohta, M.; Raidal, S.; et al. Decreased virus-neutralizing antibodies against equine herpesvirus type 1 in nasal secretions of horses after 12-hour transportation. J. Equine Vet. Sci. 2021, 103, 103665. [Google Scholar] [CrossRef]
- Pereira, L. Function of glycoprotein B homologues of the family Herpesviridae. Infect. Agents Dis. 1994, 3, 9–28. [Google Scholar] [PubMed]
- Wisner, T.W.; Wright, C.C.; Kato, A.; Kawaguchi, Y.; Mou, F.; Baines, J.D.; Roller, R.J.; Johnson, D.C. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 2009, 83, 3115–3126. [Google Scholar] [CrossRef]
- Brault, S.A.; MacLachlan, N.J. Equid gammaherpesviruses: Persistent bystanders or true pathogens? Vet. J. 2011, 187, 14–15. [Google Scholar] [CrossRef]
- Dunowska, M.; Holloway, S.A.; Wilks, C.R.; Meers, J. Genomic variability of equine herpesvirus-5. Arch. Virol. 2000, 145, 1359–1371. [Google Scholar] [CrossRef]
- Browning, G.F.; Studdert, M.J. Epidemiology of equine herpesvirus 2 (equine cytomegalovirus). J. Clin. Microbiol. 1987, 25, 13–16. [Google Scholar] [CrossRef]
- Browning, G.F.; Studdert, M.J. Physical mapping of the genomic heterogeneity of isolates of equine herpesvirus 2 (equine cytomegalovirus). Arch. Virol. 1989, 104, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Plummer, G.; Goodheart, C.R.; Studdert, M.J. Equine herpesviruses: Antigenic relationships and deoxyribonucleic acid densities. Infect. Immun. 1973, 8, 621–627. [Google Scholar] [CrossRef] [PubMed]
- OIE. OIE Expert Surveillance Panel on Equine Influenza Vaccine Composition, OIE Headquarters, 22 March 2017. Conclusions and Recommendations; World Organisation for Animal Health: Paris, France, 2017. [Google Scholar]
- Hussey, S.B.; Clark, R.; Lunn, K.F.; Breathnach, C.; Soboll, G.; Whalley, J.M.; Lunn, D.P. Detection and quantification of equine herpesvirus-1 viremia and nasal shedding by real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2006, 18, 335–342. [Google Scholar] [CrossRef]
- Diallo, I.S.; Hewitson, G.R.; de Jong, A.; Kelly, M.A.; Wright, D.J.; Corney, B.G.; Rodwell, B.J. Equine herpesvirus infections in yearlings in South-East Queensland. Arch. Virol. 2008, 153, 1643–1649. [Google Scholar] [CrossRef]
- Holloway, S.A.; Lindquester, G.J.; Studdert, M.J.; Drummer, H.E. Identification, sequence analysis and characterisation of equine herpesvirus 5 glycoprotein B. Arch. Virol. 1999, 144, 287–307. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Rambaut, A.; Lam, T.T.; Max Carvalho, L.; Pybus, O.G. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol. 2016, 2, 7. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Bielejec, F.; Rambaut, A.; Suchard, M.A.; Lemey, P. SPREAD: Spatial phylogenetic reconstruction of evolutionary dynamics. Bioinformatics 2011, 27, 2910–2912. [Google Scholar] [CrossRef]

| Infection | Virus | Negative | Positive | % Infection |
|---|---|---|---|---|
| Total | EHV1 EHV2 EHV5 | 287 | 11 | 3.69 |
| 152 | 146 | 48.99 | ||
| 139 | 159 | 53.35 | ||
| Unique infection | EHV1 EHV2 EHV5 | 293 | 5 | 1.67 |
| 249 | 49 | 16.44 | ||
| 238 | 60 | 20.13 | ||
| Co-infection | EHV1, EHV2 EHV1, EHV5 EHV2, EHV5 | 297 | 1 | 0.33 |
| 294 | 4 | 1.34 | ||
| 204 | 94 | 31.54 | ||
| Triple infection | EHV1, EHV2, EHV5 | 297 | 1 | 0.33 |
| Global incidence | EHV1, EHV2, EHV4, EHV5 | 84 | 214 | 71.81 |
| EHV1 | EHV2 | EHV5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | N° Tested | N° Positive (%) | RR | 95% CI | p-Value | N° Positive (%) | RR | 95% CI | p-Value | N° Positive (%) | RR | 95% CI | p-Value |
| Age. Year | |||||||||||||
| <5 | 170 | 6 (54.5) | 0.9035 | 0.2820–2.8951 | 0.8644 | 91 (62.3) | 1.2458 | 0.9762–1.5898 | 0.0773 | 103 (64.8) | 1.3849 | 1.0994–1.7445 | 0.0057 |
| 05–10 | 82 | 1 (9.1) | 0.2634 | 0.0343–2.0256 | 0.1999 | 44 (30.1) | 1.1363 | 0.8888–1.4527 | 0.3079 | 37 (23.3) | 0.7989 | 0.6124–1.0422 | 0.0978 |
| >10 | 30 | 3 (27.3) | 3.3500 | 0.9388–11.9544 | 0.0625 | 8 (5.5) | 0.5179 | 0.2829–0.9481 | 0.0329 | 15 (9.4) | 0.9306 | 0.6398–1.3535 | 0.7066 |
| Sex | |||||||||||||
| Male | 143 | 2 (18.2) | 0.2409 | 0.0529–1.0961 | 0.0656 | 76 (52.1) | 1.1768 | 0.9332–1.4840 | 0.1688 | 90 (56.6) | 1.4138 | 1.1390–1.7549 | 0.0017 |
| Female | 139 | 8 (72.7) | 3.0504 | 0.8253–11.2743 | 0.0945 | 67 (45.9) | 0.9701 | 0.7687–1.2244 | 0.7984 | 65 (40.9) | 0.791 | 0.6351–0.9851 | 0.0363 |
| Breed | |||||||||||||
| Arabian thoroughbred | 186 | 4 (36.4) | 0.3441 | 0.1030–1.1492 | 0.0829 | 101 (69.2) | 1.3515 | 1.0404–1.7557 | 0.024 | 102 (64.2) | 1.0775 | 0.8614–1.3479 | 0.5132 |
| English thoroughbred | 46 | 1 (9.1) | 0.5478 | 0.0718–4.1775 | 0.5615 | 23 (15.8) | 1.0244 | 0.7473–1.4043 | 0.881 | 29 (18.2) | 1.2221 | 0.9503–1.5716 | 0.1181 |
| BAB | 57 | 5 (45.5) | 0.3986 | 0.1994–0.7967 | 0.0092 | 19 (13.01) | 1.9211 | 1.1632–3.1727 | 0.0108 | 22(13.83) | 1.8198 | 1.1235–2.9476 | 0.0150 |
| Others | 9 | 1(9.1) | 0.3066 | 0.0419–2.2426 | 0.2442 | 3 (2.05) | 1.9211 | 0.4895–7.5388 | 0.3493 | 6(3.77) | 0.5719 | 0.1458–2.2443 | 0.4231 |
| Activity | |||||||||||||
| Race | 226 | 5 (45.5) | 0.2655 | 0.0835–0.8442 | 0.0246 | 125 (85.6) | 1.8963 | 1.2987–2.7690 | 0.0009 | 127 (79.9) | 1.2644 | 0.9530–1.6775 | 0.1039 |
| Leisure | 25 | 1 (9.1) | 1.0920 | 0.1457–8.1863 | 0.9318 | 9 (6.2) | 0.7174 | 0.4198–1.2259 | 0.2244 | 16 (10.1) | 1.2218 | 0.8917–1.6742 | 0.2126 |
| Breeding | 24 | 4 (36.4) | 6.5238 | 2.0544–20.7165 | 0.0015 | 7 (4.8) | 0.5749 | 0.3049–1.0842 | 0.0872 | 7 (4.4) | 0.5258 | 0.2793–0.9896 | 0.0463 |
| Show jumping | 7 | - | 1.5870 | 0.1022–24.6503 | 0.7414 | 2 (1.4) | 0.5774 | 0.1779–1.8735 | 0.3604 | 5 (3.1) | 1.3497 | 0.8344–2.1832 | 0.2216 |
| Season | |||||||||||||
| Winter | 129 | 3 (27.3) | 0.4913 | 0.1330–1.8153 | 0.2865 | 69 (47.3) | 1.174 | 0.9324–1.4781 | 0.1723 | 68 (42.8) | 0.9848 | 0.7940–1.2213 | 0.8887 |
| Spring | 84 | 2 (18.2) | 0.5661 | 0.1249–2.5660 | 0.4606 | 41 (28.1) | 0.9948 | 0.7685–1.2877 | 0.9683 | 45 (28.3) | 1.0056 | 0.7948–1.2725 | 0.9626 |
| Summer | 63 | 1 (9.1) | 0.3730 | 0.0487–2.8595 | 0.3426 | 32 (21.9) | 1.0471 | 0.7942–1.3805 | 0.7444 | 40 (25.2) | 1.2538 | 1.0004–1.5715 | 0.0496 |
| Autumn | 22 | 5 (45.5) | 10.4545 | 3.4642–31.5507 | <0.0001 | 4 (2.7) | 0.3534 | 0.1446–0.8639 | 0.0226 | 6 (3.8) | 0.492 | 0.2466–0.9814 | 0.0441 |
| Clinical signs | |||||||||||||
| Yes | 245 | 6 (54.5) | 0.2596 | 0.0823–0.8191 | 0.0214 | 126 (86.3) | 1.3629 | 0.9446–1.9664 | 0.0979 | 137 (86.2) | 1.3471 | 0.9604–1.8896 | 0.0844 |
| No | 43 | 4 (36.4) | 3.3887 | 1.0358–11.0861 | 0.0436 | 18 (12.3) | 0.8339 | 0.5744–1.2108 | 0.3398 | 21 (13.2) | 0.9024 | 0.6513–1.2504 | 0.5372 |
| Governorate | |||||||||||||
| North | 256 | 10(90.1) | 0.9429 | 0.7776–1.1433 | 0.5496 | 130 (89.04) | 0.9310 | 0.8492–1.0206 | 0.1273 | 139(87.42) | 0.9628 | 0.8772–1.0568 | 0.4255 |
| South | 30 | 1(9.1) | 1.1115 | 0.1662–7.4342 | 0.9132 | 13(8.90) | 1.2561 | 0.6329–2.4930 | 0.5145 | 13(8.17) | 1.4958 | 0.7538–2.9684 | 0.2495 |
| Imported | 12 | - | 0.9599 | 0.0598–15.4164 | 0.9769 | 3 (2.1) | 0.5 | 0.1864–1.3414 | 0.1686 | 7 (4.4) | 1.0976 | 0.6721–1.7924 | 0.7098 |
| Amplification | Virus | Region | Primers and Probes (5′-3′) | Size | References |
|---|---|---|---|---|---|
| Real time PCR | EHV1 | gB | FW: GGGGTTCTTAATTGCATTCAGACC | 106 bp | [67] |
| RV: GTAGGTGCGGTTAGATCTCACAAG | |||||
| FAM TCTCCAACGAACTCGCCAGGCTGTACC BHQ1 | |||||
| EHV2 | gB | FW: GTGGCCAGCGGGGTGTTC | 78 bp | [47] | |
| RV: CCCCCAAAGGGATTYTTGAA | |||||
| FAM CCCTCTTTGGGAGCATAGTCTCGGGG TAMRA | |||||
| EHV4 | gB | FW: TAGCAAACACCCACTAATAATAGCAAG | 78 bp | [67] | |
| RV: GCTCAAATCTCTTTATTTTATGTCATATGC | |||||
| HEXCGCAACAGGAACTCACTTCAGAGCCAGC BHQ1 | |||||
| EHV5 | gB | FW: AACCCGCCGTGCATCA | 66 bp | [47] | |
| RV: AGGCGCCACACACCCTAA | |||||
| FAMACAACACCACCAACCCCTTTCTGCTG TAMRA | |||||
| Conventional PCR | EHV2 | gB | FW: GCCAGTGTCTGCCAAGTTGATA | 444 bp | [68] |
| RV: CATGGTCTCGATGTCAAACACG | |||||
| EHV5 | gB | FW: ATGAACCTGACAGATGTGCC | 293 bp | [69] | |
| RV: CACGTTCACTATCACGTCGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, C.; Souiai, O.; Arbi, M.; El Behi, I.; Essaied, M.S.; Khosrof, I.; Benkahla, A.; Chabchoub, A.; Ghram, A. Epidemiological and Phylogeographic Study of Equid Herpesviruses in Tunisia. Pathogens 2022, 11, 1016. https://doi.org/10.3390/pathogens11091016
Badr C, Souiai O, Arbi M, El Behi I, Essaied MS, Khosrof I, Benkahla A, Chabchoub A, Ghram A. Epidemiological and Phylogeographic Study of Equid Herpesviruses in Tunisia. Pathogens. 2022; 11(9):1016. https://doi.org/10.3390/pathogens11091016
Chicago/Turabian StyleBadr, Chaima, Oussama Souiai, Marwa Arbi, Imen El Behi, Mohamed S. Essaied, Ines Khosrof, Alia Benkahla, Ahmed Chabchoub, and Abdeljelil Ghram. 2022. "Epidemiological and Phylogeographic Study of Equid Herpesviruses in Tunisia" Pathogens 11, no. 9: 1016. https://doi.org/10.3390/pathogens11091016
APA StyleBadr, C., Souiai, O., Arbi, M., El Behi, I., Essaied, M. S., Khosrof, I., Benkahla, A., Chabchoub, A., & Ghram, A. (2022). Epidemiological and Phylogeographic Study of Equid Herpesviruses in Tunisia. Pathogens, 11(9), 1016. https://doi.org/10.3390/pathogens11091016







