Promastigote-to-Amastigote Conversion in Leishmania spp.—A Molecular View
Abstract
1. Leishmania Biology and Life Cycle
1.1. Natural Life Cycle
1.2. Promastigote to Amastigote Differentiation
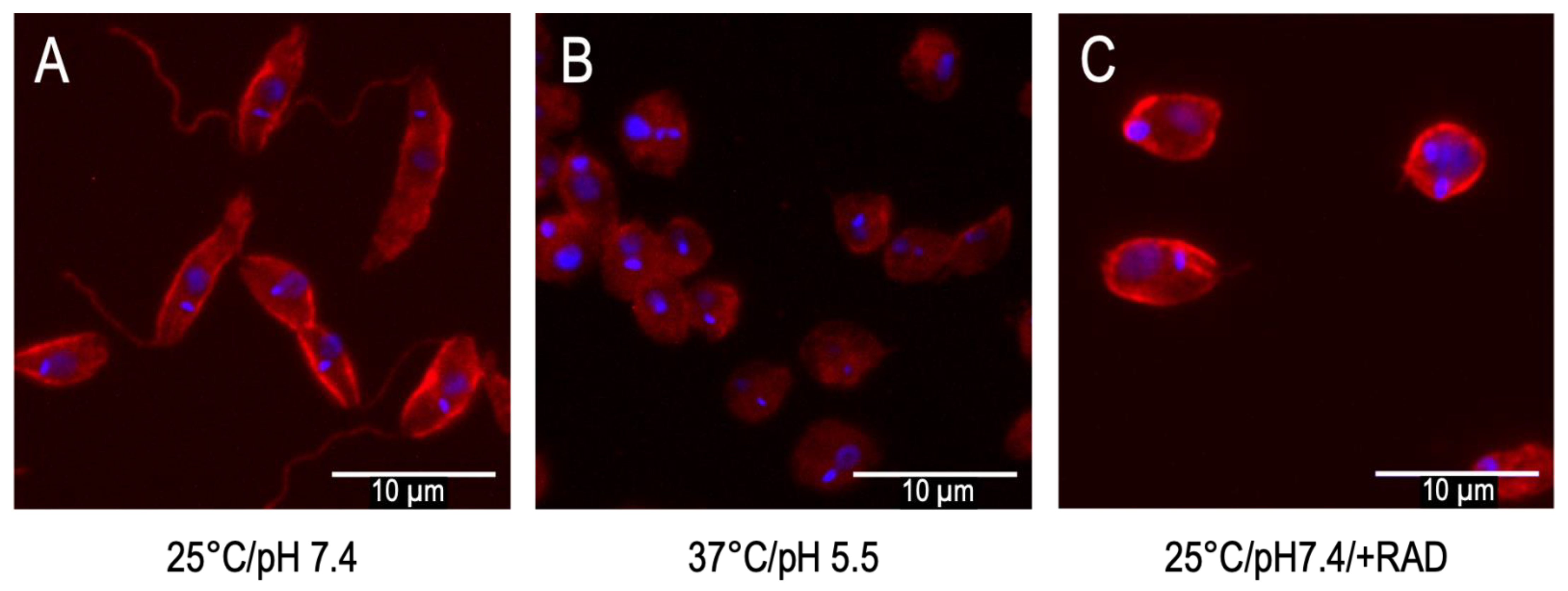
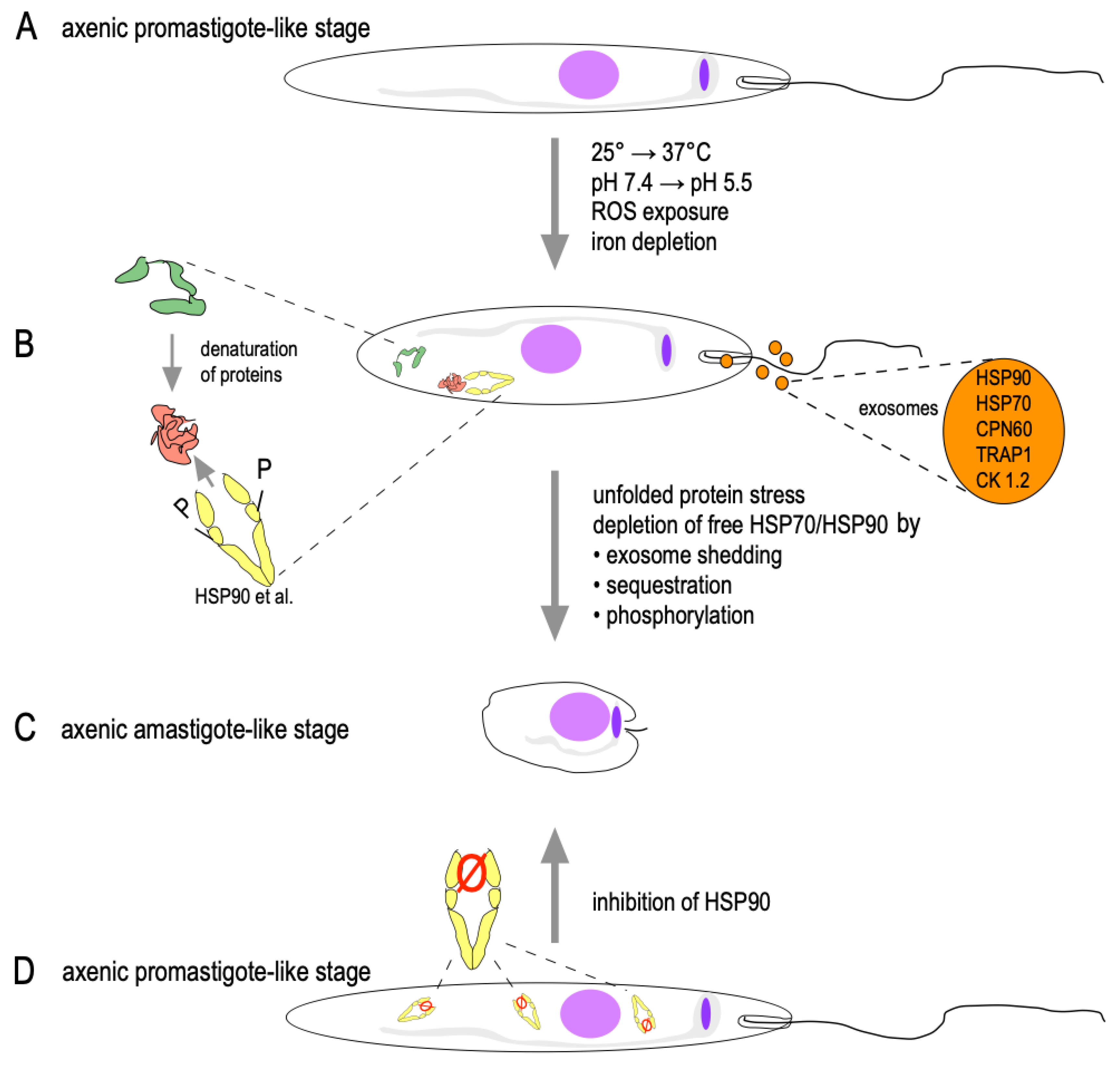
1.2.1. Temperature and pH as Triggers
1.2.2. Emerging Alternative/Additional Triggers
1.2.3. Markers of Promastigote-to-Amastigote Conversion
1.3. Limitations of In Vitro Differentiation Models
2. Transduction of Differentiation Triggers
2.1. Post-Transcriptional Gene Regulation during Stage Conversion
2.2. Protein Turnover and Leishmania Proteases in Stage Differentiation
2.3. Protein Kinases in Stage Differentiation and Intracellular Survival
2.4. Epigenetic Effects
2.5. HSPs in Stage Conversion
3. Future Directions of Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacks, D.L.; Perkins, P.V. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am. J. Trop. Med. Hyg. 1985, 34, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Gossage, S.M.; Rogers, M.E.; Bates, P.A. Two separate growth phases during the development of Leishmania in sand flies: Implications for understanding the life cycle. Int. J. Parasitol. 2003, 33, 1027–1034. [Google Scholar] [CrossRef]
- Becvar, T.; Vojtkova, B.; Siriyasatien, P.; Votypka, J.; Modry, D.; Jahn, P.; Bates, P.; Carpenter, S.; Volf, P.; Sadlova, J. Experimental transmission of Leishmania (Mundinia) parasites by biting midges (Diptera: Ceratopogonidae). PLoS Pathog. 2021, 17, e1009654. [Google Scholar] [CrossRef] [PubMed]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003, 11, 210–214. [Google Scholar] [CrossRef]
- Lambertz, U.; Silverman, J.M.; Nandan, D.; McMaster, W.R.; Clos, J.; Foster, L.J.; Reiner, N.E. Secreted virulence factors and immune evasion in visceral leishmaniasis. J. Leukoc. Biol. 2012, 91, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.A. Complete developmental cycle of Leishmania mexicanain axenic culture. Parasitology 1994, 108, 1–9. [Google Scholar] [CrossRef]
- Barak, E.; Amin-Spector, S.; Gerliak, E.; Goyard, S.; Holland, N.; Zilberstein, D. Differentiation of Leishmania donovani in host-free system: Analysis of signal perception and response. Mol. Biochem. Parasitol. 2005, 141, 99–108. [Google Scholar] [CrossRef]
- Zilberstein, D.; Nitzan Koren, R. Host-Free Systems for Differentiation of Axenic Leishmania. Methods Mol. Biol. 2019, 1971, 1–8. [Google Scholar] [CrossRef]
- Wiesgigl, M.; Clos, J. Heat Shock Protein 90 Homeostasis Controls Stage Differentiation in Leishmania donovani. Mol. Biol. Cell 2001, 12, 3307–3316. [Google Scholar] [CrossRef]
- Bente, M.; Harder, S.; Wiesgigl, M.; Heukeshoven, J.; Gelhaus, C.; Krause, E.; Clos, J.; Bruchhaus, I. Developmentally induced changes of the proteome in the protozoan parasite Leishmania donovani. Proteomics 2003, 3, 1811–1829. [Google Scholar] [CrossRef]
- Mittra, B.; Cortez, M.; Haydock, A.; Ramasamy, G.; Myler, P.J.; Andrews, N.W. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J. Exp. Med. 2013, 210, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.A.; Andrews, N.W.; Mittra, B. ROS regulate differentiation of visceralizing Leishmania species into the virulent amastigote form. Parasitol. Open 2018, 4, e19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Charest, H.; Ghedin, E.; Matlashewski, G. Identification and overexpression of the A2 amastigote-specific protein in Leishmania donovani. Mol. Biochem. Parasitol. 1996, 78, 79–90. [Google Scholar] [CrossRef]
- Zhang, W.W.; Matlashewski, G. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc. Natl. Acad. Sci. USA 1997, 94, 8807–8811. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.I.; Matlashewski, G. Localization and induction of the A2 virulence factor in Leishmania: Evidence that A2 is a stress response protein. Mol. Microbiol. 2010, 77, 518–530. [Google Scholar] [CrossRef]
- McCall, L.I.; Matlashewski, G. Involvement of the Leishmania donovani virulence factor A2 in protection against heat and oxidative stress. Exp. Parasitol. 2012, 132, 109–115. [Google Scholar] [CrossRef]
- Rosenzweig, D.; Smith, D.; Opperdoes, F.; Stern, S.; Olafson, R.W.; Zilberstein, D. Retooling Leishmania metabolism: From sand fly gut to human macrophage. FASEB J. 2008, 22, 590–602. [Google Scholar] [CrossRef]
- Kar, S.; Soong, L.; Colmenares, M.; Goldsmith-Pestana, K.; McMahon-Pratt, D. The immunologically protective P-4 antigen of Leishmania amastigotes. A developmentally regulated single strand-specific nuclease associated with the endoplasmic reticulum. J. Biol. Chem. 2000, 275, 37789–37797. [Google Scholar] [CrossRef]
- Rochette, A.; McNicoll, F.; Girard, J.; Breton, M.; Leblanc, E.; Bergeron, M.G.; Papadopoulou, B. Characterization and developmental gene regulation of a large gene family encoding amastin surface proteins in Leishmania spp. Mol. Biochem. Parasitol. 2005, 140, 205–220. [Google Scholar] [CrossRef]
- Krobitsch, S.; Clos, J. A novel role for 100 kD heat shock proteins in the parasite Leishmania donovani. Cell Stress Chaperones 1999, 4, 191–198. [Google Scholar] [CrossRef]
- Yau, W.L.; Pescher, P.; MacDonald, A.; Hem, S.; Zander, D.; Retzlaff, S.; Blisnick, T.; Rotureau, B.; Rosenqvist, H.; Wiese, M.; et al. The Leishmania donovani chaperone cyclophilin 40 is essential for intracellular infection independent of its stage-specific phosphorylation status. Mol. Microbiol. 2014, 93, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E.C.; Ng, W.W.; Kloehn, J.; Chambers, J.M.; Ng, M.; McConville, M.J. Induction of a stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog. 2014, 10, e1003888. [Google Scholar] [CrossRef] [PubMed]
- Bifeld, E.; Lorenzen, S.; Bartsch, K.; Vasquez, J.J.; Siegel, T.N.; Clos, J. Ribosome Profiling Reveals HSP90 Inhibitor Effects on Stage-Specific Protein Synthesis in Leishmania donovani. mSystems 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.B.; Alcantara, L.M. Quantification of Parasite Loads by Automated Microscopic Image Analysis. Methods Mol. Biol. 2019, 1971, 279–288. [Google Scholar] [CrossRef]
- Santarem, N.; Tavares, J.; Cordeiro-da-Silva, A. In Vitro Infections of Macrophage-Like Cell Lines with Leishmania infantum for Drug Screening. Methods Mol. Biol. 2019, 1971, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.A.; Bank, E.; Florian, C.; Forster, S.; Zimara, N.; Steinacker, J.; Klinger, M.; Reiling, N.; Ritter, U.; van Zandbergen, G. Leishmania major parasite stage-dependent host cell invasion and immune evasion. FASEB J. 2012, 26, 29–39. [Google Scholar] [CrossRef]
- Ivens, A.C.; Peacock, C.S.; Worthey, E.A.; Murphy, L.; Aggarwal, G.; Berriman, M.; Sisk, E.; Rajandream, M.A.; Adlem, E.; Aert, R.; et al. The genome of the kinetoplastid parasite, Leishmania major. Science 2005, 309, 436–442. [Google Scholar] [CrossRef]
- Clayton, C.E. Life without transcriptional control? From fly to man and back again. EMBO J. 2002, 21, 1881–1888. [Google Scholar] [CrossRef]
- Leifso, K.; Cohen-Freue, G.; Dogra, N.; Murray, A.; McMaster, W.R. Genomic and proteomic expression analysis of Leishmania promastigote and amastigote life stages: The Leishmania genome is constitutively expressed. Mol. Biochem. Parasitol. 2007, 152, 35–46. [Google Scholar] [CrossRef]
- Myler, P.J.; Beverley, S.M.; Cruz, A.K.; Dobson, D.E.; Ivens, A.C.; McDonagh, P.D.; Madhubala, R.; Martinez-Calvillo, S.; Ruiz, J.C.; Saxena, A.; et al. The Leishmania genome project: New insights into gene organization and function. Med. Microbiol. Immunol. 2001, 190, 9–12. [Google Scholar] [CrossRef]
- Hunter, K.W.; Cook, C.L.; Hayunga, E.G. Leishmanial differentiation in vitro: Induction of heat shock proteins. Biochem. Biophys. Res. Commun. 1984, 125, 755–760. [Google Scholar] [CrossRef]
- Aly, R.; Argaman, M.; Halman, S.; Shapira, M. A regulatory role for the 5’ and 3’ untranslated regions in differential expression of hsp83 in Leishmania. Nucleic Acids Res. 1994, 22, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Nandan, D.; Thomas, S.A.; Nguyen, A.; Moon, K.M.; Foster, L.J.; Reiner, N.E. Comprehensive Identification of mRNA-Binding Proteins of Leishmania donovani by Interactome Capture. PLoS ONE 2017, 12, e0170068. [Google Scholar] [CrossRef] [PubMed]
- Larreta, R.; Soto, M.; Quijada, L.; Folgueira, C.; Abanades, D.R.; Alonso, C.; Requena, J.M. The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation. BMC Mol. Biol. 2004, 5, 3. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; El Fakhry, Y.; Sereno, D.; Tamar, S.; Papadopoulou, B. A new developmentally regulated gene family in Leishmania amastigotes encoding a homolog of amastin surface proteins. Mol. Biochem. Parasitol. 2000, 110, 345–357. [Google Scholar] [CrossRef]
- McNicoll, F.; Muller, M.; Cloutier, S.; Boilard, N.; Rochette, A.; Dube, M.; Papadopoulou, B. Distinct 3’-untranslated region elements regulate stage-specific mRNA accumulation and translation in Leishmania. J. Biol. Chem. 2005, 280, 35238–35246. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Alonso, A.; Gomez, M.J.; Moreno, I.; Dominguez, M.; Parro, V.; Larraga, V. Transcriptomics throughout the life cycle of Leishmania infantum: High down-regulation rate in the amastigote stage. Int. J. Parasitol. 2010, 40, 1497–1516. [Google Scholar] [CrossRef]
- Cloutier, S.; Laverdiere, M.; Chou, M.N.; Boilard, N.; Chow, C.; Papadopoulou, B. Translational control through eIF2alpha phosphorylation during the Leishmania differentiation process. PLoS ONE 2012, 7, e35085. [Google Scholar] [CrossRef]
- Zinoviev, A.; Leger, M.; Wagner, G.; Shapira, M. A novel 4E-interacting protein in Leishmania is involved in stage-specific translation pathways. Nucleic Acids Res. 2011, 39, 8404–8415. [Google Scholar] [CrossRef]
- Rastrojo, A.; Carrasco-Ramiro, F.; Martin, D.; Crespillo, A.; Reguera, R.M.; Aguado, B.; Requena, J.M. The transcriptome of Leishmania major in the axenic promastigote stage: Transcript annotation and relative expression levels by RNA-seq. BMC Genom. 2013, 14, 223. [Google Scholar] [CrossRef]
- Dillon, L.A.; Okrah, K.; Hughitt, V.K.; Suresh, R.; Li, Y.; Fernandes, M.C.; Belew, A.T.; Corrada Bravo, H.; Mosser, D.M.; El-Sayed, N.M. Transcriptomic profiling of gene expression and RNA processing during Leishmania major differentiation. Nucleic Acids Res. 2015, 43, 6799–6813. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Dresel, A.; Clos, J. High constitutive levels of heat-shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem. J. 1995, 310 Pt 2, 225–232. [Google Scholar] [CrossRef]
- Mahat, D.B.; Kwak, H.; Booth, G.T.; Jonkers, I.H.; Danko, C.G.; Patel, R.K.; Waters, C.T.; Munson, K.; Core, L.J.; Lis, J.T. Base-pair-resolution genome-wide mapping of active RNA polymerases using precision nuclear run-on (PRO-seq). Nat. Protoc. 2016, 11, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Mottram, J.C.; Frame, M.J.; Brooks, D.R.; Tetley, L.; Hutchison, J.E.; Souza, A.E.; Coombs, G.H. The multiple cpb cysteine proteinase genes of Leishmania mexicana encode isoenzymes that differ in their stage regulation and substrate preferences. J. Biol. Chem. 1997, 272, 14285–14293. [Google Scholar] [CrossRef] [PubMed]
- Besteiro, S.; Williams, R.A.; Coombs, G.H.; Mottram, J.C. Protein turnover and differentiation in Leishmania. Int. J. Parasitol. 2007, 37, 1063–1075. [Google Scholar] [CrossRef]
- Zabala-Peñafiel, A.; Cysne-Finkelstein, L.; Conceição-Silva, F.; Fagundes, A.; Miranda, L.d.F.C.; Souza-Silva, F.; Brandt, A.A.M.L.; Dias-Lopes, G.; Alves, C.R. Novel Insights Into Leishmania (Viannia) braziliensis In Vitro Fitness Guided by Temperature Changes Along With Its Subtilisins and Oligopeptidase B. Front. Cell. Infect. Microbiol. 2022, 12, 411. [Google Scholar] [CrossRef]
- Ramu, D.; Singh, S. Potential molecular targets of Leishmania pathways in developing novel antileishmanials. Future Microbiol. 2022, 17, 41–57. [Google Scholar] [CrossRef]
- Waller, R.F.; McConville, M.J. Developmental changes in lysosome morphology and function Leishmania parasites. Int. J. Parasitol. 2002, 32, 1435–1445. [Google Scholar] [CrossRef]
- Ke, G.; Mauel, J.; Rivier, D. Leishmania mexicana: Extracellular proton concentration is a key regulator of cysteine proteinase CPb expression. Exp. Parasitol. 1998, 90, 58–64. [Google Scholar] [CrossRef]
- Gomes, C.B.; Silva, F.S.; Charret, K.D.; Pereira, B.A.; Finkelstein, L.C.; Santos-de-Souza, R.; de Castro Cortes, L.M.; Pereira, M.C.; Rodrigues de Oliveira, F.O., Jr.; Alves, C.R. Increasing in cysteine proteinase B expression and enzymatic activity during in vitro differentiation of Leishmania (Viannia) braziliensis: First evidence of modulation during morphological transition. Biochimie 2017, 133, 28–36. [Google Scholar] [CrossRef]
- Pral, E.M.; Moitinho, M.d.L.R.; Balanco, J.M.F.; Teixeira, V.R.; Milder, R.V.; Alfieri, S.C. Growth phase and medium pH modulate the expression of proteinase activities and the development of megasomes in axenically cultivated Leishmania (Leishmania) amazonensis amastigote–like organisms. J. Parasitol. 2003, 89, 35–43. [Google Scholar] [CrossRef]
- Ueda-Nakamura, T.; da Conceição Rocha Sampaio, M.; Cunha-e-Silva, N.L.; Traub-Cseko, Y.M.; de Souza, W. Expression and processing of megasome cysteine proteinases during Leishmania amazonensis differentiation. Parasitol. Res. 2002, 88, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Courret, N.; Frehel, C.; Prina, E.; Lang, T.; Antoine, J.C. Kinetics of the intracellular differentiation of Leishmania amazonensis and internalization of host MHC molecules by the intermediate parasite stages. Parasitology 2001, 122, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Yao, C. Major surface protease of trypanosomatids: One size fits all? Infect. Immun. 2010, 78, 22–31. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Casanova, M. Cell death in Leishmania. Parasite 2019, 26, 71. [Google Scholar] [CrossRef]
- Gonzalez, I.J.; Desponds, C.; Schaff, C.; Mottram, J.C.; Fasel, N. Leishmania major metacaspase can replace yeast metacaspase in programmed cell death and has arginine-specific cysteine peptidase activity. Int. J. Parasitol. 2007, 37, 161–172. [Google Scholar] [CrossRef]
- Spath, G.F.; Drini, S.; Rachidi, N. A touch of Zen: Post-translational regulation of the Leishmania stress response. Cell Microbiol. 2015, 17, 632–638. [Google Scholar] [CrossRef]
- Baker, N.; Catta-Preta, C.M.C.; Neish, R.; Sadlova, J.; Powell, B.; Alves-Ferreira, E.V.C.; Geoghegan, V.; Carnielli, J.B.T.; Newling, K.; Hughes, C.; et al. Systematic functional analysis of Leishmania protein kinases identifies regulators of differentiation or survival. Nat. Commun. 2021, 12, 1244. [Google Scholar] [CrossRef]
- Fischer Weinberger, R.; Bachmaier, S.; Dandugudumula, R.; Phan, I.Q.; Almoznino, M.; Githure, G.B.; Polatoglou, E.; Tsigankov, P.; Nitzan Koren, R.; Myler, P.J.; et al. A divergent protein kinase A in the human pathogen Leishmania is associated with developmental morphogenesis. bioRxiv 2021. under review. [Google Scholar] [CrossRef]
- Wiese, M. A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. EMBO J. 1998, 17, 2619–2628. [Google Scholar] [CrossRef]
- Agron, P.G.; Reed, S.L.; Engel, J.N. An essential, putative MEK kinase of Leishmania major. Mol. Biochem. Parasitol. 2005, 142, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Worthey, E.A.; Ward, P.N.; Mottram, J.C. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genom. 2005, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- De Lima, A.R.; Medina, R.; Uzcanga, G.L.; Noris Suarez, K.; Contreras, V.T.; Navarro, M.C.; Arteaga, R.; Bubis, J. Tight binding between a pool of the heterodimeric alpha/beta tubulin and a protein kinase CK2 in Trypanosoma cruzi epimastigotes. Parasitology 2006, 132, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Hammarton, T.C. Cell cycle regulation in Trypanosoma brucei. Mol. Biochem. Parasitol. 2007, 153, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.C.; Jesus, T.C.; Hashimoto, N.N.; Dey, M.; Schwartz, K.J.; Alves, V.S.; Avila, C.C.; Bangs, J.D.; Dever, T.E.; Schenkman, S.; et al. Novel membrane-bound eIF2alpha kinase in the flagellar pocket of Trypanosoma brucei. Eukaryot. Cell 2007, 6, 1979–1991. [Google Scholar] [CrossRef]
- Morales, M.A.; Renaud, O.; Faigle, W.; Shorte, S.L.; Spath, G.F. Over-expression of Leishmania major MAP kinases reveals stage-specific induction of phosphotransferase activity. Int. J. Parasitol. 2007, 37, 1187–1199. [Google Scholar] [CrossRef]
- Dutra, P.M.; Vieira, D.P.; Meyer-Fernandes, J.R.; Silva-Neto, M.A.; Lopes, A.H. Stimulation of Leishmania tropica protein kinase CK2 activities by platelet-activating factor (PAF). Acta Trop. 2009, 111, 247–254. [Google Scholar] [CrossRef]
- Rotureau, B.; Morales, M.A.; Bastin, P.; Späth, G.F. The flagellum-mitogen-activated protein kinase connection in Trypanosomatids: A key sensory role in parasite signalling and development? Cell Microbiol. 2009, 11, 710–718. [Google Scholar] [CrossRef]
- Madeira da Silva, L.; Beverley, S.M. Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc. Natl. Acad. Sci. USA 2010, 107, 11965–11970. [Google Scholar] [CrossRef]
- Morales, M.A.; Pescher, P.; Spath, G.F. Leishmania major MPK7 protein kinase activity inhibits intracellular growth of the pathogenic amastigote stage. Eukaryot. Cell 2010, 9, 22–30. [Google Scholar] [CrossRef]
- Morales, M.A.; Watanabe, R.; Dacher, M.; Chafey, P.; Osorio y Fortea, J.; Scott, D.A.; Beverley, S.M.; Ommen, G.; Clos, J.; Hem, S.; et al. Phosphoproteome dynamics reveal heat-shock protein complexes specific to the Leishmania donovani infectious stage. Proc. Natl. Acad. Sci. USA 2010, 107, 8381–8386. [Google Scholar] [CrossRef] [PubMed]
- Horjales, S.; Schmidt-Arras, D.; Limardo, R.R.; Leclercq, O.; Obal, G.; Prina, E.; Turjanski, A.G.; Spath, G.F.; Buschiazzo, A. The crystal structure of the MAP kinase LmaMPK10 from Leishmania major reveals parasite-specific features and regulatory mechanisms. Structure 2012, 20, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Mandal, G.; Sharma, M.; Kruse, M.; Sander-Juelch, C.; Munro, L.A.; Wang, Y.; Vilg, J.V.; Tamas, M.J.; Bhattacharjee, H.; Wiese, M.; et al. Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol. Microbiol. 2012, 85, 1204–1218. [Google Scholar] [CrossRef]
- Badjatia, N.; Ambrósio, D.L.; Lee, J.H.; Günzl, A. Trypanosome cdc2-related kinase 9 controls spliced leader RNA cap4 methylation and phosphorylation of RNA polymerase II subunit RPB1. Mol. Cell. Biol. 2013, 33, 1965–1975. [Google Scholar] [CrossRef]
- Cayla, M.; Rachidi, N.; Leclercq, O.; Schmidt-Arras, D.; Rosenqvist, H.; Wiese, M.; Spath, G.F. Transgenic analysis of the Leishmania MAP kinase MPK10 reveals an auto-inhibitory mechanism crucial for stage-regulated activity and parasite viability. PLoS Pathog. 2014, 10, e1004347. [Google Scholar] [CrossRef]
- Garg, M.; Goyal, N. MAPK1 of Leishmania donovani modulates antimony susceptibility by downregulating P-glycoprotein efflux pumps. Antimicrob. Agents Chemother. 2015, 59, 3853–3863. [Google Scholar] [CrossRef]
- Inoue, M.; Okamoto, K.; Uemura, H.; Yasuda, K.; Motohara, Y.; Morita, K.; Hiromura, M.; Reddy, E.P.; Fukuma, T.; Horikoshi, N. Identification and characterization of a cell division-regulating kinase AKB1 (associated kinase of Trypanosoma brucei 14-3-3) through proteomics study of the Tb14-3-3 binding proteins. J. Biochem. 2015, 158, 49–60. [Google Scholar] [CrossRef]
- Zylbersztejn, A.M.; de Morais, C.G.; Lima, A.K.; Souza, J.E.; Lopes, A.H.; Da-Silva, S.A.; Silva-Neto, M.A.; Dutra, P.M. CK2 Secreted by Leishmania braziliensis Mediates Macrophage Association Invasion: A Comparative Study between Virulent and Avirulent Promastigotes. Biomed. Res. Int. 2015, 2015, 167323. [Google Scholar] [CrossRef]
- Goldman-Pinkovich, A.; Balno, C.; Strasser, R.; Zeituni-Molad, M.; Bendelak, K.; Rentsch, D.; Ephros, M.; Wiese, M.; Jardim, A.; Myler, P.J.; et al. An Arginine Deprivation Response Pathway Is Induced in Leishmania during Macrophage Invasion. PLoS Pathog. 2016, 12, e1005494. [Google Scholar] [CrossRef]
- Fernandez-Cortes, F.; Serafim, T.D.; Wilkes, J.M.; Jones, N.G.; Ritchie, R.; McCulloch, R.; Mottram, J.C. RNAi screening identifies Trypanosoma brucei stress response protein kinases required for survival in the mouse. Sci. Rep. 2017, 7, 6156. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, M.; Hombach-Barrigah, A.; Clos, J.; Goyal, N. MAPK1 of Leishmania donovani interacts and phosphorylates HSP70 and HSP90 subunits of foldosome complex. Sci. Rep. 2017, 7, 10202. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Moreira-Leite, F.; Portman, N.; Gull, K. Protein diversity in discrete structures at the distal tip of the trypanosome flagellum. Proc. Natl. Acad. Sci. USA 2017, 114, E6546–E6555. [Google Scholar] [CrossRef] [PubMed]
- Hombach-Barrigah, A.; Bartsch, K.; Smirlis, D.; Rosenqvist, H.; MacDonald, A.; Dingli, F.; Loew, D.; Spath, G.F.; Rachidi, N.; Wiese, M.; et al. Leishmania donovani 90 kD Heat Shock Protein—Impact of Phosphosites on Parasite Fitness, Infectivity and Casein Kinase Affinity. Sci. Rep. 2019, 9, 5074. [Google Scholar] [CrossRef]
- Kelly, F.D.; Yates, P.A.; Landfear, S.M. Nutrient sensing in Leishmania: Flagellum and cytosol. Mol. Microbiol. 2021, 115, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Melzer, I.M.; Kruse, M.; Sander-Juelch, C.; Wiese, M. LmxMPK4, a mitogen-activated protein (MAP) kinase homologue essential for promastigotes and amastigotes of Leishmania mexicana. Kinetoplastid Biol. Dis. 2005, 4, 6. [Google Scholar] [CrossRef]
- Liu, J.; Carvalho, L.P.; Bhattacharya, S.; Carbone, C.J.; Kumar, K.G.; Leu, N.A.; Yau, P.M.; Donald, R.G.; Weiss, M.J.; Baker, D.P.; et al. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol. Cell. Biol. 2009, 29, 6401–6412. [Google Scholar] [CrossRef] [PubMed]
- Borba, J.V.B.; Silva, A.C.; Ramos, P.I.P.; Grazzia, N.; Miguel, D.C.; Muratov, E.N.; Furnham, N.; Andrade, C.H. Unveiling the Kinomes of Leishmania infantum and L. braziliensis Empowers the Discovery of New Kinase Targets and Antileishmanial Compounds. Comput. Struct. Biotechnol. J. 2019, 17, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.I. Chromatin remodelling during the life cycle of trypanosomatids. Int. J. Parasitol. 2000, 30, 679–687. [Google Scholar] [CrossRef]
- Grunebast, J.; Lorenzen, S.; Zummack, J.; Clos, J. Life Cycle Stage-Specific Accessibility of Leishmania donovani Chromatin at Transcription Start Regions. mSystems 2021, 6, e0062821. [Google Scholar] [CrossRef]
- Martinez-Calvillo, S.; Yan, S.; Nguyen, D.; Fox, M.; Stuart, K.; Myler, P.J. Transcription of Leishmania major Friedlin chromosome 1 initiates in both directions within a single region. Mol. Cell 2003, 11, 1291–1299. [Google Scholar] [CrossRef]
- Martinez-Calvillo, S.; Nguyen, D.; Stuart, K.; Myler, P.J. Transcription initiation and termination on Leishmania major chromosome 3. Eukaryot. Cell 2004, 3, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Kröber-Boncardo, C.; Grünebast, J.; Clos, J. Heat Shock Proteins in Leishmania Parasites. In Heat Shock Proteins; Asea, A., Ed.; Springer: Dordrecht, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Hubel, A.; Krobitsch, S.; Horauf, A.; Clos, J. Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol. Cell. Biol. 1997, 17, 5987–5995. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.; Ommen, G.; MacDonald, A.; Clos, J. A small heat shock protein is essential for thermotolerance and intracellular survival of Leishmania donovani. J. Cell Sci. 2014, 127, 4762–4773. [Google Scholar] [CrossRef]
- Yau, W.L.; Lambertz, U.; Colineau, L.; Pescher, P.; MacDonald, A.; Zander, D.; Retzlaff, S.; Eick, J.; Reiner, N.E.; Clos, J.; et al. Phenotypic Characterization of a Leishmania donovani Cyclophilin 40 Null Mutant. J. Eukaryot. Microbiol. 2016, 63, 823–833. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.K.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef]
- Kalesh, K.; Sundriyal, S.; Perera, H.; Cobb, S.L.; Denny, P.W. Quantitative Proteomics Reveals that Hsp90 Inhibition Dynamically Regulates Global Protein Synthesis in Leishmania mexicana. mSystems 2021, 6, e00089-21. [Google Scholar] [CrossRef]
- Masser, A.E.; Ciccarelli, M.; Andreasson, C. Hsf1 on a leash—Controlling the heat shock response by chaperone titration. Exp. Cell Res. 2020, 396, 112246. [Google Scholar] [CrossRef]
- Zimarino, V.; Wu, C. Induction of sequence specific binding of Drosophila heat shock activator protein without protein synthesis. Nature 1987, 327, 727–730. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef]
- Ommen, G.; Chrobak, M.; Clos, J. The co-chaperone SGT of Leishmania donovaniis essential for the parasite’s viability. Cell Stress Chaperones 2010, 39, 541–546. [Google Scholar] [CrossRef]
- Pratt, W.B. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Burrows, F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J. Mol. Med. 2004, 82, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Goyal, S.; Jamal, S.; Singh, A.; Grover, A. Hsp90: Friends, clients and natural foes. Biochimie 2016, 127, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Rachidi, N.; Taly, J.F.; Durieu, E.; Leclercq, O.; Aulner, N.; Prina, E.; Pescher, P.; Notredame, C.; Meijer, L.; Spath, G.F. Pharmacological assessment defines Leishmania donovani casein kinase 1 as a drug target and reveals important functions in parasite viability and intracellular infection. Antimicrob. Agents Chemother. 2014, 58, 1501–1515. [Google Scholar] [CrossRef]
- Kröber-Boncardo, C.; Lorenzen, S.; Brinker, C.; Clos, J. Casein kinase 1.2 over expression restores stress resistance to Leishmania donovani HSP23 null mutants. Sci. Rep. 2020, 10, 15969. [Google Scholar] [CrossRef]
| Family | Kinase | Reported Function | Reference |
|---|---|---|---|
| CMGC/CDK | Lmx/T.br/Tc/LmjCRK7 | No functional homologue to human CDK7 | Baker et al.,2021 [53] Badjatia et al., 2013 [70] Parsons et al., 2005 [58] |
| LmxCRK8 | Untypical regulation indicated in TrCRK8 | Baker et al., 2021 [53] Hammarton 2007 [60] | |
| CMGC/CDKL and CMGC/MAPK | Lmx/LdMPK1 | Intracellular survival, phosphorylating LdHSP70 and LdHSP90; antimony resistance | Baker et al., 2021 [53] Wiese, 1998 [56] Hombach-Barrigah et al., 2019 [79] Kaur et al., 2017 [77] Morales et al., 2010b [67] Garg and Goyal, 2015 [72] |
| LmxMPK2 | Infection; essential nutrient regulation and osmotic stress via Arginine depletion response (ADR) and AQP1-regulation; antimony resistance flagella-mediated environment sensing | Goldman-Pinkovich et al., 2016 [75] Kelly et al., 2021 [80] Mandal et al., 2012 [69] Rotureau et al. 2009 [64] | |
| LmxMPK15 | Infection | Baker et al., 2021 [53] | |
| Lmx/LmjMPK10 | Stage-specific auto-regulation and phosphorylation crucial for infection; crystal structure available; not crucial in L. major | Morales et al., 2007 [62] Horjales et al., 2012 [68] Cayla et al., 2014 [71] | |
| LmjMPK7 | Infection | Morales et al., 2010 [67] | |
| STE | Lmx/Lmj/T.brMRK1 | Cytoplasmatic MAP3K; infection; osmotic challenge in T.brucei | Baker et al., 2021 [58] Agron et al., 2005 [57] Fernandez-Cortes et al., 2017 [76] |
| CK1.2 | LdCK1.2 | Exosomal kinase; phosphorylates HSP90 and HSP23 | Hombach-Barrigah et al., 2018 [79] Kröber-Boncardo et al., 2020 [87] |
| Other/CK2 | LmxCK2A1, LmxCK2A2 | T.brCK2 linked to cytoskeletal processes; LbrCK2 secreted and ekto-forms mediating virulence | De Lima et al., 2006 [59] Zylbersztejn et al., 2015 [74] Dutra et al., 2009 [63] |
| PEK | LmxEIF2αK2 | Vital for infection; T.brEIF2αK2 linked to sensing or transport | Baker et al., 2021 [53] Moraes et al., 2007 [61] |
| PIKK related | LmxTOR3 | Infection; acidocalcisome formation and metabolic regulation | Baker et al., 2021 [53] Madeira da Silva and Beverley, 2010 [65] |
| CAMK | LmxAKB1 | Infection; T.brAKB1: cytokinesis and division | Inoue et al., 2015 [73] |
| AGC/PKA | LmxPKAC3 | Infection; morphogenesis | Fischer Weinberger et al., under review [53] |
| Other/ULK | LmxSTK36, LmxULK4 | Infection of sandfly vector and mammal. functionally linked. T.brSTK36, Tbr.ULK4: motility and flagella assembly | Baker et al., 2021 [51] Varga et al., 2017 [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clos, J.; Grünebast, J.; Holm, M. Promastigote-to-Amastigote Conversion in Leishmania spp.—A Molecular View. Pathogens 2022, 11, 1052. https://doi.org/10.3390/pathogens11091052
Clos J, Grünebast J, Holm M. Promastigote-to-Amastigote Conversion in Leishmania spp.—A Molecular View. Pathogens. 2022; 11(9):1052. https://doi.org/10.3390/pathogens11091052
Chicago/Turabian StyleClos, Joachim, Janne Grünebast, and Myrine Holm. 2022. "Promastigote-to-Amastigote Conversion in Leishmania spp.—A Molecular View" Pathogens 11, no. 9: 1052. https://doi.org/10.3390/pathogens11091052
APA StyleClos, J., Grünebast, J., & Holm, M. (2022). Promastigote-to-Amastigote Conversion in Leishmania spp.—A Molecular View. Pathogens, 11(9), 1052. https://doi.org/10.3390/pathogens11091052







