Current Topics in Dermatophyte Classification and Clinical Diagnosis
Abstract
:1. Introduction
2. Introduction to Dermatophytes
2.1. Molecular Characterization of Dermatophytes
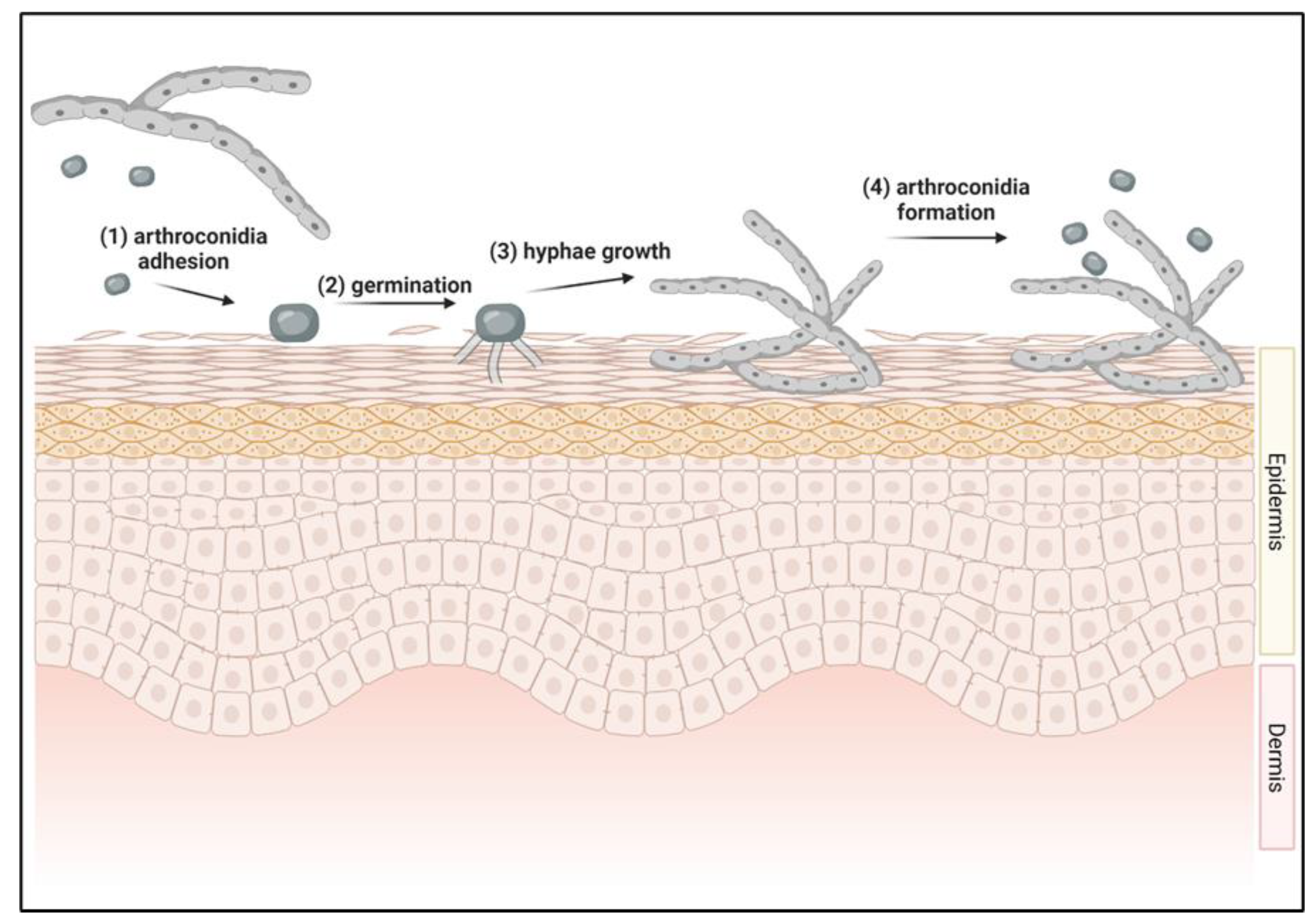
2.2. Initiation of Dermatophyte Infections
2.3. Dermatophyte Viability
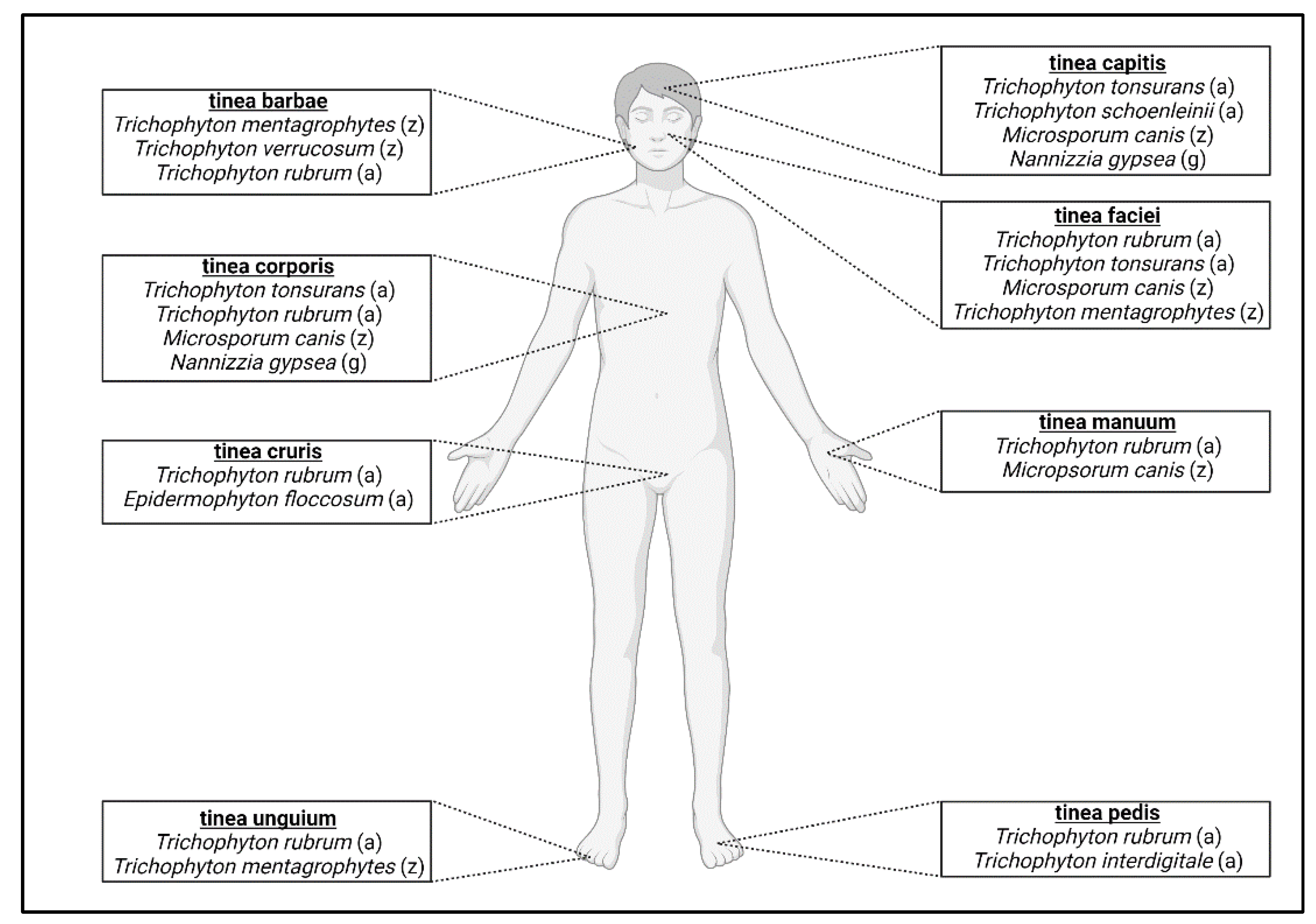
3. Dermatophyte Classification
3.1. Anthropophilic Dermatophytes
3.2. Zoophilic Dermatophytes
3.3. Geophilic Dermatophytes
4. Diagnostic Approaches to Dermatophytosis
4.1. Direct Examination
4.2. Wood’s Lamp
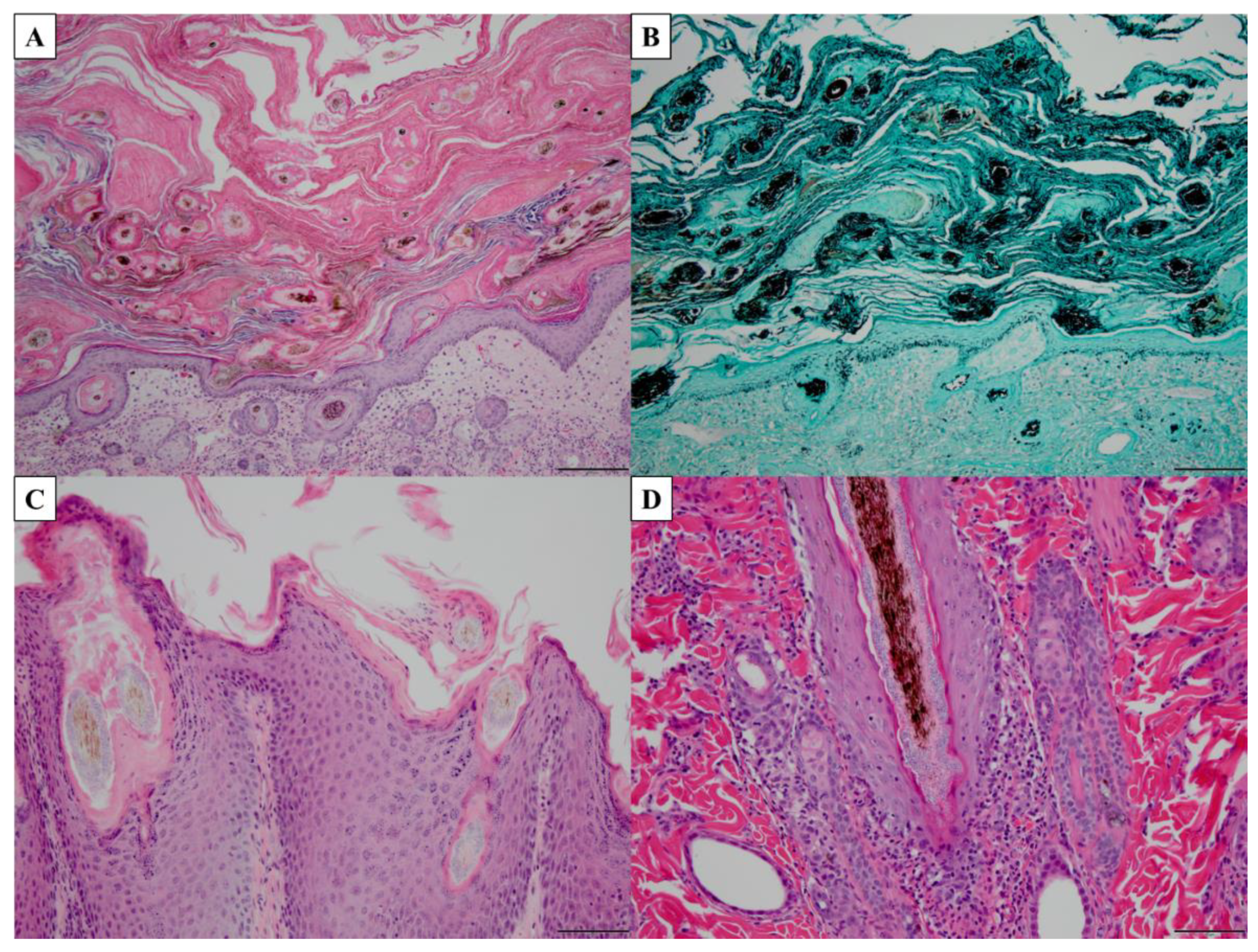
4.3. Microscopy and Histopathology
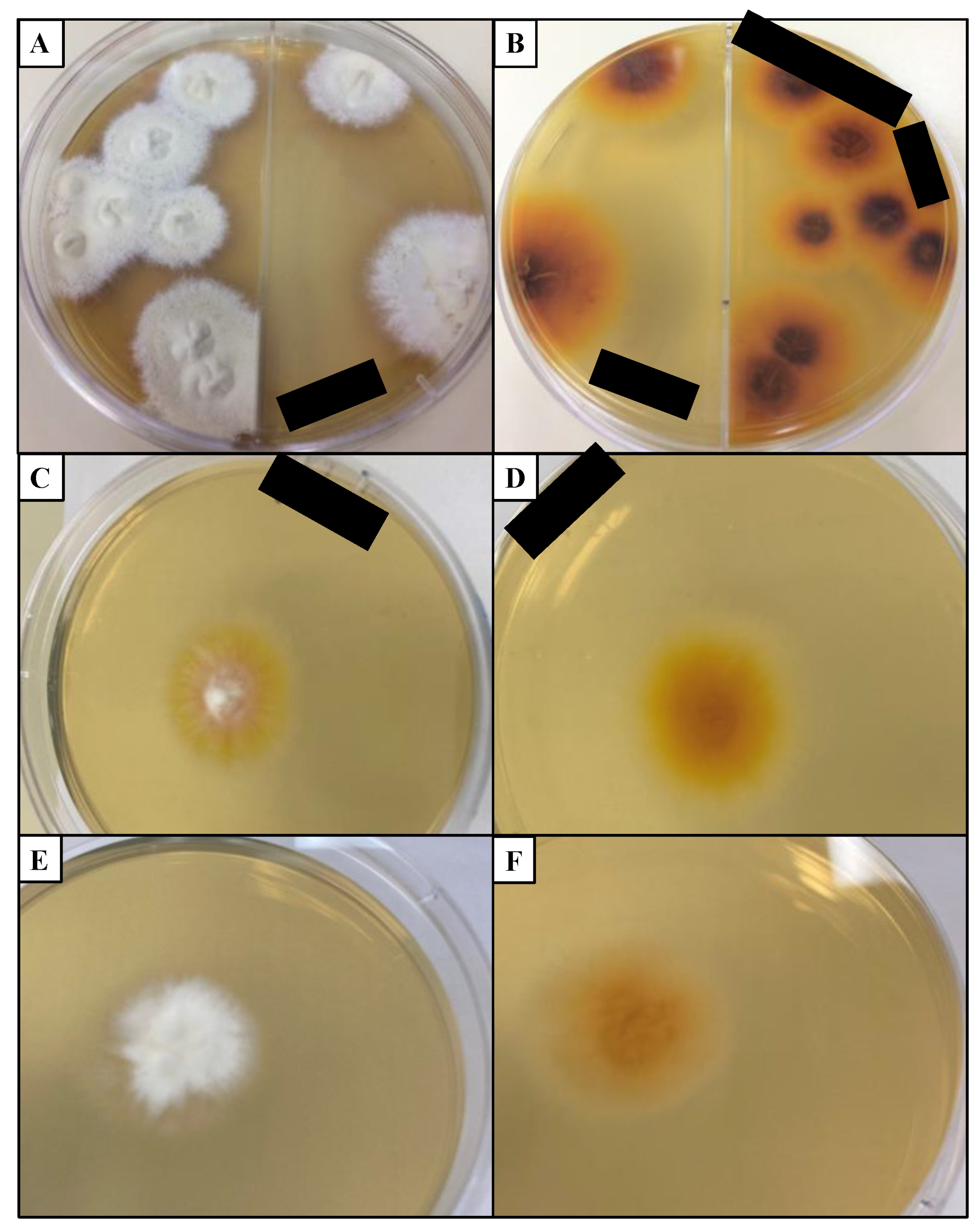
4.4. Fungal Culture
4.5. DNA-Based Assays
4.6. Antibody-Based Assays
4.7. Mass Spectrometry
5. Potential Targets of Diagnostic Assays for Dermatophytosis
5.1. Unique Dermatophytic Keratin Metabolism, Sulfite Efflux Pump (SSU1)
5.2. UV Fluorescent Metabolites
5.3. Dermatophyte-Specific Proteases
6. Introduction to Microsporum canis
6.1. Morphology and Laboratory Characteristics of M. canis
6.2. M. canis Habitat and Transmission
6.3. M. canis Distribution
6.4. M. canis Mating Types
6.5. M. canis-Associated Clinical Disease
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the Epidemiology of Dermatophyte Infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J. Tinea Capitis: Current Status. Mycopathol. Et Mycol. Appl. 2017, 182, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.A.; Dinehart, S.M.; Farmer, E.R.; Goltz, R.W.; Graham, G.F.; Hordinsky, M.K.; Lewis, C.W.; Pariser, D.M.; Skouge, J.W.; Webster, S.B.; et al. Guidelines of care for superficial mycotic infectionsof the skin: Tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. J. Am. Acad. Dermatol. 1996, 34, 282–286. [Google Scholar] [CrossRef]
- Nussipov, Y.; Markabayeva, A.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Lotti, J.; Roccia, M.G.; Fioranelli, M.; Lotti, T. Clinical and Epidemiological Features of Dermatophyte Infections in Almaty, Kazakhstan. Open Access Maced. J. Med. Sci. 2017, 5, 409–413. [Google Scholar] [CrossRef]
- Gräser, Y.; Scott, J.; Summerbell, R. The New Species Concept in Dermatophytes—a Polyphasic Approach. Mycopathologia 2008, 166, 239–256. [Google Scholar] [CrossRef]
- Garg, J.; Tilak, R.; Garg, A.; Prakash, P.; Gulati, A.K.; Nath, G. Rapid detection of dermatophytes from skin and hair. BMC Res. Notes 2009, 2, 60. [Google Scholar] [CrossRef]
- Nweze, E.; Eke, I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2017, 56, 13–28. [Google Scholar] [CrossRef]
- Richard, J.L.; Debey, M.C.; Chermette, R.; Pier, A.C.; Hasegawa, A.; Lund, A.; Bratberg, A.M.; Padhye, A.A.; Connole, M.D. Advances in veterinary mycology. J. Med Vet. Mycol. 1994, 32, 169–187. [Google Scholar] [CrossRef]
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J. Fungi 2015, 2, 4. [Google Scholar] [CrossRef]
- Marconi, V.C.; Kradin, R.; Marty, F.M.; Hospenthal, D.R.; Kotton, C.N. Disseminated dermatophytosis in a patient with hereditary hemochromatosis and hepatic cirrhosis: Case report and review of the literature. Med. Mycol. 2010, 48, 518–527. [Google Scholar] [CrossRef]
- Peres, N.T.; Maranhao, F.C.; Rossi, A.; Martinez-Rossi, N.M. Dermatophytes: Host-pathogen interaction and antifungal resistance. An. Bras. Dermatol. 2010, 85, 657–667. [Google Scholar] [CrossRef]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2018, 68, 1791–1797. [Google Scholar] [CrossRef]
- Gräser, Y.; El Fari, M.; Vilgalys, R.; Kuijpers, A.F.A.; De Hoog, G.S.; Presber, W.; Tietz, H.J. Phylogeny and taxonomy of the family Arthrodermataceae (dermatophytes) using sequence analysis of the ribosomal ITS region. Med. Mycol. 1999, 37, 105–114. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Moriello, K.A.; Coyner, K.; Paterson, S.; Mignon, B. Diagnosis and treatment of dermatophytosis in dogs and cats. Vet. Dermatol. 2017, 28, 266–268. [Google Scholar] [CrossRef]
- Graser, Y.; El Fari, M.; Presber, W.; Kuijpers, A.F.A.; De Hoog, G.S. Molecular and conventional taxonomy of the Microsporum canis complex. Med. Mycol. 2000, 38, 143–153. [Google Scholar] [CrossRef]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Segal, E.; Elad, D. Human and Zoonotic Dermatophytoses: Epidemiological Aspects. Front. Microbiol. 2021, 12, 713532. [Google Scholar] [CrossRef]
- Taylor, J.W. One Fungus = One Name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus 2011, 2, 113–120. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Crous, P.W.; Redhead, S.A.; Reynolds, D.R.; Samson, R.A.; Seifert, K.A.; Taylor, J.W.; Wingfield, M.J.; Abaci, O.; Aime, C.; et al. The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2011, 2, 105–111. [Google Scholar] [CrossRef]
- Baert, F.; Stubbe, D.; D’Hooge, E.; Packeu, A.; Hendrickx, M. Updating the Taxonomy of Dermatophytes of the BCCM/IHEM Collection According to the New Standard: A Phylogenetic Approach. Mycopathologia 2020, 185, 161–168. [Google Scholar] [CrossRef]
- Martinez, D.A.; Oliver, B.G.; Gräser, Y.; Goldberg, J.M.; Li, W.; Martinez-Rossi, N.M.; Monod, M.; Shelest, E.; Barton, R.C.; Birch, E.; et al. Comparative Genome Analysis of Trichophyton rubrum and Related Dermatophytes Reveals Candidate Genes Involved in Infection. MBio 2012, 3, e00259. [Google Scholar] [CrossRef]
- White, T.; Findley, K.; Dawson, T.; Scheynius, A.; Boekhout, T.; Cuomo, C.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef]
- Achterman, R.R.; Smith, A.R.; Oliver, B.G.; White, T.C. Sequenced dermatophyte strains: Growth rate, conidiation, drug susceptibilities, and virulence in an invertebrate model. Fungal Genet. Biol. 2011, 48, 335–341. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Yang, F.; Liu, T.; Leng, W.; Chu, Y.; Jin, Q. Recent dermatophyte divergence revealed by comparative and phylogenetic analysis of mitochondrial genomes. BMC Genom. 2009, 10, 238. [Google Scholar] [CrossRef]
- Jackson, C.J.; Barton, R.C.; Evans, E.G. Species identification and strain differentiation of dermatophyte fungi by analysis of ribosomal-DNA intergenic spacer regions. J. Clin. Microbiol. 1999, 37, 931–936. [Google Scholar] [CrossRef]
- Mochizuki, T.; Sugie, N.; Uehara, M. Random amplification of polymorphic DNA is useful for the differentiation of several anthropophilic dermatophytes. Mycoses 1997, 40, 405–409. [Google Scholar] [CrossRef]
- Kim, J.A.; Takahashi, Y.; Tanaka, R.; Fukushima, K.; Nishimura, K.; Miyaji, M. Identification and subtyping of Trichophyton mentagrophytes by random amplified polymorphic DNA. Mycoses 2001, 44, 157–165. [Google Scholar] [CrossRef]
- Sharma, R.; de Hoog, S.; Presber, W.; Gräser, Y. A virulent genotype of Microsporum canis is responsible for the majority of human infections. J. Med. Microbiol. 2007, 56, 1377–1385. [Google Scholar] [CrossRef]
- Pasquetti, M.; Peano, A.; Soglia, D.; Min, A.R.M.; Pankewitz, F.; Ohst, T.; Gräser, Y. Development and validation of a microsatellite marker-based method for tracing infections by Microsporum canis. J. Dermatol. Sci. 2013, 70, 123–129. [Google Scholar] [CrossRef] [PubMed]
- da Costa, F.V.A.; Farias, M.R.; Bier, D.; de Andrade, C.P.; de Castro, L.A.; da Silva, S.C.; Ferreiro, L. Genetic variability in Microsporum canis isolated from cats, dogs and humans in Brazil. Mycoses 2013, 56, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.; Rossi, A.; Sanches, P.R.; Bortolossi, J.C.; Martinez-Rossi, N.M. Comprehensive analysis of the dermatophyte Trichophyton rubrum transcriptional profile reveals dynamic metabolic modulation. Biochem. J. 2020, 477, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Anzawa, K.; Mochizuki, T. Molecular Epidemiology of Japanese Isolates of Microsporum canis Based on Multilocus Microsatellite Typing Fragment Analysis. Jpn. J. Infect. Dis. 2017, 70, 544–548. [Google Scholar] [CrossRef]
- Aneke, C.I.; Čmoková, A.; Hubka, V.; Rhimi, W.; Otranto, D.; Cafarchia, C. Subtyping Options for Microsporum canis Using Microsatellites and MLST: A Case Study from Southern Italy. Pathogens 2021, 11, 4. [Google Scholar] [CrossRef]
- Yamada, S.; Anzawa, K.; Mochizuki, T. Molecular Epidemiology of Microsporum canis Isolated from Japanese Cats and Dogs, and from Pet Owners by Multilocus Microsatellite Typing Fragment Analysis. Jpn. J. Infect. Diseases. 2022, 75, 105–113. [Google Scholar] [CrossRef]
- Moskaluk, A.; Darlington, L.; Kuhn, S.; Behzadi, E.; Gagne, R.B.; Kozakiewicz, C.P.; VandeWoude, S. Genetic Characterization of Microsporum canis Clinical Isolates in the United States. J. Fungi 2022, 8, 676. [Google Scholar] [CrossRef]
- Samanta, I. Veterinary Mycology; Springer: New Delhi, India, 2015. [Google Scholar]
- Roberson, R. Subcellular structure and behaviour in fungal hyphae. J. Microsc. 2020, 280, 75–85. [Google Scholar] [CrossRef]
- Brand, A.; Gow, N.A. Mechanisms of hypha orientation of fungi. Curr. Opin. Microbiol. 2009, 12, 350–357. [Google Scholar] [CrossRef]
- Köhler, J.R.; Casadevall, A.; Perfect, J. The Spectrum of Fungi That Infects Humans. Cold Spring Harb. Perspect. Med. 2014, 5, a019273. [Google Scholar] [CrossRef]
- Zurita, J.; Hay, R.J. Adherence of Dermatophyte Microconidia and Arthroconidia to Human Keratinocytes In Vitro. J. Investig. Dermatol. 1987, 89, 529–534. [Google Scholar] [CrossRef]
- Vermout, S.; Tabart, J.; Baldo, A.; Mathy, A.; Losson, B.; Mignon, B. Pathogenesis of Dermatophytosis. Mycopathologia 2008, 166, 267–275. [Google Scholar] [CrossRef]
- Baldo, A.; Monod, M.; Mathy, A.; Cambier, L.; Bagut, E.T.; Defaweux, V.; Symoens, F.; Antoine, N.; Mignon, B. Mechanisms of skin adherence and invasion by dermatophytes. Mycoses 2012, 55, 218–223. [Google Scholar] [CrossRef]
- Kasperova, A.; Kunert, J.; Raska, M. The possible role of dermatophyte cysteine dioxygenase in keratin degradation. Med. Mycol. 2013, 51, 449–454. [Google Scholar] [CrossRef]
- Aljabre, S.H.; Richardson, M.D.; Scott, E.M.; Shankland, G.S. Germination of Trichophyton mentagrophytes on human stratum corneum in vitro. J. Med. Vet. Mycol. Bi-Mon. Publ. Int. Soc. Hum. Anim. Mycol. 1992, 30, 145–152. [Google Scholar]
- Duek, L.; Kaufman, G.; Ulman, Y.; Berdicevsky, I. The pathogenesis of dermatophyte infections in human skin sections. J. Infect. 2004, 48, 175–180. [Google Scholar] [CrossRef]
- Ziółkowska, G.; Nowakiewicz, A.; Gnat, S.; Trościańczyk, A.; Zięba, P.; Majer Dziedzic, B. Molecular identification and classification of Trichophyton mentagrophytes complex strains isolated from humans and selected animal species. Mycoses 2015, 58, 119–126. [Google Scholar] [CrossRef]
- Gnat, S.; Nowakiewicz, A.; Łagowski, D.; Zięba, P. Host- and pathogen-dependent susceptibility and predisposition to dermatophytosis. J. Med. Microbiol. 2019, 68, 823–836. [Google Scholar] [CrossRef]
- DeBoer, D.J.; Moriello, K.A. Development of an experimental model of Microsporum canis infection in cats. Vet. Microbiol. 1994, 42, 289–295. [Google Scholar] [CrossRef]
- Yamada, S.; Anzawa, K.; Mochizuki, T. An Epidemiological Study of Feline and Canine Dermatophytoses in Japan. Med. Mycol. J. 2019, 60, 39–44. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Bosco, S.d.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56, 165–187. [Google Scholar] [CrossRef]
- Courtellemont, L.; Chevrier, S.; Degeilh, B.; Belaz, S.; Gangneux, J.-P.; Robert-Gangneux, F. Epidemiology of Trichophyton verrucosum infection in Rennes University Hospital, France: A 12-year retrospective study. Med. Mycol. 2017, 55, 720–724. [Google Scholar]
- Segal, E.; Frenkel, M. Dermatophyte infections in environmental contexts. Res. Microbiol. 2015, 166, 564–569. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Afshar, P.; Shokohi, T.; Aghili, R. A study on tinea gladiatorum in young wrestlers and dermatophyte contamination of wrestling mats from Sari, Iran. Br. J. Sports Med. 2007, 41, 332–334. [Google Scholar] [CrossRef]
- Nowicka, D.; Nawrot, U.; Włodarczyk, K.; Pajączkowska, M.; Patrzałek, A.; Pęcak, A.; Mozdyniewicz, P.; Fleischer, M. Detection of dermatophytes in human nail and skin dust produced during podiatric treatments in people without typical clinical signs of mycoses. Mycoses 2016, 59, 379–382. [Google Scholar] [CrossRef]
- Guirges, S.Y. Viability of Trichophyton schoenleinii in epilated hairs. Sabouraudia 1981, 19, 155–156. [Google Scholar] [CrossRef]
- McPherson, E. The influence of physical factors on dermatomycosis in domestic animals. Vet. Record. 1957, 69, 1010–1013. [Google Scholar]
- Rosenthal, S.A.; Vanbreuseghem, R. Viability of dermatophytes in epilated hairs. Arch. Dermatol. 1962, 85, 103–105. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Werrett, G.; Stokes, C.R.; Gruffydd-Jones, T.J. Microsporum canis: Inapparent carriage by cats and the viability of arthrospores. J. Small Anim. Pr. 1994, 35, 397–401. [Google Scholar] [CrossRef]
- Baker, M.; Jeffries, P. Use of Commercially Available Cryogenic Vials for Long-Term Preservation of Dermatophyte Fungi. J. Clin. Microbiol. 2006, 44, 617–618. [Google Scholar] [CrossRef]
- García-Martínez, J.; Lacomba, D.L.; Pascual, A.C. Evaluation of a Method for Long-Term Cryopreservation of Fungal Strains. Biopreservation Biobanking 2018, 16, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.A.; Bekker-Holtman, J. Viability of lyophilized fungal cultures. Antonie Van Leeuwenhoek 1976, 42, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Kwolek, W.; Haynes, W. Investigation of ultralow temperature for fungal cultures III. Viability and growth rate of mycelial cultures following cryogenic storage. Mycologia 1976, 68, 377–387. [Google Scholar] [CrossRef]
- Meyer, E. The preservation of dermatophytes at sub-freezing temperatures. Mycologia 1955, 47, 664–668. [Google Scholar] [CrossRef]
- Pasarell, L.; McGinnis, M.R. Viability of fungal cultures maintained at -70 degrees C. J. Clin. Microbiol. 1992, 30, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Stalpers, J.; Hoog, A.d.; Vlug, I.J. Improvement of the straw technique for the preservation of fungi in liquid nitrogen. Mycologia 1987, 79, 82–89. [Google Scholar] [CrossRef]
- Rippon, J.W. Medical Mycology. The Pathogenic Fungi and the Pathogenic Actinomycetes; WB Saunders Company: Philadelphia, PA, USA, 1988. [Google Scholar]
- Makimura, K.; Mochizuki, T.; Hasegawa, A.; Uchida, K.; Saito, H.; Yamaguchi, H. Phylogenetic classification of Trichophyton mentagrophytes complex strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J. Clin. Microbiol. 1998, 36, 2629–2633. [Google Scholar] [CrossRef]
- Summerbell, R.; Kushwaha, R.; Guarro, J. Biology of dermatophytes and other keratinophilic fungi. Rev. Iberoam. Micol. 2000, 44, 30–43. [Google Scholar]
- Ranganathan, S.; Balajee, S.A.M.; Raja, S.M. A survey of dermatophytosis in animals in Madras, India. Mycopathologia 1997, 140, 137–140. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Cordeiro, R.A.; Gomes, J.M.F.; Sidrim, J.J.C.; Rocha, M.F.G. Canine dermatophytosis caused by an anthropophilic species: Molecular and phenotypical characterization of Trichophyton tonsurans. J. Med. Microbiol. 2006, 55, 1583–1586. [Google Scholar] [CrossRef]
- Burstein, V.L.; Beccacece, I.; Guasconi, L.; Mena, C.J.; Cervi, L.; Chiapello, L.S. Skin Immunity to Dermatophytes: From Experimental Infection Models to Human Disease. Front. Immunol. 2020, 11, 605644. [Google Scholar] [CrossRef]
- Kidd, S.; Halliday, C.L.; Alexiou, H.; Ellis, D.H. Descriptions of Medical Fungi; CutCut Digital: Mile End, Australia, 2014. [Google Scholar]
- Kosanke, S.; Hamann, L.; Kupsch, C.; Garcia, S.M.; Chopra, A.; Gräser, Y. Unequal distribution of the mating type (MAT) locus idiomorphs in dermatophyte species. Fungal Genet. Biol. 2018, 118, 45–53. [Google Scholar] [CrossRef]
- White, T.C.; Oliver, B.G.; Gräser, Y.; Henn, M.R. Generating and Testing Molecular Hypotheses in the Dermatophytes. Eukaryot. Cell 2008, 7, 1238–1245. [Google Scholar] [CrossRef]
- Kaszubiak, A.; Klein, S.; de Hoog, G.S.; Gräser, Y. Population structure and evolutionary origins of Microsporum canis, M. ferrugineum and M. audouinii. Infect. Genet. Evol. 2004, 4, 179–186. [Google Scholar] [CrossRef]
- Hay, R.J. Dermatophytoses and Other Superficial Mycoses. In Atlas of Infectious Diseases: Fungal Infections; Mandell, G.L., Diamond, R.D., Eds.; Current Medicine Group: London, UK, 2000; pp. 191–203. [Google Scholar]
- Gupta, A.K.; Summerbell, R.C. Tinea capitis. Med. Mycol. 2000, 38, 255–287. [Google Scholar] [CrossRef]
- Daadaa, N.; Ben Tanfous, A. Favus; StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Li, H.; Wu, S.; Mao, L.; Lei, G.; Zhang, L.; Lu, A.; An, L.; Yang, G.; Abliz, P.; Meng, G. Human pathogenic fungus Trichophyton schoenleinii activates the NLRP3 inflammasome. Protein Cell. 2013, 4, 529–538. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatology. 2019, 80, 835–851. [Google Scholar] [CrossRef]
- Bodman, M.A.; Krishnamurthy, K. Onychomycosis; StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Bonifaz, A.; Ramírez-Tamayo, T.; Saúl, A. Tinea barbae (tinea sycosis): Experience with nine cases. J. Dermatol. 2003, 30, 898–903. [Google Scholar] [CrossRef]
- Rutecki, G.W.; Wurtz, R.; Thomson, R.B. From Animal to Man: Tinea Barbae. Curr. Infect. Dis. Rep. 2000, 2, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Lam, J.M.; Leong, K.F.; Hon, K.L. Tinea corporis: An updated review. Drugs Context. 2020, 9, 2020-5-6. [Google Scholar] [CrossRef]
- Belhadjali, H.; Aounallah, A.; Youssef, M.; Gorcii, M.; Babba, H.; Zili, J. Tinea faciei, underrecognized because clinically misleading. 14 cases. Presse Med. 2009, 38, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Atzori, L.; Aste, N.; Aste, N.; Pau, M. Tinea Faciei Due to Microsporum canis in Children: A Survey of 46 Cases in the District of Cagliari (Italy). Pediatr. Dermatol. 2012, 29, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Alteras, I.; Sandbank, M.; David, M.; Segal, R. 15-Year Survey of Tinea faciei in the Adult. Dermatology 1988, 177, 65–69. [Google Scholar] [CrossRef]
- Chamorro, M.J.; House, S.A. Tinea Manuum; StatPearls Publishing LLC: Tampa, FL, USA, 2021. [Google Scholar]
- Veraldi, S.; Schianchi, R.; Benzecry, V.; Gorani, A. Tinea manuum: A report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses 2019, 62, 604–608. [Google Scholar] [CrossRef]
- Taghipour, S.; Pchelin, I.M.; Mahmoudabadi, A.Z.; Ansari, S.; Katiraee, F.; Rafiei, A.; Shokohi, T.; Abastabar, M.; Taraskina, A.E.; Kermani, F.; et al. Trichophyton mentagrophytes and T interdigitale genotypes are associated with particular geographic areas and clinical manifestations. Mycoses 2019, 62, 1084–1091. [Google Scholar] [CrossRef]
- Pier, A.C.; Smith, J.M.B.; Alexiou, H.; Ellis, D.H.; Lund, A.; Pritchard, R.C. Animal ringworm—its aetiology, public health significance and control. J. Med. Vet. Mycol. 1994, 32, 133–150. [Google Scholar] [CrossRef]
- Krzyściak, P.; Al-Hatmi, A.M.S.; Ahmed, S.A.; Macura, A.B. Rare zoonotic infection with Microsporum persicolor with literature review. Mycoses 2015, 58, 511–515. [Google Scholar] [CrossRef]
- English, M.P. Trichophyton persicolor infection in the field vole and pipistrelle bat. Sabouraudia 1966, 4, 219–222. [Google Scholar] [CrossRef]
- Begum, J.; Kumar, R. Prevalence of dermatophytosis in animals and antifungal susceptibility testing of isolated Trichophyton and Microsporum species. Trop. Anim. Health Prod. 2020, 53, 3. [Google Scholar] [CrossRef]
- Veraldi, S.; Genovese, G.; Peano, A. Tinea corporis caused by Trichophyton equinum in a rider and review of the literature. Infection 2018, 46, 135–137. [Google Scholar] [CrossRef]
- Moretti, A.; Boncio, L.; Pasquali, P.; Fioretti, D.P. Epidemiological Aspects of Dermatophyte Infections in Horses and Cattle. J. Vet.-Med. B. 1998, 45, 205–208. [Google Scholar] [CrossRef]
- Chermette, R.; Ferreiro, L.; Guillot, J. Dermatophytoses in Animals. Mycopathologia 2008, 166, 385–405. [Google Scholar] [CrossRef]
- García-Agudo, L.; Espinosa-Ruiz, J.J. Tinea capitis by Microsporum gypseum, an infrequent species. Arch. Argent. Pediatr. 2018, 116, e296–e299. [Google Scholar]
- Verma, S.B.; Panda, S.; Nenoff, P.; Singal, A.; Rudramurthy, S.M.; Uhrlass, S.; Das, A.; Bisherwal, K.; Shaw, D.; Vasani, R. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 468–482. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide—A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef]
- Verma, S.B. Emergence of recalcitrant dermatophytosis in India. Lancet Infect. Dis. 2018, 18, 718–719. [Google Scholar] [CrossRef]
- Brasch, J.; Gräser, Y.; Beck-Jendroscheck, V.; Voss, K.; Torz, K.; Walther, G.; Schwarz, T. “Indian” strains of Trichophyton mentagrophytes with reduced itraconazole susceptibility in Germany. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2021, 19, 1723–1727. [Google Scholar] [CrossRef]
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Méd. 2015, 25, e44–e58. [Google Scholar] [CrossRef]
- Rees, R.G. Keratinophilic fungi from Queensland. I. Isolations from animal hair and scales. Sabouraudia 1967, 5, 165–172. [Google Scholar] [CrossRef]
- Rees, R.G. Keratinophilic Fungi from Queensland—II. Isolations from Feathers of Wild Birds. Sabouraudia 1968, 6, 14–18. [Google Scholar] [CrossRef]
- Cabañes, F. Dermatophytes in domestic animals. Rev. Iberoam. Micol. 2000, 17, 104–108. [Google Scholar]
- Dolenc-Voljč, M.; Gasparič, J. Human Infections with Microsporum gypseum Complex (Nannizzia gypsea) in Slovenia. Mycopathologia 2017, 182, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Outerbridge, C.A. Mycologic Disorders of the Skin. Clin. Tech. Small Anim. Pr. 2006, 21, 128–134. [Google Scholar] [CrossRef]
- Mackenzie, D.W.R. “Hairbrush Diagnosis” in Detection and Eradication of Non-fluorescent Scalp Ringworm. BMJ 1963, 2, 363–365. [Google Scholar] [CrossRef]
- Lim, S.S.; Shin, K.; Mun, J. Dermoscopy for cutaneous fungal infections: A brief review. Heal. Sci. Rep. 2022, 5, e464. [Google Scholar] [CrossRef]
- Lallas, A.; Kyrgidis, A.; Tzellos, T.; Apalla, Z.; Karakyriou, E.; Karatolias, A.; Lefaki, I.; Sotiriou, E.; Ioannides, D.; Argenziano, G.; et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br. J. Dermatol. 2012, 166, 1198–1205. [Google Scholar] [CrossRef]
- Bhat, Y.J.; Keen, A.; Hassan, I.; Latif, I.; Bashir, S. Can dermoscopy serve as a diagnostic tool in dermatophytosis? A pilot study. Indian Dermatol. Online J. 2019, 10, 530–535. [Google Scholar] [CrossRef]
- Piliouras, P.; Buettner, P.; Soyer, H.P. Dermoscopy use in the next generation: A survey of Australian dermatology trainees. Australas. J. Dermatol. 2014, 55, 49–52. [Google Scholar] [CrossRef]
- Zanna, G.; Auriemma, E.; Arrighi, S.; Attanasi, A.; Zini, E.; Scarampella, F. Dermoscopic evaluation of skin in healthy cats. Vet. Dermatol. 2015, 26, 14–17, 3–4. [Google Scholar] [CrossRef]
- Scarampella, F.; Zanna, G.; Peano, A.; Fabbri, E.; Tosti, A. Dermoscopic features in 12 cats with dermatophytosis and in 12 cats with self-induced alopecia due to other causes: An observational descriptive study. Vet. Dermatol. 2015, 26, 282-e63. [Google Scholar] [CrossRef]
- Dong, C.; Angus, J.; Scarampella, F.; Neradilek, M. Evaluation of dermoscopy in the diagnosis of naturally occurring dermatophytosis in cats. Vet. Dermatol. 2016, 27, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Asawanonda, P.; Taylor, C.R. Wood’s light in dermatology. Int. J. Dermatol. 1999, 38, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Klatte, J.L.; van der Beek, N.; Kemperman, P.M.J.H. 100 years of Wood’s lamp revised. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Moriello, K.A. Diagnostic techniques for dermatophytosis. Clin. Tech. Small Anim. Pract. 2001, 16, 219–224. [Google Scholar] [CrossRef]
- Begum, J.; Mir, N.A.; Lingaraju, M.C.; Buyamayum, B.; Dev, K. Recent advances in the diagnosis of dermatophytosis. J. Basic Microbiol. 2020, 60, 293–303. [Google Scholar] [CrossRef]
- Moriello, K. Feline dermatophytosis: Aspects pertinent to disease management in single and multiple cat situations. J. Feline Med. Surg. 2014, 16, 419–431. [Google Scholar] [CrossRef]
- Frymus, T.; Gruffydd-Jones, T.; Pennisi, M.G.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Dermatophytosis in Cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 598–604. [Google Scholar] [CrossRef]
- Arabatzis, M.; Van Coppenraet, L.B.; Kuijper, E.; De Hoog, G.; Lavrijsen, A.; Templeton, K.; Van Der Raaij-Helmer, E.; Velegraki, A.; Gräser, Y.; Summerbell, R. Diagnosis of common dermatophyte infections by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br. J. Dermatol. 2007, 157, 681–689. [Google Scholar] [CrossRef]
- Jacobson, L.S.; McIntyre, L.; Mykusz, J. Comparison of real-time PCR with fungal culture for the diagnosis of Microsporum canis dermatophytosis in shelter cats: A field study. J. Feline Med. Surg. 2017, 20, 103–107. [Google Scholar] [CrossRef]
- Peano, A.; Rambozzi, L.; Gallo, M.G. Development of an enzyme-linked immunosorbant assay (ELISA) for the serodiagnosis of canine dermatophytosis caused by Microsporum canis. Vet. Dermatol. 2005, 16, 102–107. [Google Scholar] [CrossRef]
- Santana, A.E.; Taborda, C.P.; Severo, J.S.; Rittner, G.M.G.; Muñoz, J.E.; Larsson, C.E., Jr.; Larsson, C.E. Development of enzyme immunoassays (ELISA and Western blot) for the serological diagnosis of dermatophytosis in symptomatic and asymptomatic cats. Med. Mycol. 2018, 56, 95–102. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef]
- Theel, E.S.; Hall, L.; Mandrekar, J.; Wengenack, N.L. Dermatophyte Identification Using Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2011, 49, 4067–4071. [Google Scholar] [CrossRef]
- Nenoff, P.; Erhard, M.; Simon, J.C.; Muylowa, G.K.; Herrmann, J.; Rataj, W.; Gräser, Y. MALDI-TOF mass spectrometry—A rapid method for the identification of dermatophyte species. Med. Mycol. 2013, 51, 17–24. [Google Scholar] [CrossRef]
- De Respinis, S.; Tonolla, M.; Pranghofer, S.; Petrini, L.; Petrini, O.; Bosshard, P. Identification of dermatophytes by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Med. Mycol. 2013, 51, 514–521. [Google Scholar] [CrossRef]
- Turin, L.; Riva, F.; Galbiati, G.; Cainelli, T. Fast, simple and highly sensitive double-rounded polymerase chain reaction assay to detect medically relevant fungi in dermatological specimens. Eur. J. Clin. Investig. 2000, 30, 511–518. [Google Scholar] [CrossRef]
- Kaplan, W.; Ajello, L. Oral treatment of spontaneous ringworm in cats with griseofulvin. J. Am. Vet.-Med. Assoc. 1959, 135, 253–261. [Google Scholar]
- Kaplan, W.; Ajello, L. Therapy of spontaneous ringworm in cats with orally administered griseofulvin. Arch. Dermatol. 1960, 81, 714–723. [Google Scholar] [CrossRef]
- Cafarchia, C.; Romito, D.; Sasanelli, M.; Lia, R.P.; Capelli, G.; Otranto, D. The epidemiology of canine and feline dermatophytoses in southern Italy. Zur Epidemiologie der Dermatophytose von Hund und Katze im Suden Italiens. Mycoses 2004, 47, 508–513. [Google Scholar] [CrossRef]
- Kaplan, W.; Georg, L.K.; Ajello, L. Recent developments in animal ringworm and their public health implications. Ann. N. Y. Acad. Sci. 1958, 70, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.D.; Smith, O.G. Ringworm in a Siamese Cattery. Can. Vet. J. La Rev. Vet. Can 1960, 1, 412–415. [Google Scholar] [PubMed]
- Keep, J.M. The epidemiology and control op microsporum canis bodin in a cat community. Aust. Vet. J. 1959, 35, 374–378. [Google Scholar] [CrossRef]
- Guillot, J.; Rojzner, K.; Fournier, C.; Touati, F.; Chermette, R.; Malandain, E.; Jankowski, F.; Seewald, W.; Schenker, R. Evaluation of the efficacy of oral lufenuron combined with topical enilconazole for the management of dermatophytosis in catteries. Vet. Rec. 2002, 150, 714–718. [Google Scholar] [CrossRef]
- DeBoer, D.J.; Moriello, K.A. Inability of two topical treatments to influence the course of experimentally induced dermatophytosis in cats. J. Am. Vet. Med. Assoc. 1995, 207, 52–57. [Google Scholar]
- Mancianti, F.; Dabizzi, S.; Nardoni, S. A lufenuron pre-treatment may enhance the effects of enilconazole or griseofulvin in feline dermatophytosis? J. Feline Med. Surg. 2009, 11, 91–95. [Google Scholar] [CrossRef]
- Newbury, S.; Moriello, K.; Verbrugge, M.; Thomas, C. Use of lime sulphur and itraconazole to treat shelter cats naturally infected with Microsporum canis in an annex facility: An open field trial. Vet. Dermatol. 2007, 18, 324–331. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Robinson, A.; MacKay, A.D.; Shaw, S.E. A study of the efficacy of topical and systemic therapy for the treatment of feline Microsporum canis infection. J. Feline Med. Surg. 2000, 2, 135–142. [Google Scholar] [CrossRef]
- Aslanzadeh, J.; Roberts, G.D. Direct microscopic examination of clinical specimens for the laboratory diagnosis of fungal infections. Clin. Microbiol. Newsl. 1991, 13, 185–188. [Google Scholar] [CrossRef]
- Jang, S.; Walker, R. Laboratory diagnosis of fungal and algal infections. GREENE CE. Infect. Dis. Dog Cat. 2006, 3, 533–542. [Google Scholar]
- Gupta, A.K.; Cooper, E.A. Dermatophytosis (Tinea) and Other Superficial Fungal Infections. In Diagnosis and Treatment of Human Mycoses; Hospenthal, D.R., Rinaldi, M.G., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 355–381. [Google Scholar]
- Leck, A. Preparation of lactophenol cotton blue slide mounts. Community Eye Health 1999, 12, 24. [Google Scholar]
- Wang, M.Z.; Guo, R.; Lehman, J.S. Correlation between histopathologic features and likelihood of identifying superficial dermatophytosis with periodic acid Schiff-diastase staining: A cohort study. J. Cutan. Pathol. 2017, 44, 152–157. [Google Scholar] [CrossRef]
- Park, Y.W.; Kim, D.Y.; Yoon, S.Y.; Park, G.Y.; Park, H.S.; Yoon, H.-S.; Cho, S. ‘Clues’ for the histological diagnosis of tinea: How reliable are they? Ann. Dermatol. 2014, 26, 286–288. [Google Scholar] [CrossRef]
- Poluri, L.V.; Indugula, J.P.; Kondapaneni, S.L. Clinicomycological Study of Dermatophytosis in South India. J. Lab. Physicians 2015, 7, 84–89. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Gruffydd-Jones, T.J.; Shaw, S.E.; Wright, A.I.; Stokes, C.R. Epidemiological and diagnostic features of canine and feline dermatophytosis in the United Kingdom from 1956 to 1991. Vet. Rec. 1993, 133, 57–61. [Google Scholar] [CrossRef]
- Guillot, J.; Latié, L.; Deville, M.; Halos, L.; Chermette, R. Evaluation of the dermatophyte test medium RapidVet-D. Vet. Dermatol. 2001, 12, 123–127. [Google Scholar] [CrossRef]
- Elwart, O.E.; Pieper, J.B.; Oh, S.; Wilcoxen, T.E.; Hoyer, L.L. Effect of light exposure on growth rate of veterinary clinical dermatophyte isolates. Vet. Dermatol. 2021, 32, 234–261. [Google Scholar] [CrossRef]
- Moriello, K.A.; Verbrugge, M.J.; Kesting, R.A. Effects of temperature variations and light exposure on the time to growth of dermatophytes using six different fungal culture media inoculated with laboratory strains and samples obtained from infected cats. J. Feline Med. Surg. 2010, 12, 988–990. [Google Scholar] [CrossRef]
- Morris, A.J.; Byrne, T.C.; Madden, J.F.; Reller, L.B. Duration of incubation of fungal cultures. J. Clin. Microbiol. 1996, 34, 1583–1585. [Google Scholar] [CrossRef]
- Brillowska-Dabrowska, A.; Michałek, E.; Saunte, D.M.L.; Søgaard Nielsen, S.; Arendrup, M.C. PCR test for Microsporum canis identification. Med. Mycol. 2013, 51, 576–579. [Google Scholar] [CrossRef]
- Wickes, B.L.; Wiederhold, N.P. Molecular diagnostics in medical mycology. Nat. Commun. 2018, 9, 5135. [Google Scholar] [CrossRef] [PubMed]
- Brillowska-Dąbrowska, A. DNA Preparation from Nail Samples; W.I.P. Organization: Copenhagen, Denmark, 2006. [Google Scholar]
- Bergman, A.; Heimer, D.; Kondori, N.; Enroth, H. Fast and specific dermatophyte detection by automated DNA extraction and real-time PCR. Clin. Microbiol. Infect. 2013, 19, E205–E211. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, A.; van der Ent, M.; Klaassen, A.; Böhm, N.; Andriesse, G.; Wintermans, R. Evaluation of a single-tube real-time PCR for detection and identification of 11 dermatophyte species in clinical material. Clin. Microbiol. Infect. 2010, 16, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Moskaluk, A.; Nehring, M.; VandeWoude, S. Serum Samples from Co-Infected and Domestic Cat Field Isolates Nonspecifically Bind FIV and Other Antigens in Enzyme-Linked Immunosorbent Assays. Pathogens 2021, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Magnarelli, L.A.; Anderson, J.F.; Barbour, A.G. Enzyme-Linked Immunosorbent Assays for Lyme Disease: Reactivity of Subunits of Borrelia burgdorferi. J. Infect. Dis. 1989, 159, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lagousi, T.; Routsias, J.; Spoulou, V. Development of an Enzyme-Linked Immunosorbent Assay (ELISA) for Accurate and Prompt Coronavirus Disease 2019 (COVID-19) Diagnosis Using the Rational Selection of Serological Biomarkers. Diagnostics 2021, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.W.; Belem, Z.R.; Lemos, E.A.; Reed, S.G.; Campos-Neto, A. Enzyme-Linked Immunosorbent Assay for Serological Diagnosis of Chagas’ Disease Employing a Trypanosoma cruzi Recombinant Antigen That Consists of Four Different Peptides. J. Clin. Microbiol. 2001, 39, 4390–4395. [Google Scholar] [CrossRef]
- Soderstrom, C.I.; Spriggs, F.P.; Song, W.; Burrell, S. Comparison of four distinct detection platforms using multiple ligand binding assay formats. J. Immunol. Methods. 2011, 371, 106–113. [Google Scholar] [CrossRef]
- Sittampalam, G.S.; Smith, W.C.; Miyakawa, T.W.; Smith, D.R.; McMorris, C. Application of experimental design techniques to optimize a competitive ELISA. J. Immunol. Methods. 1996, 190, 151–161. [Google Scholar] [CrossRef]
- Grumbt, M.; Monod, M.; Yamada, T.; Hertweck, C.; Kunert, J.; Staib, P. Keratin Degradation by Dermatophytes Relies on Cysteine Dioxygenase and a Sulfite Efflux Pump. J. Investig. Dermatol. 2013, 133, 1550–1555. [Google Scholar] [CrossRef]
- Léchenne, B.; Reichard, U.; Zaugg, C.; Fratti, M.; Kunert, J.; Boulat, O.; Monod, M. Sulphite efflux pumps in Aspergillus fumigatus and dermatophytes. Microbiology 2007, 153, 905–913. [Google Scholar] [CrossRef]
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermatophytes. Med. Mycol. 2018, 57, 13–22. [Google Scholar] [CrossRef]
- Griffith, O.W. Cysteinesulfinate metabolism. altered partitioning between transamination and decarboxylation following administration of beta-methyleneaspartate. J. Biol. Chem. 1983, 258, 1591–1598. [Google Scholar] [CrossRef]
- Irwin, S.V.; Fisher, P.; Graham, E.; Malek, A.; Robidoux, A. Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLoS ONE 2017, 12, e0186629. [Google Scholar] [CrossRef]
- Wolf, F.T.; Jones, E.A.; Nathan, H.A. Fluorescent Pigment of Microsporum. Nature 1958, 182, 475–476. [Google Scholar] [CrossRef]
- Wolf, F.T. Chemical Nature of the Fluorescent Pigment produced in Microsporum-infected Hair. Nature 1957, 180, 860–861. [Google Scholar] [CrossRef]
- Chattaway, F.W.; Barlow, A.J. Further studies of the fluorescent compounds produced in vivo by Trichophyton schoenleinii. Sabouraudia 1966, 4, 265–272. [Google Scholar] [CrossRef]
- Monod, M. Secreted Proteases from Dermatophytes. Mycopathologia 2008, 166, 285–294. [Google Scholar] [CrossRef]
- Tran, V.D.T.; De Coi, N.; Feuermann, M.; Schmid-Siegert, E.; Băguţ, E.-T.; Mignon, B.; Waridel, P.; Peter, C.; Pradervand, S.; Pagni, M.; et al. RNA Sequencing-Based Genome Reannotation of the Dermatophyte Arthroderma Benhamiae and Characterization of Its Secretome and Whole Gene Expression Profile during Infection. Msystems 2016, 1, e00036-16. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, 343–350. [Google Scholar] [CrossRef]
- Viani, F.C.; Dos Santos, J.I.; Paula, C.R.; Larson, C.E.; Gambale, W. Production of extracellular enzymes by Microsporum canis and their role in its virulence. Med. Mycol. 2001, 39, 463–468. [Google Scholar] [CrossRef]
- Baldo, A.; Mathy, A.; Tabart, J.; Camponova, P.; Vermout, S.; Massart, L.; Maréchal, F.; Galleni, M.; Mignon, B. Secreted subtilisin Sub3 from Microsporum canis is required for adherence to but not for invasion of the epidermis. Br. J. Dermatol. 2010, 162, 990–997. [Google Scholar] [CrossRef]
- Băguţ, E.T.; Baldo, A.; Mathy, A.; Cambier, L.; Antoine, N.; Cozma, V.; Mignon, B. Subtilisin Sub3 is involved in adherence of Microsporum canis to human and animal epidermis. Vet. Microbiol. 2012, 160, 413–419. [Google Scholar] [CrossRef]
- Mignon, B.; Swinnen, M.; Bouchara, J.P.; Hofinger, M.; Nikkels, A.; Pierard, G.; Gerday, C.H.; Losson, B. Purification and characterization of a 315 kDa keratinolytic subtilisin-like serine protease from and evidence of its secretion in naturally infected cats. Med. Mycol. 1998, 36, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Baldo, A.; Tabart, J.; Vermout, S.; Mathy, A.; Collard, A.; Losson, B.; Mignon, B. Secreted subtilisins of Microsporum canis are involved in adherence of arthroconidia to feline corneocytes. J. Med. Microbiol. 2008, 57, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Usui, K. Nannizzia otae sp. nov, the perfect state of Microsporum canis Bodin. Jpn. J. Med. Mycol. 1975, 16, 148–153. [Google Scholar] [CrossRef]
- Brilhante, R.; Rocha, M.; Cordeiro, R.; Rabenhorst, S.H.B.; Granjeiro, T.; Monteiro, A.; Sidrim, J. Phenotypical and molecular characterization of Microsporum canis strains in north-east Brazil. J. Appl. Microbiol. 2005, 99, 776–782. [Google Scholar] [CrossRef] [PubMed]
- McAllister, H.A. 29—Dermatophytes and Dermatophytoses 11. In Diagnostic Procedure in Veterinary Bacteriology and Mycology, 4th ed.; Carter, G.R., Cole, J.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 381–404. [Google Scholar]
- Ajello, L.; Georg, L.K. In vitro hair cultures for differentiating between atypical isolates of Trichophyton mentagrophytes and Trichophyton rubrum. Mycopathologia 1957, 8, 3–17. [Google Scholar]
- Khosravi, A.R.; Mahmoudi, M. Dermatophytes isolated from domestic animals in Iran. Dermatophyten bei Haustieren im Iran. Mycoses 2003, 46, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Burmester, A.; Tittelbach, J.; Darr-Foit, S.; Goetze, S.; Elsner, P.; Hipler, U.C. Dermatophytosis caused by rare anthropophilic and zoophilic agents. Der Hautarzt Z. Fur Dermatol. Venerol. Und Verwandte Geb. 2019, 70, 561–574. [Google Scholar] [CrossRef]
- Brilhante, R.S.; Cavalcante, C.S.; Soares-Junior, F.A.; Cordeiro, R.A.; Sidrim, J.J.; Rocha, M.F. High rate of Microsporum canis feline and canine dermatophytoses in Northeast Brazil: Epidemiological and diagnostic features. Mycopathologia 2003, 156, 303–308. [Google Scholar] [CrossRef]
- Jungerman, P.F.; Schwartzman, R.M. Veterinary Medical Mycology; Lea and Febiger: Philadelphia, PA, USA, 1972. [Google Scholar]
- Cabañes, F.J.; Abarca, M.L.; Bragulat, M.R. Dermatophytes isolated from domestic animals in Barcelona, Spain. Mycopathologia 1997, 137, 107–113. [Google Scholar] [CrossRef]
- Stenwig, H. Isolation of dermatophytes from domestic animals in Norway. Nord. Vet. 1985, 37, 161–169. [Google Scholar]
- Cabo, J.F.G.; Asensio, M.C.B.; Rodriguez, F.G.; Lázaro, J.A.A. An outbreak of dermatophytosis in pigs caused by Microsporum canis. Mycopathologia 1995, 129, 79–80. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Ben-Ami, R.; Astman, N.; Malin, A.; Baruch, Y.; Galor, I. An Outbreak of Microsporum canis infection at a military base associated with stray cat exposure and person-to-person transmission. Mycoses 2018, 61, 472–476. [Google Scholar] [CrossRef]
- Šubelj, M.; Marinko, J.S.; Učakar, V. An outbreak of Microsporum canis in two elementary schools in a rural area around the capital city of Slovenia, 2012. Epidemiol. Infect. 2014, 142, 2662–2666. [Google Scholar] [CrossRef]
- Shah, P.C.; Krajden, S.; Kane, J.; Summerbell, R.C. Tinea corporis caused by Microsporum canis: Report of a Nosocomial outbreak. Eur. J. Epidemiol. 1988, 4, 33–38. [Google Scholar] [CrossRef]
- Snider, R.; Landers, S.; Levy, M.L. The ringworm riddle: An outbreak of Microsporum canis in the nursery. Pediatr. Infect. Dis. J. 1993, 12, 145–148. [Google Scholar] [CrossRef]
- Mackenzie, D.W. The mycological diagnostic service: A five-year survey (1959–1963). Ulst. Med. J. 1964, 33, 94–100. [Google Scholar]
- Carlier, G.I.M. A seventeen-year survey of the ringworm flora of Birmingham. J. Hyg. 1963, 61, 291–305. [Google Scholar] [CrossRef]
- Al-Fouzan, A.S.; Nanda, A.; Kubec, K. Dermatophytosis of children in Kuwait: A prospective survey. Int. J. Dermatol. 1993, 32, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Ridley, M.F.; Wilson, E.; Harrington, M. The occurrence of dermatophytes in Queensland. Australas. J. Dermatol. 1961, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Marples, M.J. Some problems in the ecology of the dermatophytes. N. Z. Med. J. 1959, 58, 64–69. [Google Scholar] [PubMed]
- Zaror, L. Dermatomycosis in the south of Chile. Rev. Med. Chile. 1974, 102, 299–302. [Google Scholar]
- Londero, A.T. Dermatomycoss in the hinterland of Rio Grande do Sul (Brazil). Dermatol. Trop. Ecol. Geogr. 1964, 35, 64–68. [Google Scholar]
- Dion, W.M.; Kapica, L. Isolation of dermatophytes, Candida species and systemic fungi from dermatologic specimens in Montréal, 1963 to 1973. Can. Med. Assoc. J. 1975, 112, 712–716. [Google Scholar]
- Ross, J.B.; Butler, R.W.; Cross, R.J.; Fardy, P.W. A retrospective study of dermatophyte infection in Newfoundland for the period 1962-1968. Can. Med. Assoc. J. 1971, 104, 492–496. [Google Scholar]
- Gaisin, A.; Holzwanger, J.M.; Leyden, J.J. Endothrix tinea capitis in Philadelphia. Int. J. Dermatol. 1977, 16, 188–190. [Google Scholar] [CrossRef]
- Mahgoub, E.S. Mycoses of the Sudan. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 184–188. [Google Scholar] [CrossRef]
- Vanbreuseghem, R.; De Vroey, C. Geographic distribution of dermatophytes. Int. J. Dermatol. 1970, 9, 102–109. [Google Scholar] [CrossRef]
- Philpot, C.M. Geographical distribution of the dermatophytes: A review. J. Hyg. 1978, 80, 301–313. [Google Scholar] [CrossRef]
- Fernandes, N.C.; Akiti, T.; Barreiros, M.G. Dermatophytoses in children: Study of 137 cases. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 83–85. [Google Scholar] [CrossRef]
- Maraki, S.; Tselentis, Y. Survey on the epidemiology of Microsporum canis infections in Crete, Greece over a 5-year period. Int. J. Dermatol. 2000, 39, 21–24. [Google Scholar] [CrossRef]
- Metin, B.; Heitman, J. She Loves Me, She Loves Me Not: On the Dualistic Asexual/Sexual Nature of Dermatophyte Fungi. Mycopathologia 2020, 185, 87–101. [Google Scholar] [CrossRef]
- Li, W.; Metin, B.; White, T.C.; Heitman, J. Organization and Evolutionary Trajectory of the Mating Type (MAT) Locus in Dermatophyte and Dimorphic Fungal Pathogens. Eukaryot. Cell 2010, 9, 46–58. [Google Scholar] [CrossRef]
- Weitzman, I.; Padhye, A.A. Mating behaviour of Nannizzia otae (=Microsporum canis). Mycopathologia 1978, 64, 17–22. [Google Scholar] [CrossRef]
- Marchisio, V.F.; Gallo, M.G.; Tullio, V.; Nepote, S.; Piscozzi, A.; Cassinelli, C. Dermatophytes from cases of skin disease in cats and dogs in Turin, Italy. Mycoses 1995, 38, 239–244. [Google Scholar] [CrossRef]
- Ratajczak-Stefańska, V.; Kiedrowicz, M.; Maleszka, R.; Różewicka, M.; Mikulska, D. Majocchi’s granuloma caused by Microsporum canis in an immunocompetent patient. Clin. Exp. Dermatol. 2010, 35, 445–447. [Google Scholar] [CrossRef]
- Voisard, J.; Weill, F.; Beylot-Barry, M.; Vergier, B.; Dromer, C.; Beylot, C. Dermatophytic Granuloma Caused by Microsporum canis in a Heart-Lung Recipient. Dermatology 1999, 198, 317–319. [Google Scholar] [CrossRef]
- Moriello, K.; DeBoer, D.; Greek, J.; Kuhl, K.; Fintelman, M. The prevalence of immediate and delayed type hypersensitivity reactions to Microsporum canis antigens in cats. J. Feline Med. Surg. 2003, 5, 161–166. [Google Scholar] [CrossRef]
- Stuntebeck, R.; Moriello, K.A.; Verbrugge, M. Evaluation of incubation time for Microsporum canis dermatophyte cultures. J. Feline Med. Surg. 2017, 20, 997–1000. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Stokes, C.R.; Gruffydd-Jones, T.J. Experimental Microsporum canis infection in cats: Correlation between immunological and clinical observations. J. Med. Vet. Mycol. Bi-Mon. Publ. Int. Soc. Hum. Anim. Mycol. 1995, 33, 177–184. [Google Scholar]
| Classification | Species | Primary Host/Habitat | Main Types of Infection | Geographical Distribution | Reference |
|---|---|---|---|---|---|
| Anthropophilic | Trichophyton rubrum | Humans | Tinea pedis, tinea unguium, tinea cruris, tinea faciei, tinea corporis, tinea manuum, tinea barbae | Worldwide | [1,18,24,25,74,86,87,89] |
| Trichophyton tonsurans | Humans | Tinea capitis, tinea corporis, tinea faciei | Worldwide | [24,74,79,86] | |
| Epidermophyton floccosum | Humans | Tinea cruris | Worldwide | [74] | |
| Trichophyton digitale | Humans | Tinea pedis | Worldwide | [74,92] | |
| Trichophyton schoenleinii | Humans | Tinea capitis favosa | Asia, Europe, Africa | [74,81] | |
| Zoophilic | Microsporum canis | Cats | Ringworm | Worldwide | [16,74,93] |
| Nannizzia persicolor (former name Arthroderma persicolor) | Voles, bats | Ringworm | Africa, Australia, Europe, North America | [74,94,95] | |
| Nannizzia nana (former name Microsporum nanum) | Pigs | Ringworm | Worldwide | [18,74,96] | |
| Trichophyton equinum | Horses | Ringworm | Worldwide | [74,97] | |
| Trichophyton mentagrophytes (former name Arthroderma vanbreuseghemii) | Mice, guinea pigs | Ringworm | Worldwide | [74,93] | |
| Trichophyton verrucosum | Cattle | Ringworm | Worldwide | [74,98,99] | |
| Geophilic | Nannizzia gypsea (former name Microsporum gypseum) | Soil | Ringworm (animals), tinea capitis/tinea corporis (humans) | Worldwide | [16,19,74,100] |
| Diagnostic Method | Advantages | Disadvantages | Time to Results | Reference |
|---|---|---|---|---|
| Direct examination |
|
| Minutes | [116,117] |
| Wood’s lamp |
|
| Minutes | [119,120] |
| Microscopy |
|
| Minutes | [38,121] |
| Culture |
|
| Days–Weeks | [122,123,124] |
| PCR |
|
| Hours–Days | [16,125,126] |
| ELISA |
|
| Hours–Days | [127,128,129] |
| MALDI-ToF |
|
| Minutes–Hours | [130,131,132,133] |
| Genetic analysis |
|
| Hours–Days | [30,31,134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskaluk, A.E.; VandeWoude, S. Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens 2022, 11, 957. https://doi.org/10.3390/pathogens11090957
Moskaluk AE, VandeWoude S. Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens. 2022; 11(9):957. https://doi.org/10.3390/pathogens11090957
Chicago/Turabian StyleMoskaluk, Alex E., and Sue VandeWoude. 2022. "Current Topics in Dermatophyte Classification and Clinical Diagnosis" Pathogens 11, no. 9: 957. https://doi.org/10.3390/pathogens11090957
APA StyleMoskaluk, A. E., & VandeWoude, S. (2022). Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens, 11(9), 957. https://doi.org/10.3390/pathogens11090957





