Overview on the Infections Related to Rare Candida Species
Abstract
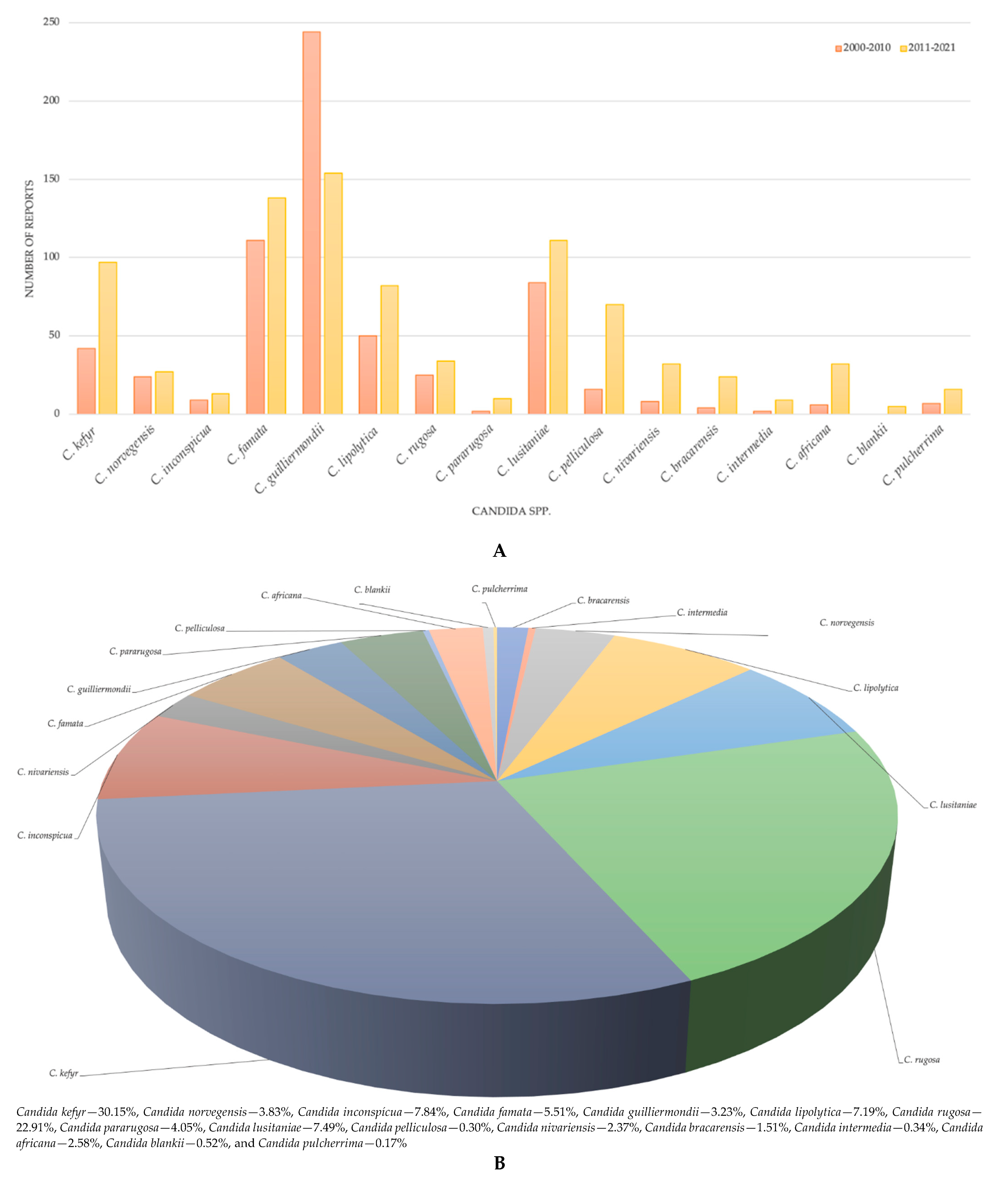
1. Introduction
2. Uncommon Candida spp.: Infections, Treatment, and Resistance
2.1. Candida kefyr (New Nomenclature: Kluyveromyces marxianus or Candida pseudotropicalis)
2.2. Candida norvegensis (New Nomenclature: Pichia norvegensis)
2.3. Candida inconspicua (New Nomenclature: Pichia cactophila)
2.4. Candida lipolytica (New Nomenclature: Yarrowia lipolytica)
2.5. Candida lusitaniae (Updated Nomenclature: Clavispora lusitaniae)
2.6. Candida famata (New Nomenclature: Debaryomyces hansenii)
2.7. Candida guilliermondii (New Nomenclature: Meyerozyma guilliermondii)
| Invasive/NonInvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Candida famata | ||||||||
| (invasive) C. famata (n = 1) | Hodgkin’s disease, blood culture | Microscopy | Voriconazole | NR | voriconazole | NA | Alive | [94] |
| (invasive) C. famata (n = 8) | Urine culture | Microscopy | Posaconazole, caspofungin | Fluconazole | Posaconazole | NA | No death | [95] |
| (invasive) C. famata (n = 2) | Blood culture | Microscopy | Reduced to echinocandins, azoles | Azole resistance reported | Liposomal amphotericin B | NA | No death | [96] |
| (invasive) C. famata (n = 1) | Blood cultures | Partial amplification and sequencing of the 26S ribosomal DNA gene | Anidulafungin and micafungin | Fluconazole | Amphotericin B | NA | Alive | [97] |
| Candida guilliermondii | ||||||||
| (non-invasive) C. guilliermondii (n = 17) | Blood sample | Amplification and sequencing of the ITS1-5.8S-ITS2 region | NR | Fluconazole and echinocandins | Amphotericin B | NA | NR | [98] |
| (invasive) C. guilliermondii (n = 1) | Blood cultures | Microscopy | NR | Fluconazole | Patient died despite of amphotericin B therapy | NA | Died despite of amphotericin B therapy | [99] |
| (invasive) C. guilliermondii (n = 47) | Blood cultures | PCR- restriction fragment length polymorphism | Caspofungin, micafungin and anidulafungin | NR | NR | NA | No | [100] |
2.8. Candida rugosa (New Nomenclature: Diutina rugosa)
2.9. Candida pararugosa (New Numenclature: Wickerhamiella pararugosa)
2.10. Candida pelliculosa (New Nomenclature: Wickerhamomyces anomalus)
2.11. Candida nivariensis (New Nomenclature: Nakaseomyces nivariensisa)
2.12. Candida bracarensis (New Nomenclature: Nakaseomyces bracarensisa)
2.13. Candida intermedia
2.14. Candida africana
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) C. africana (n = 2) | MALDI-TOF MS and PCR | Microscopy | Ketoconazole (one) Fluconazole (one) Itraconazole (both) Amphotericin B (one) | Ketoconazole (one) Fluconazole (one) Amphotericin B (one) | NR | NA | Alive | [185] |
| Candida vaginitis (n = 10) | PCR-RFLP and sequencing CHROMagar Candida | Microscopy | Fluconazole | NR | NR | NA | NA | [181] |
| (invasive) C. africana (n = 2) | CHROMagar and PCR | Microscopy | Amphotericin B, fluconazole, and itraconazole | Fluconazole and itraconazole | NR | NA | NA | [180] |
| (invasive) C. africana (n = 3) | CHROMagar MALDI-TOF MS | Microscopy | Fluconazole; voriconazole; ketoconazole; amphotericin B; anidulafungin; micafungin | NR | NR | NA | NA | [178] |
| (invasive) C. africana (n = 1) | CHROMagar and PCR | Microscopy | Amphotericin B, nystatin, fluconazole, itraconazole Voriconazole, clotrimazole, terbinafine | NR | NR | NA | NA | [186] |
| (invasive) C. africana (n = 5) | CHROMagar and PCR | Microscopy | Caspofungin, anidulafungin, micafungin, itraconazole, voriconazole, posaconazole | NR | NR | NA | All alive | [179] |
| (invasive) C. africana (n = 15) | CHROMagar and PCR | Microscopy | Fluconazole, itraconazole, miconazole, clotrimazole, | NR | NR | NA | All alive | [187] |
| (invasive) C. africana (n = 4) | CHROMagar and PCR sequencing | Microscopy | Nystatin, clotrimazole, isavuconazole, ketoconazole, miconazole and posaconazole | NR | NR | NA | All alive | [177] |
| (invasive) C. africana (n = 15) | CHROMagar and PCR sequencing | Microscopy | Amphotericin B, nystatin, Itraconazole, miconazole, econazole, and ketoconazole | NR | NA | Alive (14) Dead (1) | [183] | |
| (invasive) C. africana (n = 2) | CHROMagar and PCR | Microscopy | amphotericin B, 5-fluorocytosine, Fluconazole, itraconazole, ketoconazole, voriconazole, posaconazole and caspofungin | NR | NR | NA | Alive | [188] |
| (invasive) C. africana (n = 1) | CHROMagar and PCR | Microscopy | Amphotericin B, 5-fluorocytosine fluconazole itraconazole, ketoconazole voriconazole | NR | NR | NA | Alive | [173] |
2.15. Candida blankii
2.16. Candida pulcherrima (Updated Nomenclature: Metschnikowia pulcherrima)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Gharehbolagh, S.A.; Fallah, B.; Izadi, A.; Ardestani, Z.S.; Malekifar, P.; Borman, A.M.; Mahmoudi, S. Distribution, antifungal susceptibility pattern and intra-Candida albicans species complex prevalence of Candida africana: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0237046. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.A.; Butler, G. The Candida Pathogenic Species Complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Harrington, R.; Azie, N.; Yang, H.; Li, N.; Zhao, J.; Koo, V.; Wu, E.Q. A Risk Score for Fluconazole Failure among Patients with Candidemia. Antimicrob. Agents Chemother. 2017, 61, e02091-16. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C.F. Candida auris: A Quick Review on Identification, Current Treatments, and Challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensiv. Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
- Borman, A.M.; Johnson, E.M. Name Changes for Fungi of Medical Importance, 2018 to 2019. J. Clin. Microbiol. 2021, 59, 1811–1831. [Google Scholar] [CrossRef]
- Brandt, M.E.; Lockhart, S.R. Recent Taxonomic Developments with Candida and Other Opportunistic Yeasts. Curr. Fungal Infect. Rep. 2012, 6, 170. [Google Scholar] [CrossRef]
- Pemán, J.; Cantón, E.; Quindós, G.; Eraso, E.; Alcoba, J.; Guinea, J.; Merino, P.; Ruiz-Pérez-De-Pipaon, M.T.; Pérez-Del-Molino, L.; Linares-Sicilia, M.J.; et al. Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J. Antimicrob. Chemother. 2012, 67, 1181–1187. [Google Scholar] [CrossRef]
- Aslani, N.; Janbabaei, G.; Abastabar, M.; Meis, J.F.; Babaeian, M.; Khodavaisy, S.; Boekhout, T.; Badali, H. Identification of uncommon oral yeasts from cancer patients by MALDI-TOF mass spectrometry. BMC Infect. Dis. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Diaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Ergon, M.C.; Dereli, M.D.; Ener, B.; Atalay, M.A.; Koç, A.N.; Çerikçioğlu, N.; Erturan, Z.; Aksaray, S. Türkiye’de Altı Yıllık Zaman Dilimi İçerisinde Kan Kültürlerinden Soyutlanan Maya Mantarlarının Tür Dağılımı: Çok Merkezli Bir Çalışma. Distribution of Yeast Species Isolated from Blood Cultures for a Six Year Period in Turkey: A Multicentre Study. Mikrobiyoloji Bulteni 2020, 54, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.; Taverna, C.G.; Bosco-Borgeat, M.E.; Maldonado, I.; Vivot, W.; Szusz, W.; Garcia-Effron, G.; Córdoba, S.B. Candida glabrata species complex prevalence and antifungal susceptibility testing in a culture collection: First description of Candida nivariensis in Argentina. Mycopathologia 2016, 181, 871–878. [Google Scholar] [CrossRef]
- Arendrup, M.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J.; Andersen, C.; Arikan-Akdagli, S.; Barchiesi, F.; Chryssanthou, E.; et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef]
- Bourbeau, P.; Cerwinka, P.L.; Abramson, J.; Finn, S.; Hindiyeh, M.Y.; Loeffelholz, M.J.; Maliff, E.S.; Nugent, C.T., IV; Peat, C.R.; Sharples, N.; et al. M40-A2 CLSI Guideline—Quality Control of Microbiological Transport Systems; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2014. [Google Scholar]
- Mellinghoff, S.C.; Hoenigl, M.; Koehler, P.; Kumar, A.; Lagrou, K.; Lass-Flörl, C.; Meis, J.F.; Menon, V.; Rautemaa-Richardson, R.; Cornely, O.A. EQUAL Candida Score: An ECMM score derived from current guidelines to measure QUAlity of Clinical Candidaemia Management. Mycoses 2018, 61, 326–330. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.J.; Tullio, V.; Rodloff, A.C.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance Group. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-Year Analysis of Susceptibilities of Candida Species to Fluconazole and Voriconazole as Determined by CLSI Standardized Disk Diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef]
- Chen, S.C.-A.; Perfect, J.; Colombo, A.L.; A Cornely, O.; Groll, A.H.; Seidel, D.; Albus, K.; de Almedia, J.N.; Garcia-Effron, G.; Gilroy, N.; et al. Global guideline for the diagnosis and management of rare yeast infections: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2021, 21, e375–e386. [Google Scholar] [CrossRef]
- Dos Santos Abrantes, P.M.; McArthur, C.P.; Africa, C.W.J. Multi-drug resistant oral Candida species isolated from HIV-positive patients in South Africa and Cameroon. Diagn. Microbiol. Infect. Dis. 2014, 79, 222–227. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Obaid, K.; Joseph, L.; Chandy, R. Candida kefyr as a cause of bloodstream infection and adjunctive role of biomarkers in its diagnosis. J. Mycol. Med 2014, 25, 71–75. [Google Scholar] [CrossRef]
- Nurdin, R.S.C.; Vitayani, S.; Amin, S.; Kadir, D.; Djamaluddin, W.; Adriani, A. Cutaneous candidiasis caused by Candida kefyr. Pan Afr. Med. J. 2021, 38, 178. [Google Scholar] [CrossRef] [PubMed]
- Okmen, F.; Ekici, H.; Ari, S.A. Case Report of a Tubo-ovarian Abscess Caused by Candida kefyr. J. Obstet. Gynaecol. Can. 2018, 40, 1466–1467. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Salehi, F.; Esmaeili, M. Isolation of Candida Species from Gastroesophageal Lesions among Pediatrics in Isfahan, Iran: Identification and Antifungal Susceptibility Testing of Clinical Isolates by E-test. Adv. Biomed. Res. 2017, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Seth-Smith, H.M.B.; Büchler, A.C.; Hinic, V.; Medinger, M.; Widmer, A.F.; Egli, A. Bloodstream infection with Candida kefyr/Kluyveromyces marxianus: Case report and draft genome. Clin. Microbiol. Infect. 2020, 26, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Daneshnia, F.; Farahyar, S.; Fang, W.; Salimi, M.; Salehi, M.; Hagen, F.; Weihua, P.; Roudbary, M.; Boekhout, T. Incidence and spectrum of yeast species isolated from the oral cavity of Iranian patients suffering from hematological malignancies. J. Oral Microbiol. 2019, 11, 1601061. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, Z.; Al-Sweih, N.; Alfouzan, W.; Joseph, L.; Asadzadeh, M. Candida kefyr in Kuwait: Prevalence, antifungal drug susceptibility and genotypic heterogeneity. PLoS ONE 2020, 15, e0240426. [Google Scholar] [CrossRef]
- Dagi, H.T.; Findik, D.; Senkeles, C.; Arslan, U. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 36. [Google Scholar] [CrossRef]
- Dufresne, S.F.; Marr, K.A.; Sydnor, E.; Staab, J.F.; Karp, J.E.; Lu, K.; Zhang, S.X.; Lavallée, C.; Perl, T.M.; Neofytos, D. Epidemiology of Candida kefyr in Patients with Hematologic Malignancies. J. Clin. Microbiol. 2014, 52, 1830–1837. [Google Scholar] [CrossRef]
- Jyothi, L.; Reddy, N.P.; Naaz, S. An Unusual Case of Candida kefyr Fungemia in an Immunocompromised Patient. Cureus 2021, 13, e14138. [Google Scholar] [CrossRef]
- Nagy, F.; Bozó, A.; Tóth, Z.; Daróczi, L.; Majoros, L.; Kovács, R. In vitro antifungal susceptibility patterns of planktonic and sessile Candida kefyr clinical isolates. Med. Mycol. 2017, 56, 493–500. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Aldejohann, A.M.; Theuersbacher, J.; Haug, L.; Lamm, O.S.; Walther, G.; Kurzai, O.; Hillenkamp, J.; Kampik, D. First case of Kluyveromyces marxianus (Candida kefyr) late onset keratitis after lamellar endothelial corneal graft. Med. Mycol. Case Rep. 2021, 32, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Scapaticci, M.; Bartolini, A.; del Chierico, F.; Accardi, C.; di Girolamo, F.; Masotti, A.; Muraca, M.; Putignani, L. Phenotypic Typing and Epidemiological Survey of Antifungal Resistance of Candida Species Detected in Clinical Samples of Italian Pa-tients in a 17 Months’ Period. Germs 2018, 8, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Swarajyalakshmi, M.; Jyothilakshmi, G. Candida kefyr in Invasive Paranasal Sinusitis. Indian J. Otolaryngol. Head Neck Surg. 2012, 66, 371–374. [Google Scholar] [CrossRef]
- Fekkar, A.; Meyer, I.; Brossas, J.Y.; Dannaoui, E.; Palous, M.; Uzunov, M.; Nguyen, S.; Leblond, V.; Mazier, D.; Datry, A. Rapid Emergence of Echinocandin Resistance during Candida kefyr Fungemia Treatment with Caspofungin. Antimicrob. Agents Chemother. 2013, 57, 2380–2382. [Google Scholar] [CrossRef]
- De Freitas, E.M.; Nobre, S.A.M.; de Oliveira Pires, M.B.; Faria, R.V.J.; Batista, A.U.D.; Bonan, P.R.F. Oral Candida species in head and neck cancer patients treated by radiotherapy. Auris Nasus Larynx 2013, 40, 400–404. [Google Scholar] [CrossRef]
- Badiee, P.; Alborzi, A.; Shakiba, E.; Farshad, S.; Japoni, A. Susceptibility of Candida species isolated from immunocompromised patients to antifungal agents. East. Mediterr. Health J. 2011, 17, 425–430. [Google Scholar] [CrossRef]
- Weichert, S.; Reinshagen, K.; Zahn, K.; Geginat, G.; Dietz, A.; Kilian, A.K.; Schroten, H.; Tenenbaum, T. Candidiasis caused by Candida kefyr in a neonate: Case report. BMC Infect. Dis. 2012, 12, 61. [Google Scholar] [CrossRef]
- Pineda, C.; Kaushik, A.; Kest, H.; Wickes, B.; Zauk, A. Maternal Sepsis, Chorioamnionitis, and Congenital Candida kefyr Infection in Premature Twins. Pediatr. Infect. Dis. J. 2012, 31, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Dietrichson, E. Etude d’une collection norvégienne de levures (2e Partie) (suite et fin). Ann. Parasitol. Hum. Comp. 1954, 29, 460–498. [Google Scholar] [CrossRef]
- Nielsen, H.; Stenderup, J.; Bruun, B.; Ladefoged, J. Candida norvegensis peritonitis and invasive disease in a patient on continuous ambulatory peritoneal dialysis. J. Clin. Microbiol. 1990, 28, 1664–1665. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.V.; Moore, C.B.; Denning, D.W. Isolation of Candida norvegensis from Clinical Specimens: Four Case Reports. Clin. Infect. Dis. 1996, 23, 1185–1187. [Google Scholar] [CrossRef][Green Version]
- Kiraz, N.; Akay, O.M.; Sen, Y.; Aslan, V.; Akgun, Y.; Gulbas, Z. Candida norvegensis fungaemia in a neutropenic patient with acute non-lymphoblastic leukaemia. Mycoses 2010, 53, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Sandven, P.; Nilsen, K.; Digranes, A.; Tjade, T.; Lassen, J. Candida norvegensis: A fluconazole-resistant species. Antimicrob. Agents Chemother. 1997, 41, 1375–1376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krcmery, V.; Barnes, A.J. Non-albicans Candida spp. causing fungaemia: Pathogenicity and antifungal resistance. J. Hosp. Infect. 2002, 50, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Arikan, S.; Sancak, B.; Hascelik, G. In vitroactivity of caspofungin compared to amphotericin B, fluconazole, and itraconazole againstCandidastrains isolated in a Turkish University Hospital. Med. Mycol. 2005, 43, 171–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Monzon, A.; Buitrago, M.J.; Rodriguez-Tudela, J.L. Activity Profile In Vitro of Micafungin against Spanish Clinical Isolates of Common and Emerging Species of Yeasts and Molds. Antimicrob. Agents Chemother. 2009, 53, 2192–2195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugita, T.; Takeo, K.; Ohkusu, M.; Virtudazo, E.; Takashima, M.; Asako, E.; Ohshima, F.; Harada, S.; Yanaka, C.; Nishikawa, A.; et al. Fluconazole-Resistant Pathogens Candida inconspicua and C. norvegensis: DNA Sequence Diversity of the rRNA Intergenic Spacer Region, Antifungal Drug Susceptibility, and Extracellular Enzyme Production. Microbiol. Immunol. 2004, 48, 761–766. [Google Scholar] [CrossRef]
- Stavrou, A.A.; Pérez-Hansen, A.; Lackner, M.; Lass-Flörl, C.; Boekhout, T. Elevated Minimum Inhibitory Concentrations to Antifungal Drugs Prevail in 14 Rare Species of Candidemia-Causing Saccharomycotina Yeasts. Med. Mycol. 2020, 58, 987–995. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Fuchs, B.B.; Muhammed, M.; Coleman, J.J.; Suleiman, J.M.A.H.; Vilela, S.F.G.; Costa, A.C.B.P.; Rasteiro, V.M.C.; Jorge, A.O.C.; Mylonakis, E. Oral Candida albicans isolates from HIV-positive individuals have similar in vitro biofilm-forming ability and pathogenicity as invasive Candida isolates. BMC Microbiol. 2011, 11, 247. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Chen, W.-Y.; Li, X.; Li, H.-B.; Li, H.-Q.; Wang, L.; He, L.; Yang, X.-P.; Wang, X.-C.; Huang, Y.-L.; et al. Asymptomatic oral yeast carriage and antifungal susceptibility profile of HIV-infected patients in Kunming, Yunnan Province of China. BMC Infect. Dis. 2013, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Agwu, E.; Ihongbe, J.C.; McManus, B.A.; Moran, G.P.; Coleman, D.C.; Sullivan, D.J. Distribution of yeast species associated with oral lesions in HIV-infected patients in Southwest Uganda. Med. Mycol. 2012, 50, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Guitard, J.; Angoulvant, A.; Letscher-Bru, V.; L’Ollivier, C.; Cornet, M.; Dalle, F.; Grenouillet, F.; Lacroix, C.; Vekhoff, A.; Maury, E.; et al. Invasive infections due toCandida norvegensisandCandida inconspicua: Report of 12 cases and review of the literature. Med. Mycol. 2013, 51, 795–799. [Google Scholar] [CrossRef]
- Musso, M.; Giannella, M.; Antonini, M.; Bordi, E.; Ettorre, G.M.; Tessitore, L.; Mariano, A.; Capone, A. Invasive candidiasis due to Candida norvegensis in a liver transplant patient: Case report and literature review. Infect. Dis. Rep. 2014, 6, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente, G.; Marco, F.; Cervera, C.; Hoyo, I.; Colmenero, J.; Pitart, C.; Almela, M.; Navasa, M.; Moreno, A. Candida norvegensis fungemia in a liver transplant recipient. Rev. Iberoam. Micol. 2015, 32, 115–117. [Google Scholar] [CrossRef]
- Virgilio, E.; Mercantini, P.; Ferri, M.; Cavallini, M.; Teggi, A.; Ziparo, V. Coexistence of Diffuse Malignant Peritoneal Mesothelioma andCandida norvegensisPeritonitis. Surg. Infect. 2014, 15, 660–661. [Google Scholar] [CrossRef]
- Mixão, V.; Hansen, A.P.; Saus, E.; Boekhout, T.; Lass-Florl, C.; Gabaldón, T. Whole-Genome Sequencing of the Opportunistic Yeast Pathogen Candida inconspicua Uncovers Its Hybrid Origin. Front. Genet. 2019, 10, 383. [Google Scholar] [CrossRef]
- Egue, L.A.; N’Guessan, F.K.; Aka-Gbezo, S.; Bouatenin, J.-P.K.; Koussemon-Camara, M. Candida species in tchapalo and bangui, two traditional alcoholic beverages from Côte d’Ivoire. Fungal Biol. 2018, 122, 283–292. [Google Scholar] [CrossRef]
- Kovács, R.; Gesztelyi, R.; Berényi, R.; Domán, M.; Kardos, G.; Juhász, B.; Majoros, L. Killing rates exerted by caspofungin in 50 % serum and its correlation with in vivo efficacy in a neutropenic murine model against Candida krusei and Candida inconspicua. J. Med. Microbiol. 2014, 63, 186–194. [Google Scholar] [CrossRef]
- Pérez-Hansen, A.; Lass-Flörl, C.; Lackner, M. Antifungal Susceptibility Profiles of Rare Ascomycetous Yeasts. J. Antimicrob. Chemother. 2019, 74, 2649–2656. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. (Eds.) CANDIDA | Yarrowia (Candida) lipolytica. In The Yeasts, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, p. xiii. [Google Scholar]
- Walsh, T.J.; Salkin, I.F.; Dixon, D.M.; Hurd, N.J. Clinical, microbiological, and experimental animal studies of Candida lipolytica. J. Clin. Microbiol. 1989, 27, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, H.; Chtara, K.; Khemakhem, N.; Néji, S.; Cheikhrouhou, F.; Sellami, H.; Guidara, R.; Makni, F.; Bouaziz, M.; Ayadi, A. Fungemia Caused by Yarrowia lipolytica. Mycopathologia 2015, 179, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Zinjarde, S.S. Food-related applications of Yarrowia lipolytica. Food Chem. 2014, 152, 1–10. [Google Scholar] [CrossRef]
- Liu, W.-C.; Chan, M.-C.; Lin, T.-Y.; Hsu, C.-H.; Chiu, S.-K. Candida lipolytica candidemia as a rare infectious complication of acute pancreatitis: A case report and literature review. J. Microbiol. Immunol. Infect. 2013, 46, 393–396. [Google Scholar] [CrossRef][Green Version]
- Lai, C.-C.; Lee, M.-R.; Hsiao, C.-H.; Tan, C.-K.; Lin, S.-H.; Liao, C.-H.; Huang, Y.-T.; Hsueh, P.-R. Infections caused by Candida lipolytica. J. Infect. 2012, 65, 372–374. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, J.F.-W.; Tsang, C.-C.; Wang, H.; Guo, D.; Pan, Y.; Xiao, Y.; Yue, N.; Chen, J.H.-K.; Lau, S.K.-P.; et al. Clinical Characteristics, Laboratory Identification, and In Vitro Antifungal Susceptibility of Yarrowia (Candida) lipolytica Isolates Causing Fungemia: A Multicenter, Prospective Surveillance Study. J. Clin. Microbiol. 2015, 53, 3639–3645. [Google Scholar] [CrossRef]
- Özdemir, H.; Karbuz, A.; Çiftçi, E.; Dinçaslan, H.U.; Ince, E.; Aysev, D.; Yavuz, G.; Doğru, Ü. Successful treatment of central venous catheter infection due to Candida lipolytica by caspofungin-lock therapy. Mycoses 2011, 54, e647–e649. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Letscher-Bru, V.; Neuvéglise, C.; Dromer, F. Yarrowia lipolytica causes sporadic cases and local outbreaks of infections and colonisation. Mycoses 2020, 63, 737–745. [Google Scholar] [CrossRef]
- Diekema, D.J.; Messer, S.A.; Boyken, L.B.; Hollis, R.J.; Kroeger, J.; Tendolkar, S.; Pfaller, M.A. In Vitro Activity of Seven Systemically Active Antifungal Agents against a Large Global Collection of Rare Candida Species as Determined by CLSI Broth Microdilution Methods. J. Clin. Microbiol. 2009, 47, 3170–3177. [Google Scholar] [CrossRef]
- Blanco, M.T.; Garcia-Martos, P.; García-Tapia, A.; Fernández, C.; Navarro, J.; Guerrero, F. Fungemia por Candida lipolytica: A propósito de 2 casos. Rev. Iberoam. Micol. 2009, 26, 211–212. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, D.; Romano, F.; Pontieri, E.; Fioritoni, G.; Caracciolo, C.; Bianchini, S.; Olioso, P.; Staniscia, T.; Sferra, R.; Boccia, S.; et al. Catheter-Related Candidemia Caused by Candida lipolytica in a Patient Receiving Allogeneic Bone Marrow Transplantation. J. Clin. Microbiol. 2002, 40, 1381–1386. [Google Scholar] [CrossRef]
- Shin, J.H.; Kook, H.; Shin, D.H.; Hwang, T.J.; Kim, M.; Suh, S.P.; Ryang, D.W. Nosocomial Cluster of Candida lipolytica Fungemia in Pediatric Patients. Eur. J. Clin. Microbiol. 2000, 19, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Abbes, S.; Amouri, I.; Trabelsi, H.; Neji, S.; Sellami, H.; Rahmouni, F.; Makni, F.; Rebai, T.; Ayadi, A. Analysis of virulence factors and in vivo biofilm-forming capacity of Yarrowia lipolytica isolated from patients with fungemia. Med. Mycol. 2016, 55, 193–202. [Google Scholar] [CrossRef]
- Bahloul, M.; Chtara, K.; Turki, O.; Bouaziz, N.K.; Regaieg, K.; Hammami, M.; Ben Amar, W.; Chabchoub, I.; Ammar, R.; Ben Hamida, C.; et al. Yarrowia lipolytica fungemia in patients with severe polytrauma requiring intensive care admission: Analysis of 32 cases. Intensiv. Care Med. 2017, 43, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Taj-Aldeen, S.J.; Abdulwahab, A.; Kolecka, A.; Deshmukh, A.; Meis, J.F.; Boekhout, T. Uncommon opportunistic yeast bloodstream infections from Qatar. Med. Mycol. 2014, 52, 552–556. [Google Scholar] [CrossRef]
- Viudes, A.; Pemán, J.; Cantón, E.; Úbeda, P.; Lopez-Ribot, J.L.; Gobernado, M. Candidemia at a Tertiary-Care Hospital: Epidemiology, Treatment, Clinical Outcome and Risk Factors for Death. Eur. J. Clin. Microbiol. 2002, 21, 767–774. [Google Scholar] [CrossRef]
- EUCAST Breakpoint Tables for Interpretation of MICs, Version 10.0, Valid from 4 February 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf (accessed on 18 August 2022).
- Pappagianis, D.; Collins, M.S.; Hector, R.; Remington, J. Development of Resistance to Amphotericin B in Candida lusitaniae Infecting a Human. Antimicrob. Agents Chemother. 1979, 16, 123–126. [Google Scholar] [CrossRef]
- Peyron, F.; Favel, A.; Michel-Nguyen, A.; Gilly, M.; Regli, P.; Bolmström, A. Improved Detection of Amphotericin B-Resistant Isolates of Candida lusitaniae by Etest. J. Clin. Microbiol. 2001, 39, 339–342. [Google Scholar] [CrossRef]
- Minari, A.; Hachem, R.; Raad, I. Candida lusitaniae: A Cause of Breakthrough Fungemia in Cancer Patients. Clin. Infect. Dis. 2001, 32, 186–190. [Google Scholar] [CrossRef]
- Merz, W.G. Candida lusitaniae: Frequency of recovery, colonization, infection, and amphotericin B resistance. J. Clin. Microbiol. 1984, 20, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Vazquez, J.A.; Steffan, P.E.; Sobel, J.D.; Akins, R.A. High-Frequency, In Vitro Reversible Switching of Candida lusitaniae Clinical Isolates from Amphotericin B Susceptibility to Resistance. Antimicrob. Agents Chemother. 1999, 43, 836–845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodrigues, C.F.; Henriques, M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens 2017, 6, 62. [Google Scholar] [CrossRef]
- Viudes, A.; Pemán, J.; Cantón, E.; Salavert, M.; Úbeda, P.; Lopez-Ribot, J.; Gobernado, M. Two Cases of Fungemia due to Candida lusitaniae and a Literature Review. Eur. J. Clin. Microbiol. 2002, 21, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Bariola, J.R.; Saccente, M. Candida lusitaniae septic arthritis: Case report and review of the literature. Diagn. Microbiol. Infect. Dis. 2008, 61, 61–63. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Khan, S.; Joseph, L. Candida lusitaniae in Kuwait: Prevalence, antifungal susceptibility and role in neonatal fungemia. PLoS ONE 2019, 14, e0213532. [Google Scholar] [CrossRef]
- Pietrucha-Dilanchian, P.; Lewis, R.E.; Ahmad, H.; Lechin, A.E. Candida Lusitaniae Catheter-Related Sepsis. Ann. Pharmacother. 2001, 35, 1570–1574. [Google Scholar] [CrossRef]
- Hawkins, J.L.; Baddour, L.M. Candida lusitaniae Infections in the Era of Fluconazole Availability. Clin. Infect. Dis. 2003, 36, e14–e18. [Google Scholar] [CrossRef]
- Blinkhorn, R.J.; Adelstein, D.; Spagnuolo, P.J. Emergence of a new opportunistic pathogen, Candida lusitaniae. J. Clin. Microbiol. 1989, 27, 236–240. [Google Scholar] [CrossRef]
- Wawrysiuk, S.; Rechberger, T.; Futyma, K.; Miotła, P. Candida lusitaniae—A case report of an intraperitoneal infection. Menopausal Rev. 2018, 17, 94–96. [Google Scholar] [CrossRef]
- Elkamouni, Y.; Lmimouni, B.; Doghmi, K.; Elouenass, M. Candida famata candidemia in immunosuppressed patient: Report of a case with literature review. Ann. Biol. Clin. 2011, 69, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Diba, K.; Makhdoomi, K.; Nasri, E.; Vaezi, A.; Javidnia, J.; Gharabagh, D.J.; Jazani, N.H.; Chavshin, A.R.; Badiee, P.; Badali, H.; et al. Emerging Candida species isolated from renal transplant recipients: Species distribution and susceptibility profiles. Microb. Pathog. 2018, 125, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Beyda, N.D.; Lewis, R.E.; Garey, K.W. Echinocandin Resistance in Candida Species: Mechanisms of Reduced Susceptibility and Therapeutic Approaches. Ann. Pharmacother. 2012, 46, 1086–1096. [Google Scholar] [CrossRef]
- Karapetsa, M.; Tsolaki, V.; Arabatzis, M.; Petinaki, E.; Velegraki, A.; Zakynthinos, E. Septic shock due to Candida famata (Debaryomyces hansenii) candidemia in an ICU immunocompetent trauma-patient. J. Infect. Public Health 2019, 12, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Puig-Asensio, M.; Pérez-García, F.; Escribano, P.; Sánchez-Carrillo, C.; Zaragoza, O.; Padilla, B.; Cuenca-Estrella, M.; Almirante, B.; Martín-Gómez, M.T.; et al. Candida guilliermondii Complex Is Characterized by High Antifungal Resistance but Low Mortality in 22 Cases of Candidemia. Antimicrob. Agents Chemother. 2017, 61, e00099-17. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.D.; Rosengard, B.R.; Merz, W.G.; Stuart, R.K.; Hutchins, G.M.; Saral, R. Fatal Disseminated Candidiasis Due to Amphotericin-B-Resistant Candida guilliermondii. Ann. Intern. Med. 1985, 102, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Huang, S.-Y.; Tang, J.-L.; Tsay, W.; Yao, M.; Ko, B.-S.; Chou, W.-C.; Tien, H.-F.; Hsueh, P.-R. Clinical features of patients with infections caused by Candida guilliermondii and Candida fermentati and antifungal susceptibility of the isolates at a medical centre in Taiwan, 2001–2010. J. Antimicrob. Chemother. 2013, 68, 2632–2635. [Google Scholar] [CrossRef][Green Version]
- Nucci, M.; Marr, K.A. Emerging Fungal Diseases. Clin. Infect. Dis. 2005, 41, 521–526. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Rare and Emerging Opportunistic Fungal Pathogens: Concern for Resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419. [Google Scholar] [CrossRef]
- Reinhardt, J.F.; Ruane, P.J.; Walker, L.J.; George, W.L. Intravenous catheter-associated fungemia due to Candida rugosa. J. Clin. Microbiol. 1985, 22, 1056–1057. [Google Scholar] [CrossRef]
- Sugar, A.; Stevens, D.A. Candida rugosa in immunocompromised infection case reports, drug susceptibility, and review of the literature. Cancer 1985, 15, 318–320. [Google Scholar] [CrossRef]
- Dubé, M.P.; Heseltine, P.N.R.; Rinaldi, M.G.; Evans, S.; Zawacki, B. Fungemia and Colonization with Nystatin-Resistant Candida rugosa in a Burn Unit. Clin. Infect. Dis. 1994, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Azevedo Melo, A.S.; Crespo Rosas, R.F.; Salomão, R.; Briones, M.; Hollis, R.J.; Messer, S.A.; Pfaller, M.A. Outbreak of Candida rugosa candidemia: An emerging pathogen that may be refractory to amphotericin B therapy. Diagn. Microbiol. Infect. Dis. 2003, 46, 253–257. [Google Scholar] [CrossRef]
- Rosas, R.; Nucci, M.; Castelo, A.; Colombo, A.L. Predictive value of Candida spp. colonization in the diagnosis of candidemia in intensive care unit patients (abstract M-269). In Proceedings of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington DC, USA, 30 October–2 November 2004. [Google Scholar]
- Behera, B.; Singh, R.I.; Xess, I.; Mathur, P.; Hasan, F.; Misra, M.C. Candida rugosa: A possible emerging cause of candidaemia in trauma patients. Infection 2010, 38, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Guimarães, T.; Silva, L.R.B.F.; de Almeida Monfardini, L.P.; Cunha, A.K.B.; Rady, P.; Alves, T.; Rosas, R.C. Prospective Observational Study of Candidemia in São Paulo, Brazil: Incidence Rate, Epidemiology, and Predictors of Mortality. Infect. Control Hosp. Epidemiol. 2007, 28, 570–576. [Google Scholar] [CrossRef]
- da Matta, D.A.; de Almeida, L.P.; Machado, A.M.; Azevedo, A.C.; Kusano, E.J.U.; Travassos, N.F.; Salomão, R.; Colombo, A.L. Antifungal susceptibility of 1000 Candida bloodstream isolates to 5 antifungal drugs: Results of a multicenter study conducted in São Paulo, Brazil, 1995–2003. Diagn. Microbiol. Infect. Dis. 2007, 57, 399–404. [Google Scholar] [CrossRef]
- Minces, L.R.; Ho, K.S.; Veldkamp, P.J.; Clancy, C.J. Candida rugosa: A distinctive emerging cause of candidaemia. A case report and review of the literature. Scand. J. Infect. Dis. 2009, 41, 892–897. [Google Scholar] [CrossRef]
- Piatti, G.; Feltrin, S.; Fellini, E.; Barbero, V.; Ballestrero, A. Catheter-Related Sepsis by Candida Pararugosa in an Adult Patient under Chemotherapy Regimen. Case Rep. Infect. Dis. 2021, 2021, 8858157. [Google Scholar] [CrossRef]
- El Helou, G.; Palavecino, E. Candida pararugosa: First Reported Bloodstream Infection in an Adult. Cureus 2017, 9, e1283. [Google Scholar] [CrossRef]
- Noni, M.; Stathi, A.; Velegraki, A.; Malamati, M.; Kalampaliki, A.; Zachariadou, L.; Michos, A. Rare Invasive Yeast Infections in Greek Neonates and Children, a Retrospective 12-Year Study. J. Fungi 2020, 6, 194. [Google Scholar] [CrossRef]
- Arastehfar, A.; Shaban, T.; Zarrinfar, H.; Roudbary, M.; Ghazanfari, M.; Hedayati, M.-T.; Sedaghat, A.; Ilkit, M.; Najafzadeh, M.J.; Perlin, D.S. Candidemia among Iranian Patients with Severe COVID-19 Admitted to ICUs. J. Fungi 2021, 7, 280. [Google Scholar] [CrossRef]
- Oliveira, V.K.P.; Ruiz, L.d.S.; Oliveira, N.A.J.; Moreira, D.; Hahn, R.C.; de Azevedo Melo, A.S.; Nishikaku, A.S.; Paula, C.R. Fun-gemia Caused by Candida Species in a Children’s Public Hospital in the City of São Paulo, Brazil: Study in the Period 2007-Revista do Instituto de Medicina Tropical de São Paulo. Rev. Inst. Med. Trop. São Paulo 2014, 56, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Peremalo, T.; Madhavan, P.; Hamzah, S.; Than, L.; Wong, E.H.; Nasir, M.D.M.; Chong, P.P.; Ng, K.P. Antifungal susceptibilities, biofilms, phospholipase and proteinase activities in the Candida rugosa complex and Candida pararugosa isolated from tertiary teaching hospitals. J. Med. Microbiol. 2019, 68, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.W.; Zhang, J.P.; Lawhon, S.; Fothergill, S.D.; Cano, A.W.; Sutton, J.; DA Paredes, K. Molecular Identification and Antifungal Susceptibility Testing of Clinical Isolates of the Candida Rugosa Species Complex and Proposal of the New Spe-cies Candida Neorugosa. J. Clin. Microbiol. 2012, 50, 2397–2403. [Google Scholar]
- Epis, S.; Capone, A.; Martin, E.; Paolucci, M.; Bazzocchi, C.; Valzano, M.; Bozic, J.; Novati, S.; Favia, G.; Ricci, I. A rapid qPCR method to investigate the circulation of the yeast Wickerhamomyces anomalus in humans. New Microbiol. 2015, 38, 577–581. [Google Scholar]
- Passoth, V.; Olstorpe, M.; Schnürer, J. Past, present and future research directions with Pichia anomala. Antonie Leeuwenhoek 2010, 99, 121–125. [Google Scholar] [CrossRef]
- Medina, I.R.; Fuentes, L.R.; Arteaga, M.B.; Valcárcel, F.R.; Arbelo, F.A.; del Castillo, D.P.; Suárez, S.D.; Quintana, O.F.; Gutiérrez, B.V.; Sergent, F.S.; et al. Pigeons and their droppings as reservoirs of Candida and other zoonotic yeasts. Rev. Iberoam. Micol. 2017, 34, 211–214. [Google Scholar] [CrossRef]
- Yılmaz-Semerci, S.; Demirel, G.; Tastekin, A. Wickerhamomyces anomalus blood stream infection in a term newborn with pneumonia. Turk. J. Pediatr. 2017, 59, 349–351. [Google Scholar] [CrossRef]
- Jung, J.; Moon, Y.S.; Yoo, J.A.; Lim, J.-H.; Jeong, J.; Jun, J.-B. Investigation of a nosocomial outbreak of fungemia caused by Candida pelliculosa (Pichia anomala) in a Korean tertiary care center. J. Microbiol. Immunol. Infect. 2018, 51, 794–801. [Google Scholar] [CrossRef]
- Suhr, M.J.; Gomes-Neto, J.C.; Banjara, N.; Florescu, D.F.; Mercer, D.F.; Iwen, P.C.; Hallen-Adams, H.E. Epidemiological In-vestigation of Candida Species Causing Bloodstream Infection in Paediatric Small Bowel Transplant Recipients. Mycoses 2017, 60, 366–374. [Google Scholar] [CrossRef]
- Lin, H.-C.; Lin, H.-Y.; Su, B.-H.; Ho, M.-W.; Ho, C.-M.; Lee, C.-Y.; Lin, M.-H.; Hsieh, H.-Y.; Lin, H.-C.; Li, T.-C.; et al. Reporting an outbreak of Candida pelliculosa fungemia in a neonatal intensive care unit. J. Microbiol. Immunol. Infect. 2013, 46, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, Y.; Li, Y.; Chen, X.; Ding, C.; Liu, Y. Risk Factors and Biofilm Formation Analyses of Hospital-Acquired In-fection of Candida Pelliculosa in a Neonatal Intensive Care Unit. BMC Infect. Dis. 2021, 21, 620. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, W.; Ding, L.; Yang, L.; Su, J.; Wu, B. Two different clones of Candida pelliculosa bloodstream infection in a tertiary neonatal intensive care unit. J. Infect. Dev. Ctries. 2021, 15, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, L.; Bednářová, D.; Hamal, P. The Prevalence of Candida Pelliculosa, Candida Utilis, and Candida Fabianii in the Olomouc University Hospital: Epidemiological Study. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Pro. Epidemiologii A Mikrobiol. Ceske Lek. Spol. JE Purkyne 2016, 65, 34–38. [Google Scholar]
- Cecarini, V.; Cuccioloni, M.; Bonfili, L.; Ricciutelli, M.; Valzano, M.; Cappelli, A.; Amantini, C.; Favia, G.; Eleuteri, A.M.; Angeletti, M.; et al. Identification of a Killer Toxin from Wickerhamomyces anomalus with β-Glucanase Activity. Toxins 2019, 11, 568. [Google Scholar] [CrossRef]
- Parafati, L.; Cirvilleri, G.; Restuccia, C.; Wisniewski, M. Potential Role of Exoglucanase Genes (WaEXG1 and WaEXG2) in the Biocontrol Activity of Wickerhamomyces anomalus. Microb. Ecol. 2016, 73, 876–884. [Google Scholar] [CrossRef]
- Harit, T.; Bellaouchi, R.; Rokni, Y.; Riahi, A.; Malek, F.; Asehraou, A. Synthesis, Characterization, Antimicrobial Activity, and Docking Studies of New Triazolic Tripodal Ligands. Chem. Biodivers. 2017, 14, e1700351. [Google Scholar] [CrossRef]
- Paris, A.P.; Persel, C.; Serafin, C.F.; Simão, R.d.C.G.; Gandra, R.F. Susceptibility of Candida albicans Isolated from Blood to Wickerhamomyces anomalous Mycocins. Curr. Microbiol. 2016, 73, 878–884. [Google Scholar] [CrossRef]
- Tay, S.-T.; Lim, S.-L.; Tan, H.-W. Growth inhibition of Candida species by Wickerhamomyces anomalus mycocin and a lactone compound of Aureobasidium pullulans. BMC Complement. Altern. Med. 2014, 14, 439. [Google Scholar] [CrossRef]
- Vasconcelos, N.M.; Fontes, J.M.; Lins, M.; Bernardo, G.R.B.; Araújo, J.M.; Lima, G.M.S. Streptomyces ansochromogenes Tur-10 produces a substance with antifungal bioactivity. Genet. Mol. Res. 2015, 14, 5435–5444. [Google Scholar] [CrossRef]
- Esgin, H.; Bulut, E.; Örüm, Ç. Candida pelliculosa endophthalmitis after cataract surgery: A case report. BMC Res. Notes 2014, 7, 169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanada, K.; Miyokawa, N.; Sano, A.; Igarashi, S.; Yoshida, A. Fungal Dacryocystitis with Cacosmia after Penetrating Kera-toplasty—Taxonomy and Identification of Pathogenic Fungi Based on DNA Sequence Analysis. Nippon Ganka Gakkai Zasshi 2012, 116, 1144–1149. [Google Scholar] [PubMed]
- Dutra, V.R.; Silva, L.F.; Oliveira, A.N.M.; Beirigo, E.F.; Arthur, V.M.; Da Silva, R.B.; Ferreira, T.B.; Andrade-Silva, L.; Silva, M.V.; Fonseca, F.M.; et al. Fatal Case of Fungemia by Wickerhamomyces anomalus in a Pediatric Patient Diagnosed in a Teaching Hospital from Brazil. J. Fungi 2020, 6, 147. [Google Scholar] [CrossRef]
- Kamoshita, M.; Matsumoto, Y.; Nishimura, K.; Katono, Y.; Murata, M.; Ozawa, Y.; Shimmura, S.; Tsubota, K. Wickerhamo-myces Anomalus Fungal Keratitis Responds to Topical Treatment with Antifungal Micafungin. J. Infect. Chemother. 2015, 21, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, L.; Davies, J.; Anson, J.; Hales, S.; Beeching, N.J.; Beadsworth, M.B.J. Candida Pelliculosa Meningitis as an Oppor-tunistic Infection in HIV: The First Reported Case. Int. J. STD AIDS 2011, 22, 54–56. [Google Scholar] [CrossRef]
- Cartier, N.; Chesnay, A.; N’Diaye, D.; Thorey, C.; Ferreira, M.; Haillot, O.; Bailly, É.; Desoubeaux, G. Candida nivariensis: Identification strategy in mycological laboratories. J. Mycol. Médicale 2020, 30, 101042. [Google Scholar] [CrossRef]
- Tay, S.T.; Lotfalikhani, A.; Sabet, N.S.; Ponnampalavanar, S.; Sulaiman, S.; Na, S.L.; Ng, K.P. Occurrence and Characteriza-tion of Candida Nivariensis from a Culture Collection of Candida Glabrata Clinical Isolates in Malaysia. Mycopathologia 2014, 178, 307–314. [Google Scholar] [CrossRef]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. Fluconazole Resistance in Isolates of Uncommon Pathogenic Yeast Species from the United Kingdom. Antimicrob. Agents Chemother. 2019, 63, e00211-19. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Salehi, M.-R.; Zarrinfar, H.; Khodavaisy, S.; Haas, P.-J.; Roudbary, M.; Najafzadeh, M.-J.; Zomorodian, K.; Charsizadeh, A. Corrigendum: Molecular Characterization and Antifungal Susceptibility Testing of Can-dida Nivariensis from Blood Samples-an Iranian Multicentre Study and a Review of the Literature. J. Med. Microbiol. 2019, 68, 1695. [Google Scholar] [CrossRef]
- Enache-Angoulvant, A.; Guitard, J.; Grenouillet, F.; Martin, T.; Durrens, P.; Fairhead, C.; Hennequin, C. Rapid Discrimination between Candida glabrata, Candida nivariensis, and Candida bracarensis by Use of a Singleplex PCR. J. Clin. Microbiol. 2011, 49, 3375–3379. [Google Scholar] [CrossRef]
- Decat, E.; Van Mechelen, E.; Saerens, B.; Vermeulen, S.J.T.; Boekhout, T.; De Blaiser, S.; Vaneechoutte, M.; Deschaght, P. Rapid and Accurate Identification of Isolates of Candida Species by Melting Peak and Melting Curve Analysis of the Inter-nally Transcribed Spacer Region 2 Fragment (ITS2-MCA). Res. Microbiol. 2013, 164, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xu, J.; Shao, Y.; Gong, J.; Zhao, F.; He, L.; Shan, X. Rapid identification of the Candida glabrata species complex by high-resolution melting curve analysis. J. Clin. Lab. Anal. 2020, 34, e23226. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, Y.; Fan, S.; Vitagliano, A.; Liu, X.; Liao, y.; Liang, Y.; Vitale, S.G. Clinical Characteristics and Antifungal Susceptibility of Candida nivariensis from Vulvovaginal Candidiasis. Gynecol. Obstet. Investig. 2020, 85, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Ortiz, A.; Mateo, E.; Ortega-Riveros, M.; De-La-Pinta, I.; Quindós, G.; Eraso, E. Caenorhabditis elegans as a Model System To Assess Candida glabrata, Candida nivariensis, and Candida bracarensis Virulence and Antifungal Efficacy. Antimicrob. Agents Chemother. 2020, 64, e00824-20. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Gupta, S.; Chaudhary, M.; Raj, A.T.; Awan, K.H.; Patil, S. Oral candida prevalence and species specificity in leprosy. Disease-a-Month 2020, 66, 100920. [Google Scholar] [CrossRef]
- López-Soria, L.M.; Bereciartua, E.; Santamaría, M.; Soria, L.M.; Hernández-Almaraz, J.L.; Mularoni, A.; Nieto, J.; Montejo, M. First Case Report of Catheter-Related Fungemia by Candida Nivariensis in the Iberian Peninsula. Rev. Iberoam. Micol. 2012, 30, 69–71. [Google Scholar] [CrossRef]
- Sikora, M.; Kuthan, R.; Piskorska-Malolepsza, K.; Golas-Pradzynska, M.; Domański, D.; Augustynowicz-Kopeć, E.; Swoboda-Kopec, E. Prevalence and Antifungal Susceptibility of the Emerging Fungal Species, Candida nivariensis, Isolated in a Teaching Hospital in Poland. Pol. J. Microbiol. 2019, 68, 303–308. [Google Scholar] [CrossRef]
- Li, J.; Shan, Y.; Fan, S.; Liu, X. Prevalence of Candida nivariensis and Candida bracarensis in Vulvovaginal Candidiasis. Mycopathologia 2014, 178, 279–283. [Google Scholar] [CrossRef]
- Morales-López, S.; Dudiuk, C.; Vivot, W.; Szusz, W.; Córdoba, S.B.; Garcia-Effron, G. Phenotypic and Molecular Evaluation of Echinocandin Susceptibility of Candida glabrata, Candida bracarensis, and Candida nivariensis Strains Isolated during 30 Years in Argentina. Antimicrob. Agents Chemother. 2017, 61, e00170-17. [Google Scholar] [CrossRef]
- Treviño-Rangel, R.D.J.; Espinosa-Pérez, J.F.; Villanueva-Lozano, H.; Montoya, A.M.; Andrade, A.; Bonifaz, A.; González, G.M. First report of Candida bracarensis in Mexico: Hydrolytic enzymes and antifungal susceptibility pattern. Folia Microbiol. 2018, 63, 517–523. [Google Scholar] [CrossRef]
- Correia, A.; Sampaio, P.; James, S.; Pais, C. Candida bracarensis sp. nov., a novel anamorphic yeast species phenotypically similar to Candida glabrata. Int. J. Syst. Evol. Microbiol. 2006, 56, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Silva, S.; Botelho, C.; Sampaio, P.; Pais, C.; Henriques, M. Candida bracarensis: Evaluation of Virulence Factors and its Tolerance to Amphotericin B and Fluconazole. Mycopathologia 2015, 180, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lotfali, E.; Fattahi, A.; Sayyahfar, S.; Ghasemi, R.; Rabiei, M.M.; Fathi, M.; Vakili, K.; Deravi, N.; Soheili, A.; Toreyhi, H.; et al. A Review on Molecular Mechanisms of Antifungal Resistance in Candida glabrata: Update and Recent Advances. Microb. Drug Resist. 2021, 27, 1371–1388. [Google Scholar] [CrossRef] [PubMed]
- Telleria, O.; Ezpeleta, G.; Herrero, O.; Miranda-Zapico, I.; Quindós, G.; Cisterna, R. Validation of the PCR–dHPLC method for rapid identification of Candida glabrata phylogenetically related species in different biological matrices. J. Chromatogr. B 2012, 893–894, 150–156. [Google Scholar] [CrossRef]
- Miranda-Zapico, I.; Eraso, E.; Hernández-Almaraz, J.L.; López-Soria, L.M.; Carrillo-Muñoz, A.J.; Hernández-Molina, J.M.; Quindós, G. Prevalence and antifungal susceptibility patterns of new cryptic species inside the species complexes Candida parapsilosis and Candida glabrata among blood isolates from a Spanish tertiary hospital. J. Antimicrob. Chemother. 2011, 66, 2315–2322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swoboda-Kopeć, E.; Sikora, M.; Golas, M.; Piskorska, K.; Gozdowski, D.; Netsvyetayeva, I. Candida nivariensis in comparison to different phenotypes of Candida glabrata. Mycoses 2014, 57, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Małek, M.; Mrowiec, P.; Klesiewicz, K.; Skiba-Kurek, I.; Szczepański, A.; Białecka, J.; Żak, I.; Bogusz, B.; Kędzierska, J.; Budak, A.; et al. Prevalence of human pathogens of the clade Nakaseomyces in a culture collection—the first report on Candida bracarensis in Poland. Folia Microbiol. 2018, 64, 307–312. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Alanazi, A.F.; Ahmad, S.; Al-Sweih, N.; Khan, Z. Lack of detection of Candida nivariensis and Candida bracarensis among 440 clinical Candida glabrata sensu lato isolates in Kuwait. PLoS ONE 2019, 14, e0223920. [Google Scholar] [CrossRef]
- Hou, X.; Xiao, M.; Chen, S.C.; Kong, F.; Wang, H.; Fan, X.; Zhao, Y.-P.; Xu, Y.-C. Identification of Candida glabrata complex species: Use of Vitek MS® RUO & Bruker ClinproTools®. Future Microbiol. 2018, 13, 645–657. [Google Scholar] [CrossRef]
- Morales-lópez, S.; Dudiuk, C.; Vivot, W.; Szusz, W. Crossm Phenotypic and Molecular Evaluation of Echinocandin Suscepti-bility of Candida. Antimicrob. Agents Chemother. 2017, 61, 7–10. [Google Scholar]
- Hasejima, N.; Kamei, K.; Matsubayashi, M.; Kawabe, R.; Shimura, C.; Hijikata, N.; Oda, T.; Matsushima, H. The first case of bloodstream infection by Candida intermedia in Japan: The importance of molecular identification. J. Infect. Chemother. 2011, 17, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Charsizadeh, A.; Mirhendi, H.; Nikmanesh, B.; Eshaghi, H.; Makimura, K. Microbial epidemiology of candidaemia in neonatal and paediatric intensive care units at the Children’s Medical Center, Tehran. Mycoses 2017, 61, 22–29. [Google Scholar] [CrossRef]
- Shirkhani, S.; Sepahvand, A.; Mirzaee, M.; Anbari, K. Phospholipase and proteinase activities of Candida spp. isolates from vulvovaginitis in Iran. J. Mycol. Médicale 2016, 26, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hesham, A.E.-L.; Gupta, V.K.; Singh, B. Use of PCR-denaturing gradient gel electrophoresis for the discrimination of Candida species isolated from natural habitats. Microb. Pathog. 2018, 120, 19–22. [Google Scholar] [CrossRef]
- Tietz, H.J.; Küssner, A.; Thanos, M.; de Andrade, M.P.; Presber, W.; Schönian, G. Phenotypic and genotypic characterization of unusual vaginal isolates of Candida albicans from Africa. J. Clin. Microbiol. 1995, 33, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Al-Hedaithy, S.S.A.; Fotedar, R. Recovery and studies on chlamydospore-negativeCandida albicansisolated from clinical specimens. Med. Mycol. 2002, 40, 301–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romeo, O.; Criseo, G. Molecular Epidemiology of Candida albicans and Its Closely Related Yeasts Candida dubliniensis and Candida africana. J. Clin. Microbiol. 2009, 47, 212–214. [Google Scholar] [CrossRef]
- Romeo, O.; Criseo, G. Morphological, biochemical and molecular characterisation of the first ItalianCandida africanaisolate. Mycoses 2009, 52, 454–457. [Google Scholar] [CrossRef]
- Alonso-Vargas, R.; Elorduy, L.; Eraso, E.; Cano, J.F.; Guarro, J.; Pontón, J.; Quindós, G. Isolation of Candida Africana, Proba-ble Atypical Strains of Candida Albicans, from a Patient with Vaginitis. Med. Mycol. 2008, 46, 167–170. [Google Scholar] [CrossRef]
- Odds, F.C.; Bougnoux, M.-E.; Shaw, D.J.; Bain, J.M.; Davidson, A.D.; Diogo, D.; Jacobsen, M.D.; Lecomte, M.; Li, S.-Y.; Tavanti, A.; et al. Molecular Phylogenetics of Candida albicans. Eukaryot. Cell 2007, 6, 1041–1052. [Google Scholar] [CrossRef]
- Tietz, H.-J.; Hopp, M.; Schmalreck, A.; Sterry, W.; Czaika, V. Candida africana sp. nov., a new human pathogen or a variant of Candida albicans? Mycoses 2001, 44, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Forche, A.; Schönianb, G.; Gräserb, Y.; Vilgalys, R.; Mitchell, T.G. Genetic Structure of Typical and Atypical Populations of Candida albicans from Africa. Fungal Genet. Biol. 1999, 28, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Muralidhar, S.; Xu, J.; Meis, J.F.; Chowdhary, A. Multilocus sequence typing of Candida Africana from patients with vulvovaginal candidiasis in New Delhi, India. Mycoses 2014, 57, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Hazirolan, G.; Altun, H.U.; Gumral, R.; Gursoy, N.C.; Otlu, B.; Sancak, B. Prevalence of Candida africana and Candida dubliniensis, in vulvovaginal candidiasis: First Turkish Candida africana isolates from vulvovaginal candidiasis. J. Mycol. Med. 2017, 27, 376–381. [Google Scholar] [CrossRef]
- Yazdanparast, S.A.; Khodavaisy, S.; Fakhim, H.; Shokohi, T.; Haghani, I.; Nabili, M.; Gholami, H.; Ahmadi, I.; Badali, H. Molecular Characterization of Highly Susceptible Candida africana from Vulvovaginal Candidiasis. Mycopathologia 2015, 180, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Majdabadi, N.; Falahati, M.; Heidarie-Kohan, F.; Farahyar, S.; Rahimi-Moghaddam, P.; Ashrafi-Khozani, M.; Razavi, T.; Mohammadnejad, S. Effect of 2-Phenylethanol as Antifungal Agent and Common Antifungals (Amphotericin B, Fluconazole, and Itraconazole) on Candida Species Isolated from Chronic and Recurrent Cases of Candidal Vulvovaginitis. ASSAY Drug Dev. Technol. 2018, 16, 141–149. [Google Scholar] [CrossRef]
- Naeimi, B.; Mirhendi, H.; Khamisipour, G.; Sadeghzadeh, F.; Ahmadi, B. Candida Africana in Recurrent Vulvovaginal Can-didiasis (RVVC) Patients: Frequency and Phenotypic and Genotypic Characteristics. J. Med. Microbiol. 2018, 67, 1601–1607. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, A.; Chen, X.; Wang, G.; Feng, X. Molecular Characterization ofCandida africanain Genital Specimens in Shanghai, China. BioMed Res. Int. 2015, 2015, 185387. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Linton, C.J.; Palmer, M.D.; Brown, P.; Johnson, E.M. Epidemiology, Antifungal Susceptibility, and Pathogenicity of Candida Africana Isolates from the United Kingdom. J. Clin. Microbiol. 2013, 51, 967–972. [Google Scholar] [CrossRef]
- Romeo, O.; Criseo, G. Candida africana and its closest relatives. Mycoses 2010, 54, 475–486. [Google Scholar] [CrossRef]
- Ngouana, T.K.; Krasteva, D.; Drakulovski, P.; Toghueo, R.K.; Kouanfack, C.; Ambe, A.; Reynes, J.; Delaporte, E.; Boyom, F.F.; Mallié, M.; et al. Investigation of minor species Candida africana, Candida stellatoidea and Candida dubliniensis in the Candida albicans complex among Yaoundé (Cameroon) HIV-infected patients. Mycoses 2015, 58, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Theill, L.; Dudiuk, C.; Morano, S.; Gamarra, S.; Nardin, M.E.; Méndez, E.; Garcia-Effron, G. Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina. Rev. Argent. Microbiol. 2016, 48, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Fan, S.; Liu, X.; Li, J. Prevalence of Candida albicans-closely related yeasts, Candida africana and Candida dubliniensis, in vulvovaginal candidiasis. Med. Mycol. 2014, 52, 636–640. [Google Scholar] [CrossRef]
- Nnadi, E.; Ayanbimpe, G.; Scordino, F.; Okolo, M.O.; Enweani, I.B.; Criseo, G.; Romeo, O. Isolation and molecular characterization ofCandida africanafrom Jos, Nigeria. Med. Mycol. 2012, 50, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Buckley, H.R.; van Uden, N. Five new Candida species. Mycopathologia 1968, 36, 257–266. [Google Scholar] [CrossRef]
- De Almeida, J.N.; Campos, S.V.; Thomaz, D.Y.; Thomaz, L.; de Almeida, R.K.G.; del Negro, G.M.B.; Gimenes, V.F.; Grenfell, R.C.; Motta, A.L.; Rossi, F.; et al. Candida Blankii: An Emergent Opportunistic Yeast with Reduced Susceptibility to Antifungals Correspondence. Emerg. Microbes Infect. 2018, 7, 1–3. [Google Scholar] [CrossRef]
- Chowdhary, A.; Stielow, J.B.; Upadhyaya, G.; Singh, P.K.; Singh, A.; Meis, J.F. Candida blankii: An emerging yeast in an outbreak of fungaemia in neonates in Delhi, India. Clin. Microbiol. Infect. 2020, 26, 648.e5–648.e8. [Google Scholar] [CrossRef]
- Al-Haqqan, A.; Al-Sweih, N.; Ahmad, S.; Khan, S.; Joseph, L.; Varghese, S.; Khan, Z. Azole-resistant Candida blankii as a newly recognized cause of bloodstream infection. New Microbes. New Infect. 2018, 26, 25–29. [Google Scholar] [CrossRef]
- Kollu, V.S.; Kalagara, P.K.; Islam, S.; Gupte, A. A Report of Candida blankii Fungemia and Possible Endocarditis in an Immunocompetent Individual and the Review of Literature. Cureus 2021, 13, e14945. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Dinh, K.V.; Nguyen, V.D. Biodiversity and Enzyme Activity of Marine Fungi with 28 New Records from the Tropical Coastal Ecosystems in Vietnam. Mycobiology 2021, 49, 559–581. [Google Scholar] [CrossRef]
- Bereczki, L.; Bartha, N.; Kocsube, S.; Soki, J.; Lengyel, G.; Tálosi, G.; Mader, K.; Deák, J.; Doczi, I. Fungaemia caused by Candida pulcherrima. Med. Mycol. 2012, 50, 522–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mpakosi, A.; Siopi, M.; Falaina, V.; Siafakas, N.; Roilides, E.; Kimouli, M.; Theodoraki, M.; Karle, P.; Meletiadis, J. Successful therapy of Candida pulcherrima fungemia in a premature newborn with liposomal amphotericin B and micafungin. Med. Mycol. Case Rep. 2016, 12, 24–27. [Google Scholar] [CrossRef]
- Deconinck, L.; Meybeck, A.; Pradier, M.; Patoz, P.; Melliez, H.; Senneville, E. Community acquired fungemia caused by Candida pulcherrima: Diagnostic contribution of MALDI-TOF mass spectrometry. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Önal, U.; Metin, D.Y.; Karaca, C.; Polat, S.H.; Ersin, S.; Taşbakan, M.I. Retrospective evaluation of candidemic patients among general surgery department in a tertiary care university hospital. Turk. J. Surg. 2019, 35, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Marín Martínez, E.M.; Aller García, A.I.; Martín-Mazuelos, E. Epidemiología, factores de riesgo y sensibilidad in vitro en candidemias por especies diferentes de Candida albicans. Rev. Iberoam. Micol. 2016, 33, 248–252. [Google Scholar] [CrossRef]
- Türkel, S.; Ener, B. Isolation and Characterization of New Metschnikowia Pulcherrima Strains as Producers of the Antimicrobial Pigment Pulcherrimin. 2009. Available online: http://uu245-211.uludag.edu.tr/handle/11452/22813 (accessed on 6 July 2022).
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Isla, G.; Rodriguez, D.; Almirante, B.; Pahissa, A.; Rodriguez-Tudela, J.L.; Group, B.C.P.S. Prevalence of Candida Bracarensis and Candida Nivariensis in a Spanish Collection of Yeasts: Comparison of Re-sults from a Reference Centre and from a Population-Based Surveillance Study of Candidemia. Med. Mycol. 2011, 49, 525–529. [Google Scholar]

| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (noninvasive) Cutaneous candidiasis (n = 1) | Carbohydrate fermentation test | Microscopy | Yes | NR | Miconazole cream and fusidic cream | Tenofovir, lamivudine, and efavirenz | Alive (n = 1) | [22] |
| (invasive) Bloodstream infection (n = 1) | Culture | Microscopy | No at low dose (100-200 mg fluconazole) | Yes | Fluconazole (high and prolonged dose) | NR | Successfully treated with 3 months use of fluconazole (n = 1) | [30] |
| (noninvasive) Infection at the interface of graft and host cornea (n = 1) | MIC | Optical coherence tomography | Yes | No | Fluconazole, voriconazole | Dexamethasone | Alive (n = 1) | [33] |
| (invasive) Infection at blood, respiratory, and urine samples (n = 69) | PCR, antifungal susceptibility testing | NR | Some strains were susceptible | Fluconazole and voriconazole, caspofungin and micafungin, amphotericin B | Fluconazole, voriconazole, caspofungin, micafungin, amphotericin B | NR | NA | [27] |
| (invasive) Bloodstream infection (n = 1) | MIC, genome sequencing | MRI | Yes | NR | Fluconazole, posaconazole | Trimethoprim, sulfamethoxazole | Alive (n = 1) | [25] |
| (invasive) Tubo-ovarian abscess (n = 1) | Culture | CT scan | Yes | No | Fluconazole | Consumption of organic dairy products | Alive (n = 1) | [23] |
| (invasive and noninvasive) Superficial and/or invasive infections (n = 2) | Anti-fungal susceptibility testing, Mass spectrometry | NR | Yes | No | Amphotericin B, itraconazole, voriconazole, posaconazole, fluconazole, caspofungin micafungin, anidulafungin | NR | NA | [34] |
| (invasive) Derived from blood, urine, bronchus, abdominal, and throat samples (n = 10) | Antifungal susceptibility testing, | Scanning electron microscopy | Lower in vitro susceptibility | Development of resistance | Fluconazole, amphotericin B, caspofungin, micafungin | No | NA | [31] |
| (noninvasive) Mucocutaneous candidiasis (n = 10) | Antifungal susceptibility testing, PCR, Sequencing | NR | Yes | No | Fluconazole, itraconazole amphotericin B | No | NA | [24] |
| (invasive) Bloodstream infection (n = 3) | Biochemical and molecular methods | NR | Susceptible to most of the antifungals | Amphotericin B | Fluconazole, voriconazole, caspo/anidulafungin, amphotericin B | No | NA | [28] |
| (invasive) Bloodstream infection (n = 1) | PCR, sequencing of the ITS region ofrDNA | NR | Yes | No | Amphotericin B, itraconazole, voriconazole, posaconazole, fluconazole, caspofungin micafungin, anidulafungin | No | NA | [21] |
| (invasive) Bloodstream infection (n = 2) | Gram staining, and germ tube test | Microscopy | Yes | No | Azoles, echinocandins | No | NA | [20] |
| (invasive) (n = 83) | Antifungal susceptibility testing, PCR | NR | No | Yes | Micafungin, liposomal amphotericin B, flucytosine | Yogurt | All alive (n = 83) | [29] |
| (invasive) Fungal sinusitis (n = 1) | Germ tube and sugar assimilation test | NR | Yes | No | Amphotericin B | No | Recovered completely (n = 1) | [35] |
| (invasive) Blood, bile and stool infection (n = 1) | Antifungal susceptibility testing, PCR | NR | No | Yes | Caspofungin, micafungin, and anidulafungin | No | Recovered completely (n = 1) | [36] |
| Saliva (n = 92) | Antifungal susceptibility testing, RAPD | NR | Yes | No | Fluconazole and itraconazole | No | Alive (n = 92) | [37] |
| (invasive and noninvasive) Blood, saliva, urine, broncho alveolar lavage (n = 410) | Germ tube and chlamydospore production tests | NR | Yes | Resistant to Itraconazole | Ketoconazole, itraconazole, voriconazole, caspofungin, amphotericin B | No | No death reported | [38] |
| (invasive) Systemic candidiasis (n = 1) | Ellipsometer test, PCR | NR | Yes | No | Liposomal amphotericin B, fluconazole | Broad spectrum antibiotics | Recovered completely (n = 1) | [39] |
| (invasive) Blood and urine samples (n = 3) | Culture, RAPD, ophthalmologic | Echocardiography, ultrasound check | Yes | No | Liposomal amphotericin B, fluconazole, and itraconazole | No | No death reported (n = 3) | [40] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) Clinical isolates (n = 14) | MIC, Fungicidal and fungistatic activity | NR | Amphotericin B | Fluconazole, itraconazole, voriconazole, posaconazole | NA | NA | NA | [50] |
| (invasive) Oral and systemic candidiasis of HIV patients (n = 1) | Growth on Hicrome Candida, germ tube test, clamydospore formation on corn meal agar, and API20C for sugar assimilation | NR | Amphotericin B, fluconazole | NA | NA | NA | NA | [51] |
| (invasive) Clinical isolate from HIV patient (n = 1) | Culture | microscopy | Fluconazole itraconazole voriconazole amphotericin B | NA | No treatment with antifungal or antimicrobial agents | NA | NA | [52] |
| (invasive) Oropharyngeal candidiasis in HIV patient (2.9% had C. norvegensis infection) (n = 4) | Culture, germ tube and chlamydosporulation tests | microscopy | ND | ND | Nystatin and clotrimazole | NA | NA | [53] |
| (invasive) Clinical Isolates from oral cavity, stools/anal, respiratory, urine and, blood/catheter of Candidemia patients (n = 2) | Aux- anogram panel ID 32C Gene sequencing | NR | Itraconazole, voriconazole, amphotericin B, caspofungine, posaconazole | Fluconazole | Fluconazole | Antibiotic | All alive | [54] |
| (invasive candidiasis) in HCV-related cirrhosis and hepatocarcinoma (n = 1) | blood cultures polymerase chain reaction-sequencing | NR | Anidulafungin | Azoles | Anidulafungin | Vancomycin and piperacillin/tazobactam, and linezolid plus meropenem | Alive | [55] |
| (invasive candidiasis) in hepatocarcinoma (n = 1) | Blood cultures MALDI-TOF MS | NR | Amphotericin B, itraconazole, voriconazole, caspofungin | Flucytosine Fluconazole | Fluconazole, anidulafungin | Meropenem, vancomycin, amikacin, and prophylactic | Died | [56] |
| (invasive candidiasis) in peritonitis (n = 1) | Blood culture | CT Scan-abdomen | Voriconazole | NA | Fluconazole, itraconazole | NA | Alive | [57] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (noninvasive) Sample collected from alcoholic beverages (n = NR) | PCR, Sequencing, Enzyme profiling | NR | Amphotericin B and micafungin | Itraconazole, fluonazole, | Itraconazole, fluconazole, amphotericin B and micafungin | No | NA | [40] |
| (invasive and noninvasive) Samples from oral cavity, anal/stools, urine respiratory, blood/catheter (n = 12) | Ellipsometer test, MIC | NR | Caspofungin | Fluconazole-resistant | Itraconazole, voriconazole, posaconazole isavuconazole, fluconazole, amphotericin B and caspofungin | Broad-spectrum antibiotic | Died (n = 1) | [42] |
| (invasive) Blood sample (n = 2) | MIC, Fungicidal and fungistatic activity | NR | Echinocandins | Azoles | Fluconazole, caspofungin | No | NA | [43] |
| (invasive) Systemic mycosis (n = 168) | Antifungal susceptibility and Ellipsometer test, MALDI-TOF MS | NR | Susceptible to echinocandins, polyenes | Azoles | Itraconazole, voriconazole, posaconazole, isavuconazole, fluconazole, Caspofungin, micafungin, anidulafungin, Amphotericin B, | No | NA | [44] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) Clinical isolates (n = 27) | MIC, Fungicidal and fungistatic activity | NR | Amphotericin B, voriconazole | Anidulafungin, micafungin | NR | NA | NR | [50] |
| (invasive) Catheter-related candidemia/Acute pancreatitis (n = 1) | Blood culture using VITEK 2 YST system | NR | Itraconazole, voriconazole, 5-flucytosine, amphotericin B | NA | Fluconazole, micafungin | Flomoxef | Alive | [67] |
| (invasive) Catheter-related candidemia/severe oral mucositis (n = 1) | Blood cultures | Microscopy | Fluconazole, itraconazole, amphotericin B and caspofungin | 5-flucytosine | Caspofungin, fluconazole | Cefoperazone-sulbactam and amikacin meropenem, teicoplanin | Alive | [70] |
| (invasive) Bloodstream infections/ (invasive) Clinical isolates (n = 20) (endocarditis, fungemia) | Blood cultures and RNA Sequencing | Microscopy | Amphotericin B, posaconazole, voriconazole, caspofungin | Fluconazole, flucytosine | NR | NA | NR | [71] |
| (invasive) Yarrowia lipolytica fungemia (n = 13) | Blood cultures | Microscopy | Voriconazole, caspofungin, micafungin, anidulafungin, amphotericin B | Fluconazole, itraconazole and posaconazole | Fluconazole | NA | Alive (n = 10) 3 patients died even after treatment with fluconazole | [69] |
| Invasive candidiasis (n = 16) isolates of C. lipolytica | Vitek and API yeast identification systems | NR | Voriconazole echinocandins | Amphotericin B, fluconazole | Amphotericin B | NA | NR | [72] |
| (invasive) Fungemia (n = 2) | Blood cultures | Microscopy | Voriconazole, caspofungin, amphotericin B, posaconazole, itraconazole, ketoconazole | Fluconazole | Caspofungin, voriconazole | NA | All alive | [73] |
| (invasive) Catheter-Related Candidemia caused by C. lipolytica/blood and the central venous catheter (n = 1) | Blood cultures Biochemical tests | NR | Amphotericin B | Azoles | Trimethoprim-sulfamethoxazole, amphotericin B | Cyclosporine, acyclovir | Died | [74] |
| (invasive) Catheter-Related Fungemia caused by C. lipolytica (n = 3) | Blood culturesBiochemical tests (2) Corneal biopsy culture (1) | NR | Fluconazole, Micafungin (n = 2); Itraconazole, voriconazole, amphotericin B (n = 1) | Fluconazole and 5-flucytosine (n = 1) | Fluconazole | Natamycin, imipenem | All alive | [68] |
| (invasive) Candida lipolytica fungemia (n = 5) (paediatric patients) | Blood cultures | Microscopy | Amphotericin B. Very low susceptibility to fluconazole and itraconazole | NR | Patient 1 and 5: fluconazole Patient 2: no treatment Patient 3 and 4: amphotericin B | NA | All alive | [75] |
| (invasive) Fungemia/Clinical isolates (n = 58) from blood samples, urine, and vaginal site | Blood culture Biochemical tests DNA sequencing | Microscopy | Fluconazole, posaconazole, itraconazole | Low susceptibility to flucytosine, amphotericin B (n = 3), ketoconazole (n = 2), caspofungin (n = 2), both voriconazole and caspofungin (n = 1) both amphotericin B and ketoconazole (n = 21) | NR | NA | NA | [76] |
| (invasive) C. lipolytica fungemia/septicemia (n = 32) | Blood cultures | Microscopy | Amphotericin B (97% of the isolates), fluconazole (69% of the isolates) | NR | NR | NA | Died (n = 12) Alive (n = 20) | [77] |
| (invasive) C. lipolytica fungemia (n = 1) | Diabetic mellitus, renal failure/blood cultures | Microscopy | Itraconazole, voriconazole, amphotericin B, posaconazole, isavuconazole, anidulafungin | Fluconazole | Caspofungin | NA | Died | [78] |
| (invasive) Y. lipolytica fungemia associated with central venous catheter (n = 14) | Blood cultures sequencing | Microscopy | Caspofungin, micafungin, anidulafungi, amphotericin B | Azoles | Fluconazole | NA | Died (n = 3) Alive (n = 11) | [69] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) C. lusitaniae (n = 2) | Blood culture Urine culture | Microscopy Chest radiograph | Liposomal Amphotericin-B, Fluconazole, 5-flucytosine | NR | Liposomal Amphotericin B Vancomycin Amphotericin B deoxycholate | NA | Died (n = 1) Alive (n = 1) | [87] |
| (invasive) C. lusitaniae (n = 1) | Blood culture | Microscopy | NR | NR | Fluconazole | Vancomycin, piperacillin/tazobactam, and levofloxacin | Alive | [88] |
| (invasive) C. lusitaniae (n = 8) | Blood culture | Microscopy PCR | Amphotericin B, fluconazole, voriconazole, caspofungin, micafungin, anidulafungin | Fluconazole (Only two isolates) | Amphotericin B Fluconazole, Caspofungin (in 2 only) | Ampicillin and amikacin | Died (n = 3) Alive (n = 5) | [89] |
| (invasive) C. lusitaniae Candidemia (n = 1) | Blood culture | Microscopy | Fluconazole | Amphotericin B | Amphotericin B, fluconazole | NA | Alive | [90] |
| (invasive) C. lusitaniae Candidemia (n = 1) | Blood culture | Microscopy | Amphotericin B, fluconazole 5-flucytosine Itraconazole, | NR | Fluconazole | NA | Alive | [91] |
| (invasive) C. lusitaniae Candidemia (n = 2) | Blood culture Stool culture | Microscopy | AmphotericinB flucytosine | Ketoconazole and miconazole | Amphotericin B ketoconazole | NA | Died (n = 1) Alive (n = 1) | [92] |
| (invasive) C. lusitaniae fingemia (n = 1) | Blood culture Stool culture | Microscopy | NR | AmphotericinB | Fluconazole | Doxycycline clarithromycin | Alive | [93] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) C. rugosa bloodstream infection (n = 19) | Blood culture PCR | Microscopy | 5-flucytosine, voriconazole and amphotericin B | Fluconazole (only 4) | Fluconazole amphotericin B | NA | Died (n = 13) Alive (n = 6) | [108] |
| (invasive) C. rugosa bloodstream infection (n = 25) | Blood culture PCR | Microscopy | 5-flucytosine, voriconazole | Fluconazole and itraconazole (only 4) | Fluconazole amphotericin B | NA | Died (n = 18) Alive (n = 7) | [109] |
| (invasive) C. rugosa Candidemia (n = 6) | Blood culture PCR | Microscopy | Amphotericin B, fluconazole, and 5-flucytosine | NR | Amphotericin B | NA | Died (n = 5) Alive (n = 1) | [106] |
| (invasive) C. rugosa bloodstream infection (n = 25) | Blood culture | Microscopy | 5-flucytosine, voriconazole, fluconazole amphotericin B itraconazole | Voriconazole, flluconazole | Fluconazole amphotericin B | NA | NR | [110] |
| (invasive) C. rugosa fungemia (n = 1) | Blood culture | Microscopy | NR | NR | Fluconazole | NA | Alive | [111] |
| Invasive/Non- invasive Candidiasis (No. of Human Cases/Strains/Isolates) | Identification Methods | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|
| (invasive) Candidemia (n = 1) | Blood culture, Vitek-2 kit | Fluconazole, flucytosine caspofungin, voriconazole, and amphotericin B | No | Intravenous fluconazole for 2 weeks | NA | Alive (n = 1) | [122] |
| (noninvasive) Endophthalmitis (n = 1) | Culture of anterior chamber | Intraocular amphotericin B | Topical fluconazole | Multiple intraocular amphotericin B | Netilmicin sulfate cyclopentolate HCl eye drops | Alive (n = 1) | [135] |
| (noninvasive) Dacryocystitis with cacosmia (n = 1) | fungal hyphae observed on the excised lacrimal sac wall. DNA sequencing | NR | NR | Antifungal agent and washing of the nasolacrimal duct | NA | Alive (n = 1) | [136] |
| (invasive) Candidemia (n = 6) | Blood culture, API-32C and Mini API system, RAPD | 2 isolates were resistant to Amphotericin B and low susceptibility to Itraconazole | Fluconazole, voriconazole, and micafungin | Amphotericin B and fluconazole | NA | Alive (n = 5) Died (n = 1) | [125] |
| (invasive) Candidemia (n = 1) | Blood culture, VITEC2, (ITS) region amplification and sequencing | Amphotericin B, ketoconazole, itraconazole, voriconazole, and fluconazole | No | Fluconazole and amphotericin B | Ceftriaxone, cefepime. and oxacillin | Alive (n = 1) | [137] |
| (noninvasive) Fungal keratitis (n = 1) | Morphological characteristics and ITS region amplification and sequencing | NR | NR | Topical micafungin | No | Alive (n = 1) | [138] |
| (invasive) Meningitis in HIV patient caused by C. pelliculosa (n = 1) | Phenotypic and molecular methods, Histopathological staining(PAS) from autopsy | NR | NR | NR | Combination antiretroviral therapy (tenofovir, abacavir and atazanavir/ritonavir) | Died (n = 1) | [139] |
| Invasive/Noninvasive Candidiasis (n Human/Cases/Isolate) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments(e.g., Probiotics, Natural Compounds, Antivirals, etc) | Outcome (n) | Reference (s) |
|---|---|---|---|---|---|---|---|---|
| Candida bracarensis | ||||||||
| (noninvasive) Vulvovaginal candidiasis (n = 1) | API Candida system; ITS1 region and the 5.8S ribosomal RNA gene; sequencing | Microscopy (Germ tube test, chlamydospore test) | Susceptible to nystatin and azoles (fluconazole, itraconazole, miconazole, clotrimazole) | NR | NR | NR | NR | [152] |
| (invasive) Peripheral neuropathy in type 1 diabetes (patient’s stool positive for C. bracarensis) (n = 1) | CHROMagar Candida; multiplex PCR; sequencing; MALDI-TOF MS analysis | NR | Amphotericin B, flucytosine, fluconazole, voriconazole, anidulafungin and caspofungin | Itraconazole (MIC ≥ 32 mg/L), posaconazole (MIC ≥ 32 mg/L) | NR | NR | NR | [161] |
| Candida intermedia | ||||||||
| (invasive) Candidemia (with diabetes bloody sputum, fever, and dyspnea) (n = 1) | API ID32C; molecular identification - D1/D2 domain of the large-subunit 26S rRNA gene | NR | Amphotericin B, flucytosine, fluconazole, itraconazole, miconazole, micafungin | NR | Several antifungals | Antibiotic treatment, mechanical ventilation, steroid therapy | Alive, discharged on the 34th hospital day (n = 1) | [165] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other Treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) C. blankii bloodstream infection (n = 1) | Blood sample, CHROMagar Candida, PCR sequencing of rDNA | Microscopy | Voriconazole, itraconazole, posaconazole, amphotericin B, caspofungin, Micafungin, anidulafungin, | Reduced susceptibility to fluconazole b (≥12 μg/mL) | Amphotericin B, fluconazole, caspofungin | Amikacin, ampicillin, cefotaxime, meropenem, teicoplanin, Piperacillin/tazobactam, vancomycin | Died (n = 1) | [192] |
| (invasive) C. blankii fungaemia (n = 9) | ITS and D1/D2 region sequencing | NR | Isavuconazole, posaconazole, itraconazole, voriconazole, micafungin, | Fluconazole had higher MIC (8 μg/mL), Anidulafungin MIC (2 μg/mL) had high MICs | Fluconazole | Broad-spectrum antibiotics | Alive (n = 5), Died (n = 4) | [191] |
| (invasive) C. blankii fungemia and possible endocarditis (n = 1) | Blood culture | NR | Amphotericin B, anidulafungin, 5-flucytosine, itraconazole, micafungin, posaconazole, voriconazole | Fluconazole had higher MIC (16 μg/mL), caspofungin higher MIC (1 μg/mL), | Fluconazole, micafungin and liposomal amphotericin B, voriconazole | Vancomycin, aztreonam, linezolid, daptomycin, meropenem | Alive (n = 1) | [193] |
| (invasive) C. blankii bloodstream infection (n = 1) | Blood cultures, CHROMagar, sequence analysis of the ITS1, and D1D2of the rRNA | NR | Voriconazole, amphotericin B, micafungin | Fluconazole had higher MIC (16 μg/mL), anidulafungin (1 μg/mL) | Liposomal amphotericin B, micafungin | Teicoplanin, meropenem, cotrimoxazole | Alive (n = 1) | [190] |
| Invasive/Noninvasive Candidiasis (n Human Cases/Strains/Isolates) | Identification Methods | Imaging Test | Antifungal Susceptibility | Antifungal Resistance | Antifungal Treatment | Other treatments (e.g., Probiotics, Natural Compounds, Antivirals) | Outcome (n) | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| (invasive) C.pulcherrima (n = 1) | Blood culture | Microscopy and ITS sequencing | Amphotericin B lipid complex | NR | Amphotericin B lipid complex | NA | Alive | [195] |
| (invasive) C.pulcherimma (n = 1) | Blood culture | MALDI-TOF MS | Fluconazole | NR | Fluconazole | NA | Alive | [197] |
| (invasive) C. pulcherimma (n = 1) | Blood culture | Microscopy and ITS sequencing | Liposomal amphotericin B, fluconazole, voriconazole, posoconazole, micafungin, anidulafungin | NR | Liposomal amphotericin B and micafungin | NA | Alive | [196] |
| (invasive) C. pulcherimma (n = 1) | Blood cultures | Microscopy and MALDI-TOF MS | Amphotericin B, azoles, echinocandins | NR | Amphotericin B, voriconazole, echinocandins | NA | Alive | [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Černáková, L.; Rodrigues, C.F. Overview on the Infections Related to Rare Candida Species. Pathogens 2022, 11, 963. https://doi.org/10.3390/pathogens11090963
Kumar S, Kumar A, Roudbary M, Mohammadi R, Černáková L, Rodrigues CF. Overview on the Infections Related to Rare Candida Species. Pathogens. 2022; 11(9):963. https://doi.org/10.3390/pathogens11090963
Chicago/Turabian StyleKumar, Sunil, Awanish Kumar, Maryam Roudbary, Rasoul Mohammadi, Lucia Černáková, and Célia Fortuna Rodrigues. 2022. "Overview on the Infections Related to Rare Candida Species" Pathogens 11, no. 9: 963. https://doi.org/10.3390/pathogens11090963
APA StyleKumar, S., Kumar, A., Roudbary, M., Mohammadi, R., Černáková, L., & Rodrigues, C. F. (2022). Overview on the Infections Related to Rare Candida Species. Pathogens, 11(9), 963. https://doi.org/10.3390/pathogens11090963






