Pulmonary Fibrosis and Hypereosinophilia in TLR9-/- Mice Infected by Cryptococcus gattii
Abstract
:1. Introduction
2. Results
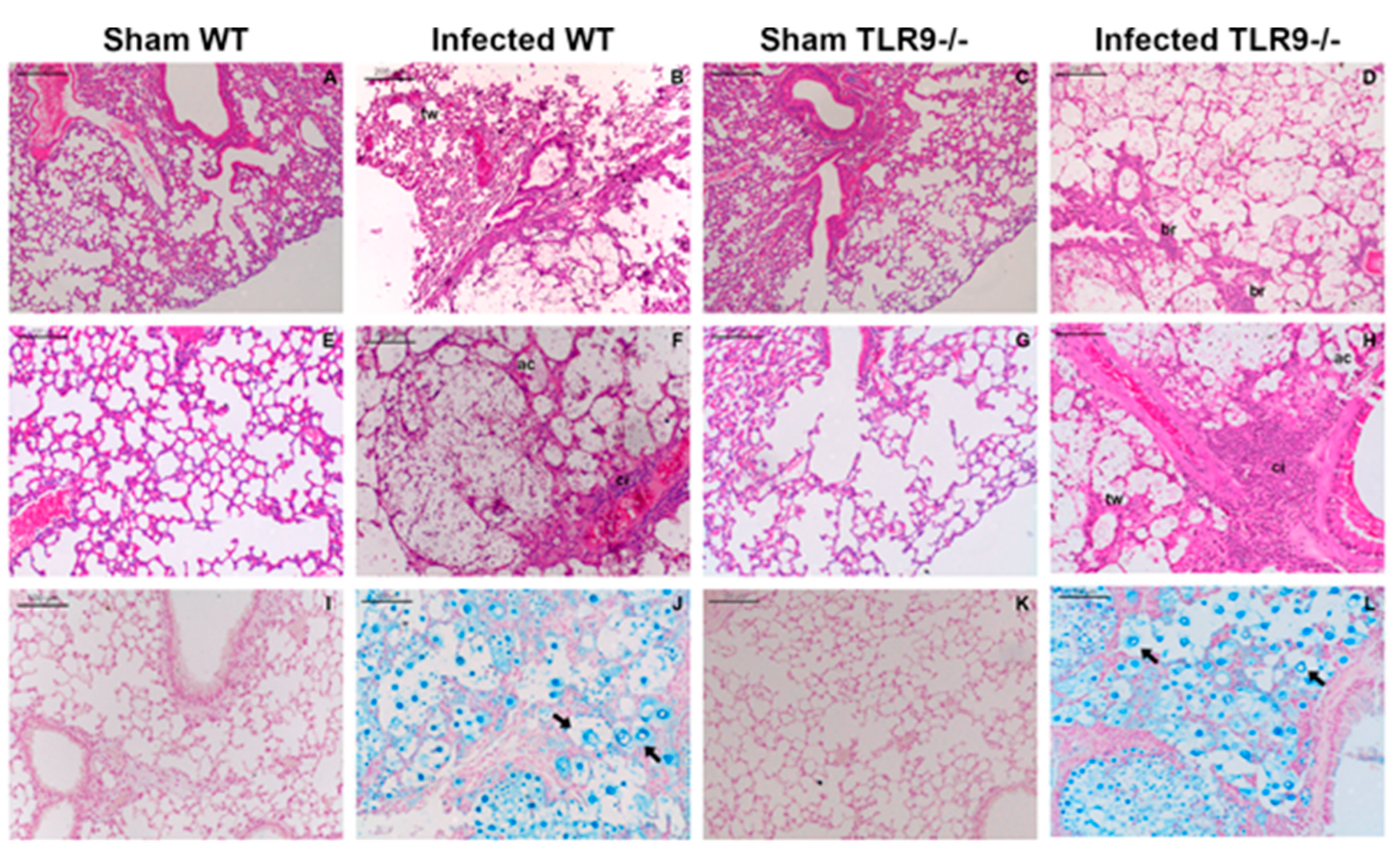
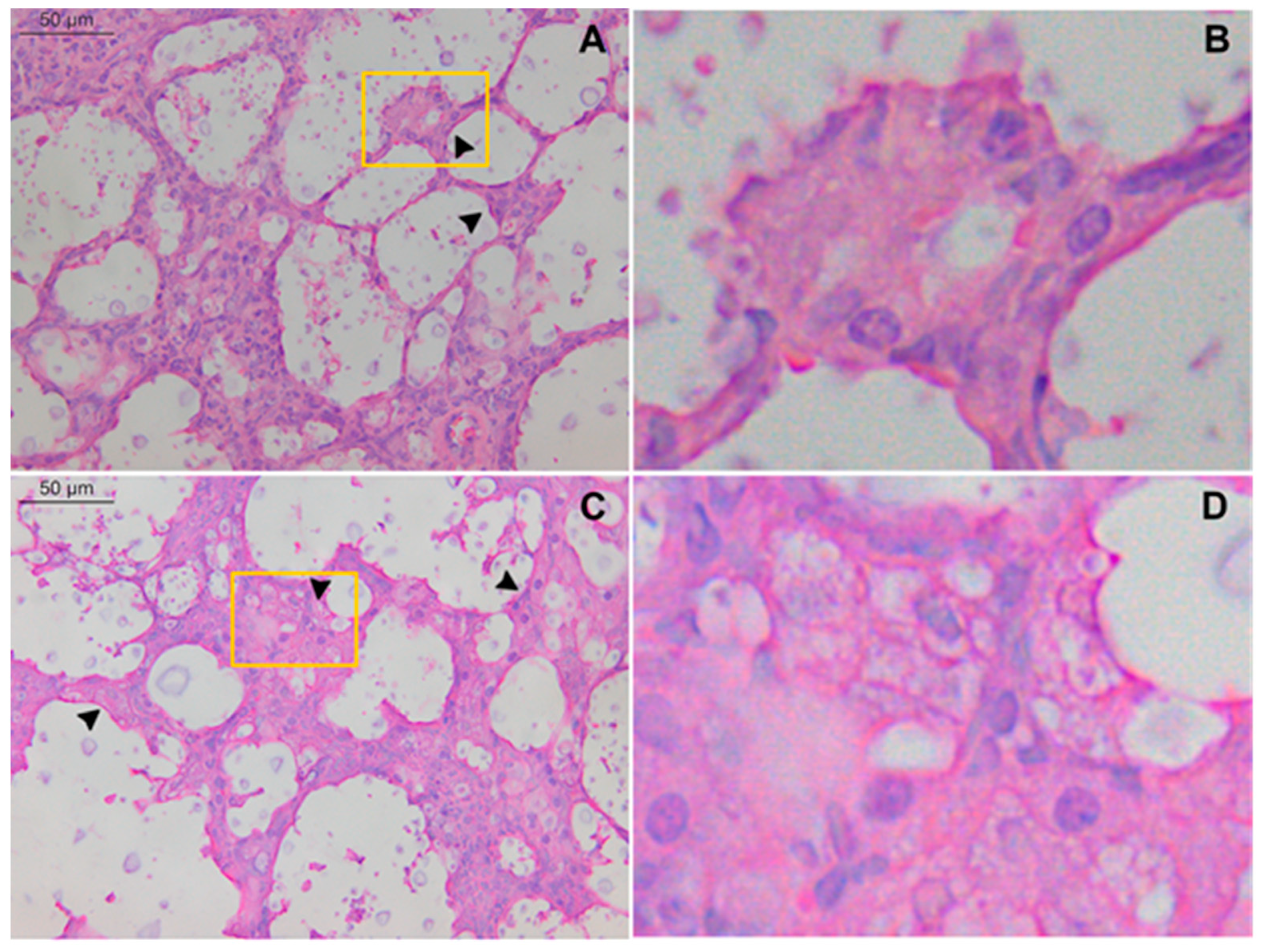
2.1. Intratracheal Infection by C. gattii Causes a Severe Lung Injury with Epithelial Destruction
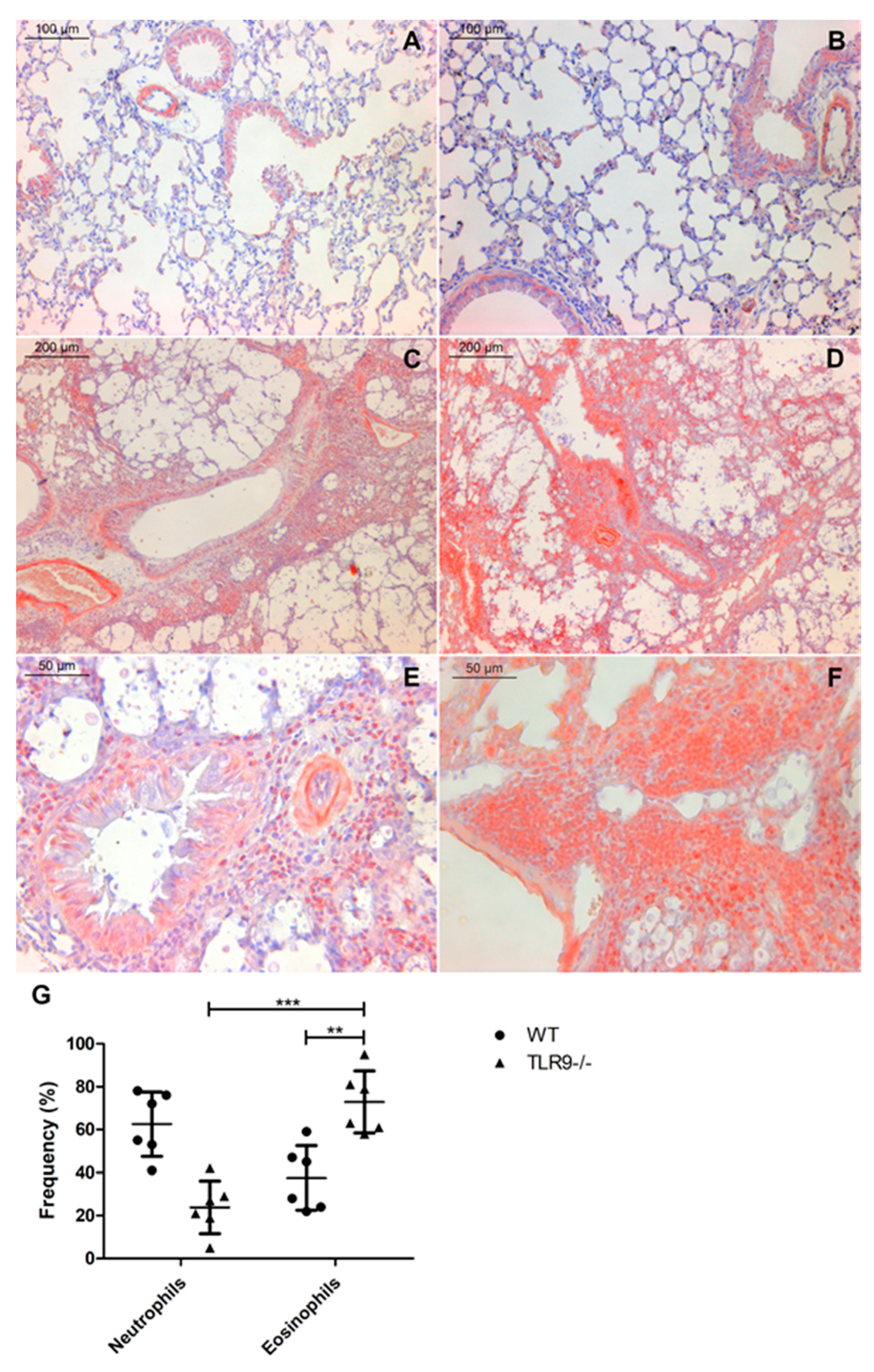
2.2. TLR9-/- Infected Mice Have Hypereosinophilia in Mixed Inflammatory Infiltrate
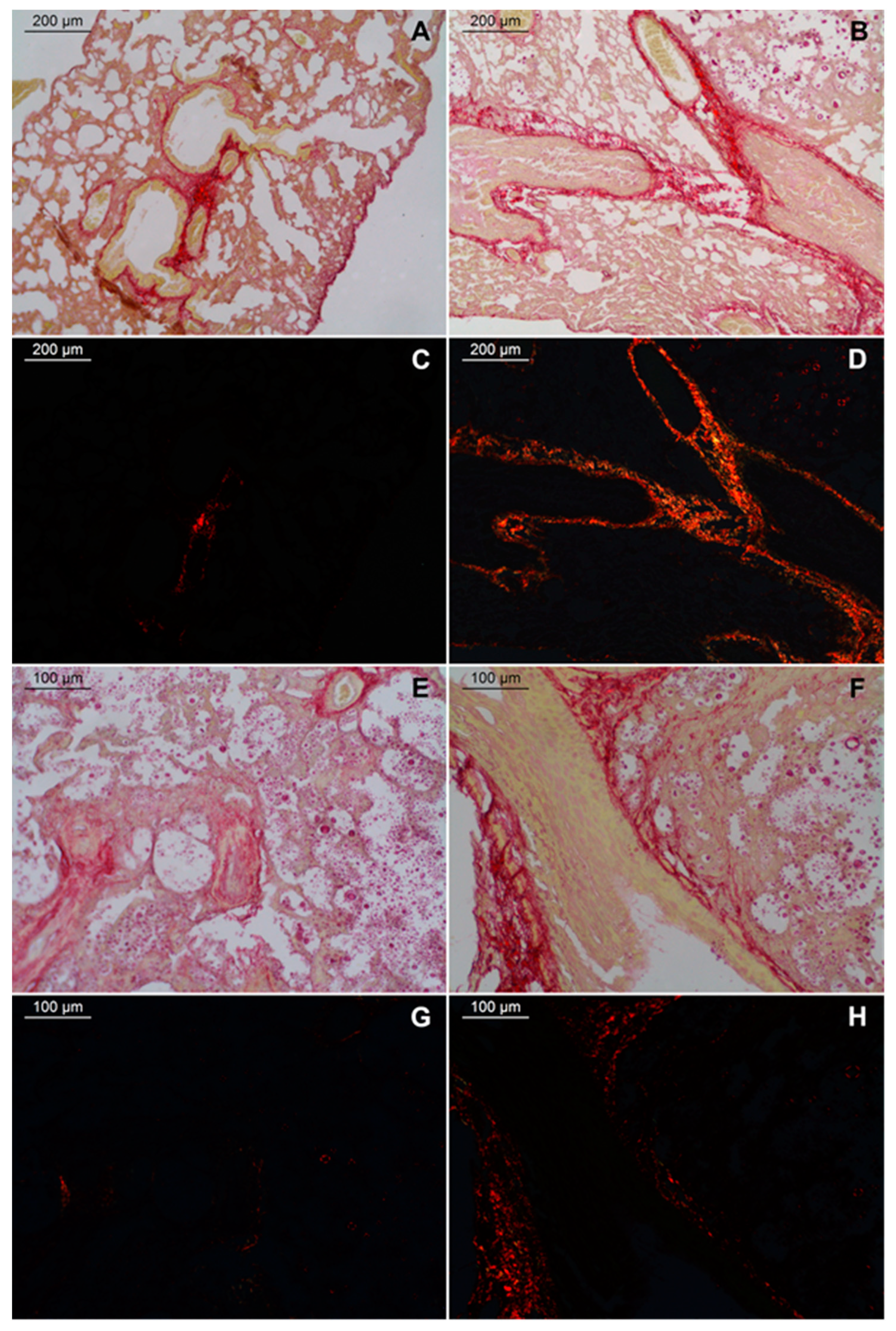
2.3. Large Fibrosis in the Lungs of TLR9-/- Infected Mice
3. Discussion
4. Materials and Methods
4.1. Cryptococcus Strain
4.2. Inoculum Preparation
4.3. Mice and Infection Model
4.4. Anesthesia and Analgesia
4.5. Intratracheal Infection
4.6. Histological Sections
4.7. Eosinophils and Neutrophils Frequency
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis. Clin. N. Am. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Zaragoza, O. Basic principles of the virulence of Cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Lima, I.; Fonseca, L.M.d.; Silva-Junior, E.B.d.; Guimarães-de-Oliveira, J.C.; Freire-de-Lima, L.; Nascimento, D.O.; Morrot, A.; Previato, J.O.; Mendonça-Previato, L.; Decote-Ricardo, D.; et al. Cryptococcus: History, Epidemiology and Immune Evasion. Appl. Sci. 2022, 12, 7086. [Google Scholar] [CrossRef]
- Chen, S.C.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, K.H.; Kidd, S.E.; Kronstad, J.W. The emergence of Cryptococcus gattii in British Columbia and the Pacific Northwest. Curr. Infect. Dis. Rep. 2008, 10, 58–65. [Google Scholar] [CrossRef]
- Gullo, F.P.; Rossi, S.A.; Sardi Jde, C.; Teodoro, V.L.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Cryptococcosis: Epidemiology, fungal resistance, and new alternatives for treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1377–1391. [Google Scholar] [CrossRef]
- Decote-Ricardo, D.; LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nascimento, D.O.; Nunes, M.P.; Morrot, A.; Freire-de-Lima, L.; Previato, J.O.; Mendonca-Previato, L.; Freire-de-Lima, C.G. Immunomodulatory Role of Capsular Polysaccharides Constituents of Cryptococcus neoformans. Front. Med. 2019, 6, 129. [Google Scholar] [CrossRef]
- Diniz-Lima, I.; da Fonseca, L.M.; Dos Reis, J.S.; Rodrigues da Costa Santos, M.A.; da Costa, K.M.; do Nascimento Santos, C.A.; Barcelos, P.M.; Guimaraes-Pinto, K.; Filardy, A.A.; Freire-de-Lima, M.E.; et al. The Sweet Side of Fungal Infections: Structural Glycan Diversity and Its Importance for Pathogenic Adaptation. Medicines 2022, 9, 37. [Google Scholar] [CrossRef]
- Casadevall, A.; Coelho, C.; Cordero, R.J.B.; Dragotakes, Q.; Jung, E.; Vij, R.; Wear, M.P. The capsule of Cryptococcus neoformans. Virulence 2019, 10, 822–831. [Google Scholar] [CrossRef]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl Microbiol 2009, 68, 133–216. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, K.L.; Murphy, J.W. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 1998, 4, 71–83. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Nosanchuk, J.D.; Casadevall, A. Vesicular Trans-Cell Wall Transport in Fungi: A Mechanism for the Delivery of Virulence-Associated Macromolecules? Lipid Insights 2008, 2, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Freire-de-Lima, C.G.; Nosanchuk, J.D.; Casadevall, A.; Rodrigues, M.L.; Nimrichter, L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 2010, 78, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Tompkins, K.C.; McNamara, A.; Jain, A.V.; Moore, B.B.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am. J. Pathol. 2009, 175, 2489–2500. [Google Scholar] [CrossRef]
- Angkasekwinai, P.; Sringkarin, N.; Supasorn, O.; Fungkrajai, M.; Wang, Y.H.; Chayakulkeeree, M.; Ngamskulrungroj, P.; Angkasekwinai, N.; Pattanapanyasat, K. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect. Immun. 2014, 82, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Teitz-Tennenbaum, S.; Chen, G.H.; Dils, A.J.; Malachowski, A.N.; Curtis, J.L.; Olszewski, M.A.; Osterholzer, J.J. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J. Immunol. 2014, 193, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Urai, M.; Takatsuka, S.; Abe, M.; Miyazaki, Y.; Kinjo, Y. Immunization with Antigen-Pulsed Dendritic Cells Against Highly Virulent Cryptococcus gattii Infection: Analysis of Cytokine-Producing T Cells. Methods Mol. Biol. 2017, 1625, 327–339. [Google Scholar] [CrossRef]
- Mansour, M.K.; Latz, E.; Levitz, S.M. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J. Immunol. 2006, 176, 3053–3061. [Google Scholar] [CrossRef]
- Jamil, K.; Polyak, M.J.; Feehan, D.D.; Surmanowicz, P.; Stack, D.; Li, S.S.; Ogbomo, H.; Olszewski, M.; Ganguly, A.; Mody, C.H. Phagosomal F-Actin Retention by Cryptococcus gattii Induces Dendritic Cell Immunoparalysis. mBio 2020, 11, e01821-20. [Google Scholar] [CrossRef]
- Diniz-Lima, I.; da Rosa, P.R.; da Silva-Junior, E.B.; Guimarães-de-Oliveira, J.C.; de Freitas, E.O.; de Oliveira Nascimento, D.; Morrot, A.; Nimrichter, L.; Previato, J.O.; Mendonça-Previato, L.; et al. X-linked immunodeficient (XID) mice exhibit high susceptibility to Cryptococcus gattii infection. Sci. Rep. 2021, 11, 18397. [Google Scholar] [CrossRef]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; DosReis, G.A.; Previato, J.O.; Mendonca-Previato, L.; Freire-de-Lima, C.G. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell. Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.D.; Nascimento, M.T.; Decote-Ricardo, D.; Corte-Real, S.; Morrot, A.; Heise, N.; Nunes, M.P.; Previato, J.O.; Mendonca-Previato, L.; DosReis, G.A.; et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci. Rep. 2015, 5, 8008. [Google Scholar] [CrossRef] [PubMed]
- Heyen, L.; Muller, U.; Siegemund, S.; Schulze, B.; Protschka, M.; Alber, G.; Piehler, D. Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog. Dis. 2016, 74, ftw086. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Boyd, M.B.; Street, N.E.; Lipscomb, M.F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 1998, 160, 2393–2400. [Google Scholar] [PubMed]
- Holmer, S.M.; Evans, K.S.; Asfaw, Y.G.; Saini, D.; Schell, W.A.; Ledford, J.G.; Frothingham, R.; Wright, J.R.; Sempowski, G.D.; Perfect, J.R. Impact of surfactant protein D, interleukin-5, and eosinophilia on Cryptococcosis. Infect. Immun. 2014, 82, 683–693. [Google Scholar] [CrossRef]
- Kindermann, M.; Knipfer, L.; Obermeyer, S.; Muller, U.; Alber, G.; Bogdan, C.; Schleicher, U.; Neurath, M.F.; Wirtz, S. Group 2 Innate Lymphoid Cells (ILC2) Suppress Beneficial Type 1 Immune Responses During Pulmonary Cryptococcosis. Front. Immunol. 2020, 11, 209. [Google Scholar] [CrossRef]
- Flaczyk, A.; Duerr, C.U.; Shourian, M.; Lafferty, E.I.; Fritz, J.H.; Qureshi, S.T. IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J. Immunol. 2013, 191, 2503–2513. [Google Scholar] [CrossRef]
- Piehler, D.; Grahnert, A.; Eschke, M.; Richter, T.; Kohler, G.; Stenzel, W.; Alber, G. T1/ST2 promotes T helper 2 cell activation and polyfunctionality in bronchopulmonary mycosis. Mucosal. Immunol. 2013, 6, 405–414. [Google Scholar] [CrossRef]
- Arora, S.; Hernandez, Y.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 2005, 174, 6346–6356. [Google Scholar] [CrossRef]
- Hernandez, Y.; Arora, S.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 2005, 174, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- McDermott, A.J.; Tumey, T.A.; Huang, M.; Hull, C.M.; Klein, B.S. Inhaled Cryptococcus neoformans elicits allergic airway inflammation independent of Nuclear Factor Kappa B signalling in lung epithelial cells. Immunology 2018, 153, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J. TLR9 in health and disease. Int Rev. Immunol. 2006, 25, 155–181. [Google Scholar] [CrossRef]
- Pratti, J.E.S.; da Fonseca Martins, A.M.; da Silva, J.P.; Ramos, T.D.; Pereira, J.C.; Firmino-Cruz, L.; Oliveira-Maciel, D.; Vieira, T.S.S.; Lacerda, L.L.; Vale, A.M.; et al. The role of TLR9 on Leishmania amazonensis infection and its influence on intranasal LaAg vaccine efficacy. PLoS Negl. Trop. Dis. 2019, 13, e0007146. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Campos, C.; Burguete-Garcia, A.I.; Madrid-Marina, V. Role of TLR9 in Oncogenic Virus-Produced Cancer. Viral Immunol. 2017, 30, 98–105. [Google Scholar] [CrossRef]
- Kasperkovitz, P.V.; Cardenas, M.L.; Vyas, J.M. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J. Immunol. 2010, 185, 7614–7622. [Google Scholar] [CrossRef]
- Khan, N.S.; Kasperkovitz, P.V.; Timmons, A.K.; Mansour, M.K.; Tam, J.M.; Seward, M.W.; Reedy, J.L.; Puranam, S.; Feliu, M.; Vyas, J.M. Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing beta-1,3 Glucan. J. Immunol. 2016, 196, 2249–2261. [Google Scholar] [CrossRef]
- Edwards, L.; Williams, A.E.; Krieg, A.M.; Rae, A.J.; Snelgrove, R.J.; Hussell, T. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur. J. Immunol. 2005, 35, 273–281. [Google Scholar] [CrossRef]
- Qiu, Y.; Zeltzer, S.; Zhang, Y.; Wang, F.; Chen, G.H.; Dayrit, J.; Murdock, B.J.; Bhan, U.; Toews, G.B.; Osterholzer, J.J.; et al. Early induction of CCL7 downstream of TLR9 signaling promotes the development of robust immunity to cryptococcal infection. J. Immunol. 2012, 188, 3940–3948. [Google Scholar] [CrossRef]
- LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nunes, M.P.; Oliveira, P.A.V.; Nascimento, D.O.; Freire-de-Lima, L.; Takiya, C.M.; Morrot, A.; Decote-Ricardo, D.; Previato, J.O.; et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci. Rep. 2018, 8, 16378. [Google Scholar] [CrossRef] [Green Version]
- da Silva-Junior, E.B.; Firmino-Cruz, L.; Guimaraes-de-Oliveira, J.C.; De-Medeiros, J.V.R.; de Oliveira Nascimento, D.; Freire-de-Lima, M.; de Brito-Gitirana, L.; Morrot, A.; Previato, J.O.; Mendonca-Previato, L.; et al. The role of Toll-like receptor 9 in a murine model of Cryptococcus gattii infection. Sci. Rep. 2021, 11, 1407. [Google Scholar] [CrossRef]
- Hommel, B.; Mukaremera, L.; Cordero, R.J.B.; Coelho, C.; Desjardins, C.A.; Sturny-Leclere, A.; Janbon, G.; Perfect, J.R.; Fraser, J.A.; Casadevall, A.; et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018, 14, e1006982. [Google Scholar] [CrossRef] [PubMed]
- Trevijano-Contador, N.; de Oliveira, H.C.; Garcia-Rodas, R.; Rossi, S.A.; Llorente, I.; Zaballos, A.; Janbon, G.; Arino, J.; Zaragoza, O. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog. 2018, 14, e1007007. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Nielsen, K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell 2012, 11, 820–826. [Google Scholar] [CrossRef]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Shi, M. Neutrophil swarming toward Cryptococcus neoformans is mediated by complement and leukotriene B4. Biochem. Biophys. Res. Commun. 2016, 477, 945–951. [Google Scholar] [CrossRef]
- Ueno, K.; Yanagihara, N.; Otani, Y.; Shimizu, K.; Kinjo, Y.; Miyazaki, Y. Neutrophil-mediated antifungal activity against highly virulent Cryptococcus gattii strain R265. Med. Mycol. 2019, 57, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Brito, P.K.M.; Rezende, C.P.; Almeida, F.; Roque-Barreira, M.C.; da Silva, T.A. iNOS/Arginase-1 expression in the pulmonary tissue over time during Cryptococcus gattii infection. Innate Immun. 2020, 26, 117–129. [Google Scholar] [CrossRef]
- Tiwary, M.; LeMessurier, K.S.; Samarasinghe, A.E. Murine Models of Eosinophil Function in Fungal and Viral Infections. Methods Mol. Biol. 2021, 2241, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Ueki, S.; Tamari, M.; Imoto, Y.; Fujieda, S.; Taniguchi, M. Adult-onset eosinophilic airway diseases. Allergy 2020, 75, 3087–3099. [Google Scholar] [CrossRef]
- Krishack, P.A.; Hollinger, M.K.; Kuzel, T.G.; Decker, T.S.; Louviere, T.J.; Hrusch, C.L.; Sperling, A.I.; Verhoef, P.A. IL-33-mediated Eosinophilia Protects against Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2021, 64, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Dion, J.; Van Dyken, S.; Ricardo-Gonzalez, R.R.; Danel, C.J.; Taille, C.; Mouthon, L.; Locksley, R.M.; Terrier, B. A role for IL-33-activated ILC2s in eosinophilic vasculitis. JCI Insight 2021, 6, e143366. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, Y.; Yu, J.; Dong, L.; Husain, A.N.; Shen, L.; Weber, C.R. Idiopathic pulmonary fibrosis is associated with tight junction protein alterations. Biochim. Biophys. Acta. Biomembr. 2020, 1862, 183205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Bhan, U.; Huffnagle, G.B.; Toews, G.B.; Standiford, T.J.; Olszewski, M.A. TLR9 signaling is required for generation of the adaptive immune protection in Cryptococcus neoformans-infected lungs. Am. J. Pathol. 2010, 177, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Herbst, S.; Shah, A.; Mazon Moya, M.; Marzola, V.; Jensen, B.; Reed, A.; Birrell, M.A.; Saijo, S.; Mostowy, S.; Shaunak, S.; et al. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol. Med. 2015, 7, 240–258. [Google Scholar] [CrossRef]
- Schoffelen, T.; Illnait-Zaragozi, M.T.; Joosten, L.A.; Netea, M.G.; Boekhout, T.; Meis, J.F.; Sprong, T. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS ONE 2013, 8, e55579. [Google Scholar] [CrossRef]
- Garcia-Barbazan, I.; Trevijano-Contador, N.; Rueda, C.; de Andres, B.; Perez-Tavarez, R.; Herrero-Fernandez, I.; Gaspar, M.L.; Zaragoza, O. The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell. Microbiol. 2016, 18, 111–124. [Google Scholar] [CrossRef]
- Zaragoza, O.; Nielsen, K. Titan cells in Cryptococcus neoformans: Cells with a giant impact. Curr. Opin. Microbiol. 2013, 16, 409–413. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Roselletti, E.; Garcia-Rodas, R.; Vecchiarelli, A.; Zaragoza, O. Role of IL-17 in Morphogenesis and Dissemination of Cryptococcus neoformans during Murine Infection. Microorganisms 2022, 10, 373. [Google Scholar] [CrossRef]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef] [Green Version]
- Piehler, D.; Eschke, M.; Schulze, B.; Protschka, M.; Muller, U.; Grahnert, A.; Richter, T.; Heyen, L.; Kohler, G.; Brombacher, F.; et al. The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal. Immunol. 2016, 9, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.B.; Hogaboam, C.M. Murine models of pulmonary fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L152–L160. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, N.; Ma, Q.; Zhu, R.; Chen, Z.; Wang, Z.; Qian, Y.; Wu, C.; Hu, F.; Huang, M.; et al. Epithelial-Mesenchymal Transition in Asthma Airway Remodeling Is Regulated by the IL-33/CD146 Axis. Front. Immunol. 2020, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Saikumar Jayalatha, A.K.; Hesse, L.; Ketelaar, M.E.; Koppelman, G.H.; Nawijn, M.C. The central role of IL-33/IL-1RL1 pathway in asthma: From pathogenesis to intervention. Pharmacol. Ther. 2021, 225, 107847. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef] [PubMed]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. IL-33/ST2 Axis in Organ Fibrosis. Front. Immunol. 2018, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Cherniak, R.; Reiss, E.; Slodki, M.E.; Plattner, R.D.; Blumer, S.O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol. Immunol. 1980, 17, 1025–1032. [Google Scholar] [CrossRef]
- Cheng, P.Y.; Sham, A.; Kronstad, J.W. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect. Immun. 2009, 77, 4284–4294. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Grouls, V.; Helpap, B. Selective staining of eosinophils and their immature precursors in tissue sections and autoradiographs with Congo red. Stain Technol. 1981, 56, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.D.; Fullmer, H.M. Histopathologic Technic and Practical Histochemistry, 4th ed.; McGraw-Hill: New York, NY, USA, 1976; p. 942. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva-Junior, E.B.; Diniz-Lima, I.; Silva, A.C.; Guimarães-de-Oliveira, J.C.; Morrot, A.; Freire-de-Lima, L.; da Fonseca, L.M.; de Brito-Gitirana, L.; Decote-Ricardo, D.; de Matos Guedes, H.L.; et al. Pulmonary Fibrosis and Hypereosinophilia in TLR9-/- Mice Infected by Cryptococcus gattii. Pathogens 2022, 11, 987. https://doi.org/10.3390/pathogens11090987
da Silva-Junior EB, Diniz-Lima I, Silva AC, Guimarães-de-Oliveira JC, Morrot A, Freire-de-Lima L, da Fonseca LM, de Brito-Gitirana L, Decote-Ricardo D, de Matos Guedes HL, et al. Pulmonary Fibrosis and Hypereosinophilia in TLR9-/- Mice Infected by Cryptococcus gattii. Pathogens. 2022; 11(9):987. https://doi.org/10.3390/pathogens11090987
Chicago/Turabian Styleda Silva-Junior, Elias Barbosa, Israel Diniz-Lima, Amanda Couto Silva, Joyce Cristina Guimarães-de-Oliveira, Alexandre Morrot, Leonardo Freire-de-Lima, Leonardo Marques da Fonseca, Lycia de Brito-Gitirana, Debora Decote-Ricardo, Herbert Leonel de Matos Guedes, and et al. 2022. "Pulmonary Fibrosis and Hypereosinophilia in TLR9-/- Mice Infected by Cryptococcus gattii" Pathogens 11, no. 9: 987. https://doi.org/10.3390/pathogens11090987
APA Styleda Silva-Junior, E. B., Diniz-Lima, I., Silva, A. C., Guimarães-de-Oliveira, J. C., Morrot, A., Freire-de-Lima, L., da Fonseca, L. M., de Brito-Gitirana, L., Decote-Ricardo, D., de Matos Guedes, H. L., & Freire-de-Lima, C. G. (2022). Pulmonary Fibrosis and Hypereosinophilia in TLR9-/- Mice Infected by Cryptococcus gattii. Pathogens, 11(9), 987. https://doi.org/10.3390/pathogens11090987












