Synergistic Antibiofilm Activity between Synthetic Peptides and Ciprofloxacin against Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biologic Material
2.2. Peptide Sequence
2.3. Antibiofilm Assay
2.4. Biofilm Integrity Determined by Fluorescence Microscopy
2.5. Overproduction of Reactive Oxygen Species (ROS)
2.6. Hemolytic Assay
2.7. Statistical Analysis
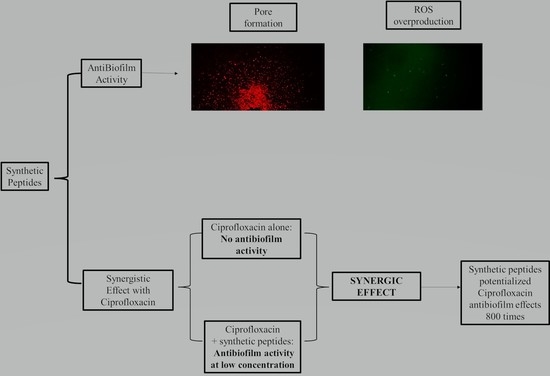
3. Results
3.1. Combined Antibiofilm Activity of Synthetic Peptides and Ciprofloxacin against S. aureus
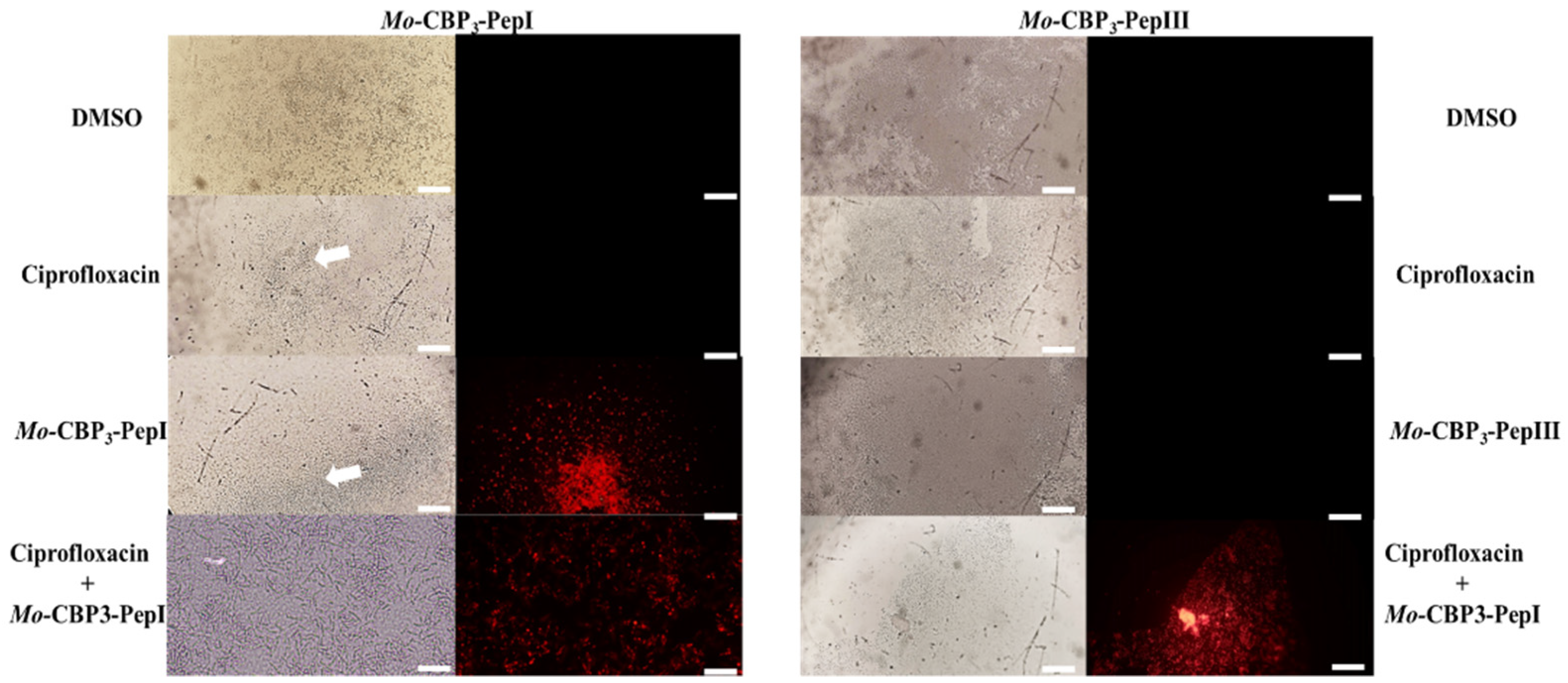
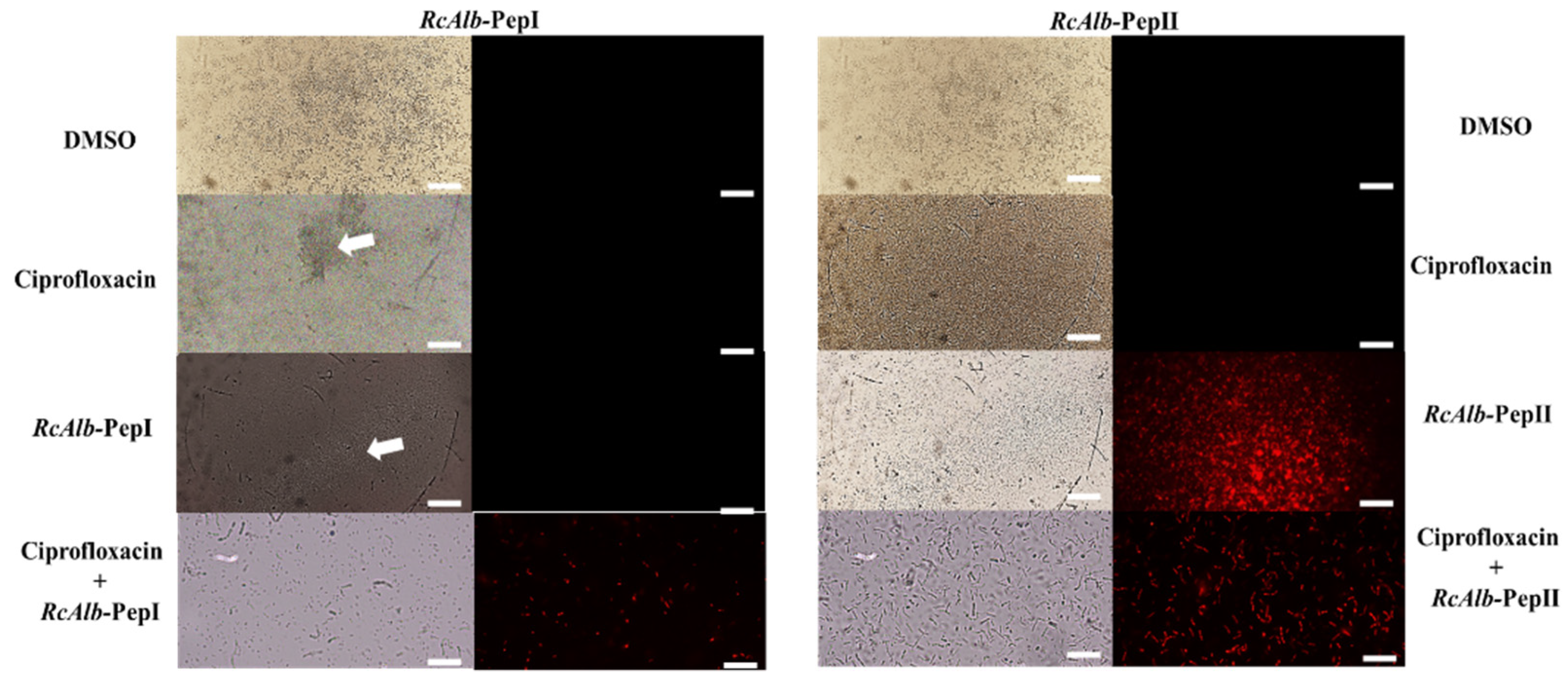
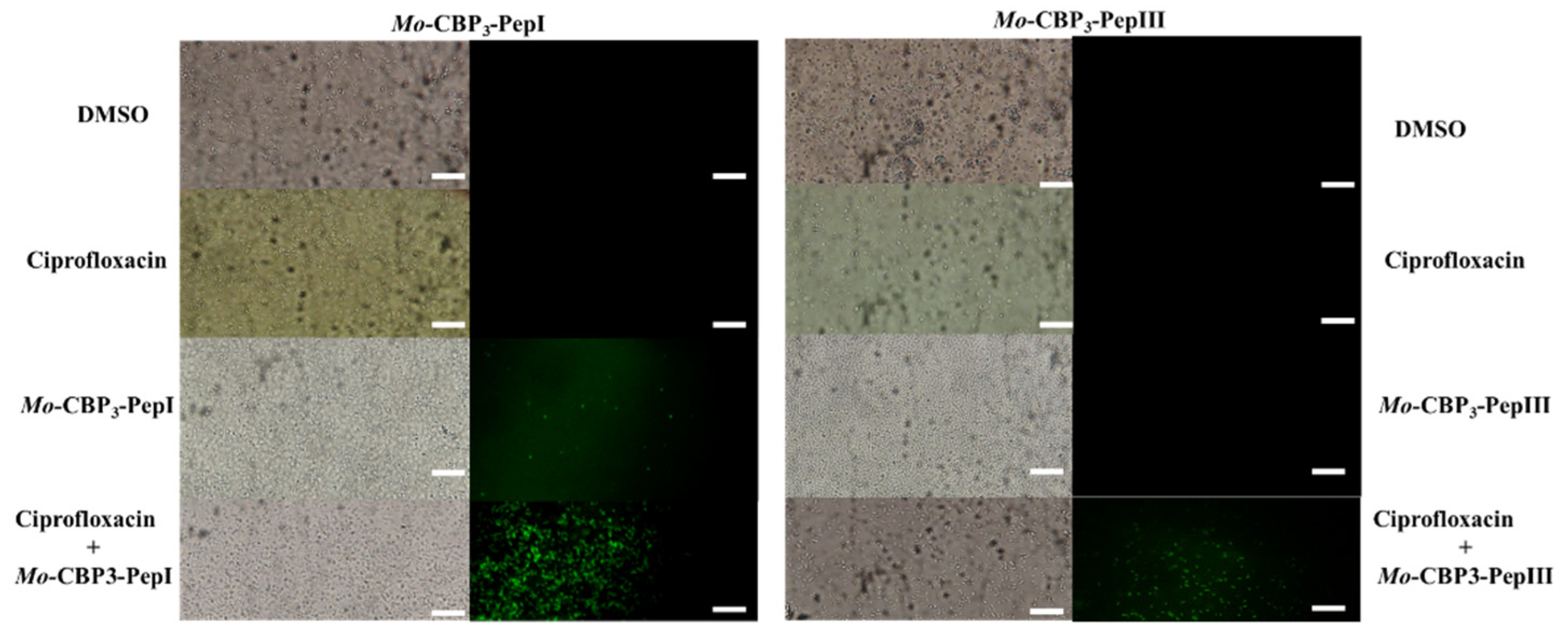
3.2. Action Mechanisms of Synthetic Peptides
3.2.1. Membrane Pore Formation by Propidium Iodide Uptake
3.2.2. ROS Overproduction
3.3. Hemolytic Action
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.L.; Santos, D.O.; de Freitas, C.C.; Ferreira, B.L.A.; Afonso, I.F.; Rodrigues, C.R.; Castro, H.C. Staphylococcus aureus: Visitando uma cepa de importância hospitalar. J. Bras. Patol. Med. Lab. 2007, 43, 413–423. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs:Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus bloodstream infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Chegini, Z.; Van Belkum, A.; Mirzaii, M.; Khoramrooz, S.S.; Darban-Sarokhalil, D. The global prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-resistant Staphylococcus aureus and coagulase–negative staphylococci strains: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 1–20. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef]
- Kundu, R. Cationic amphiphilic peptides: Synthetic antimicrobial agents inspired by nature. ChemMedChem 2020, 15, 1887–1896. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Marques, L.S.M.; Oliveira, J.T.A.; Lima, P.G.; Dias, L.P.; Neto, N.A.S.; Lopes, F.E.S.; Sousa, J.S.; Silva, A.F.B.; Caneiro, R.F.; et al. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020, 175, 132–145. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Souza, P.F.; Vasconcelos, I.M.; Dias, L.P.; Martins, T.F.; Van Tilburg, M.F.; Guedes, M.I.; Sousa, D.O. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability. Biochimie 2019, 157, 10–21. [Google Scholar] [CrossRef]
- Bezerra, L.P.; Freitas, C.D.T.; Silva, A.F.B.; Amaral, J.L.; Neto, N.A.S.; Silva, R.G.G.; Parra, A.L.C.; Goldman, G.H.; Oliveira, J.T.A.; Mesquita, F.P.; et al. Synergistic Antifungal Activity of Synthetic Peptides and Antifungal Drugs against Candida albicans and C. parapsilosis Biofilms. Antibiotics 2022, 11, 553. [Google Scholar] [CrossRef]
- Dias, L.P.; Souza, P.F.; Oliveira, J.T.; Vasconcelos, I.M.; Araújo, N.M.; Tilburg, M.F.; Guedes, M.I.; Carneiro, R.F.; Lopes, J.L.; Sousa, D.O. RcAlb-PepII, a synthetic small peptide bioinspired in the 2S albumin from the seed cake of Ricinus communis, is a potent antimicrobial agent against Klebsiella pneumoniae and Candida parapsilosis. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1862, 183092. [Google Scholar] [CrossRef]
- Bezerra, L.P.; Silva, A.F.; Santos-Oliveira, R.; Alencar, L.M.; Amaral, J.L.; Neto, N.A.; Silva, R.G.; O Belém, M.; de Andrade, C.R.; Oliveira, J.T.; et al. Combined antibiofilm activity of synthetic peptides and antifungal drugs against Candida spp. Future Microbiol. 2022, 17, 1133–1146. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef]
- Howden, B.P.; McEvoy, C.R.E.; Allen, D.L.; Chua, K.; Gao, W.; Harrison, P.; Bell, J.; Coombs, G.; Bennett-Wood, V.; Porter, J.L.; et al. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog. 2011, 7, e1002359. [Google Scholar] [CrossRef]
- Ward, P.B.; Johnson, P.D.R.; A Grabsch, E.; Mayall, B.C.; Grayson, M.L.; BAppSc, P.B.W.; Fracp, P.D.R.J.; Mph, E.A.G.B.; Frcpa, B.C.M.F.; Fafphm, M.L.G.F. Treatment failure due to methicillin-resistant Staphylococcus aureus (MRSA) with reduced susceptibility to vancomycin. Med. J. Aust. 2001, 175, 480–483. [Google Scholar] [CrossRef]
- Young, B.C.; Golubchik, T.; Batty, E.M.; Fung, R.; Larner-Svensson, H.; Votintseva, A.A.; Miller, R.R.; Godwin, H.; Knox, K.; Everitt, R.G.; et al. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc. Natl. Acad. Sci. USA 2012, 109, 4550–4555. [Google Scholar] [CrossRef]
- Rong, M.; Zheng, X.; Ye, M.; Bai, J.; Xie, X.; Jin, Y.; He, X. Phenotypic plasticity of Staphylococcus Aureus in liquid medium containing vancomycin. Front. Microbiol. 2019, 10, 809. [Google Scholar] [CrossRef]
- Gardete, S.; Tomasz, A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Investig. 2014, 124, 2836–2840. [Google Scholar] [CrossRef]
- Lima, P.G.; Souza, P.F.; Freitas, C.D.; Bezerra, L.P.; Neto, N.A.; Silva, A.F.; Oliveira, J.T.; Sousa, D.O. Synthetic peptides against Trichophyton mentagrophytes and T. rubrum: Mechanisms of action and efficiency compared to griseofulvin and itraconazole. Life Sci. 2021, 265, 118803. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Lima, P.G.; Freitas, C.D.T.; Sousa, D.O.B.; Neto, N.A.S.; Dias, L.P.; Vasconcelos, I.M.; Freitas, L.B.N.; Silva, R.G.G.; Sousa, J.S.; et al. Antidermatophytic activity of synthetic peptides: Action mechanisms and clinical application as adjuvants to enhance the activity and decrease the toxicity of Griseofulvin. Mycoses 2020, 63, 979–992. [Google Scholar] [CrossRef]
- Souza, P.F.N.; vanTilburg, M.F.; Mesquita, F.P.; Amaral, J.L.; Lima, L.B.; Montenegro, R.C.; Lopes, F.E.S.; Martins, R.X.; Vieira, L.; Farias, D.F.; et al. Neutralizing Effect of Synthetic Peptides toward SARS-CoV-2. ACS Omega 2022, 7, 16222–16234. [Google Scholar] [CrossRef]
- Bessa, L.J.; Eaton, P.; Dematei, A.; Plácido, A.; Vale, N.; Gomes, P.; Delerue-Matos, C.; Leite, J.R.S.; Gameiro, P. Synergistic and antibiofilm properties of ocellatin peptides against multidrug-resistant Pseudomonas aeruginosa. Future Microbiol. 2018, 13, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Gonçalves, S.; Felício, M.R.; Maturana, P.; Santos, N.C.; Semorile, L.; Hollmann, A.; Maffía, P.C. Synergistic and antibiofilm activity of the antimicrobial peptide P5 against carbapenem-resistant Pseudomonas aeruginosa. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1329–1337. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, X.; Li, Z.; Yuan, J.; Shen, F.; Zhang, S. Synergistic effect and antibiofilm activity of an antimicrobial peptide with traditional antibiotics against multi-drug resistant bacteria. Microb. Pathog. 2021, 158, 105056. [Google Scholar] [CrossRef]
- Thai, T.; Salisbury, B.H.; Zito, P.M. Ciprofloxacin; StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Ciprofloxacin Monograph for Professionals—Drugs.Com. Available online: https://www.drugs.com/monograph/ciprofloxacin.html (accessed on 4 August 2022).
- Reis, A.C.C.; Santos, S.R.d.S.; de Souza, S.C.; Saldanha, M.G.; Pitanga, T.N.; Oliveira, R.R. Ciprofloxacin resistance pattern among bacteria isolated from patients with community-acquired urinary tract infection. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 53. [Google Scholar] [CrossRef]
- Smith, N.; Fackrell, R.; Henderson, E. Ciprofloxacin-associated bilateral iliopsoas tendon rupture: A case report. Age Ageing 2016, 45, 734–735. [Google Scholar] [CrossRef]
- Shimatsu, K.; Subramaniam, S.; Sim, H.; Aronowitz, P. Ciprofloxacin-induced tendinopathy of the gluteal tendons. J. Gen. Intern. Med. 2014, 29, 1559–1562. [Google Scholar] [CrossRef]
- Blandeau, J.M. Expanded activity and utility of the new fluoroquinolones: A review. Clin. Ther. 1999, 21, 3–40. [Google Scholar] [CrossRef]
- Campion, J.J.; McNamara, P.J.; Evans, M.E. Evolution of ciprofloxacin-resistant Staphylococcus aureus in In Vitro pharmacokinetic environments. Antimicrob. Agents Chemother. 2004, 48, 4733–4744. [Google Scholar] [CrossRef] [Green Version]
- Lima, P.G.; Souza, P.F.; Freitas, C.D.; Oliveira, J.T.; Dias, L.P.; Neto, J.X.; Vasconcelos, I.M.; Lopes, J.L.; Sousa, D.O. Anticandidal activity of synthetic peptides: Mechanism of action revealed by scanning electron and fluorescence microscopies and synergism effect with nystatin. J. Pept. Sci. 2020, 26, e3249. [Google Scholar] [CrossRef]
- Čáp, M.; Váchová, L.; Palková, Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative Med. Cell. Longev. 2012, 2012, 976753. [Google Scholar] [CrossRef]
- Maurya, I.K.; Pathak, S.; Sharma, M.; Sanwal, H.; Chaudhary, P.; Tupe, S.G.; Deshpande, M.; Chauhan, V.S.; Prasad, R. Antifungal activity of novel synthetic peptides by accumulation of reactive oxygen species (ROS) and disruption of cell wall against Candida albicans. Peptides 2011, 32, 1732–1740. [Google Scholar] [CrossRef]
- Lim, S.; Alam, M.G. Ciprofloxacin-induced acute interstitial nephritis and autoimmune hemolytic anemia. Ren. Fail. 2003, 25, 647–651. [Google Scholar] [CrossRef]


| Peptides/Combinations | % Hemolysis | ||
|---|---|---|---|
| Type-A Blood | Type-B Blood | Type-O Blood | |
| 0.1% Triton X-100 | 100 ± 0.001 | 100 ± 0.001 | 100 ± 0.005 |
| DMSO-NaCl Solution | 0 | 0 | 0 |
| Ciprofloxacin (1000 µg mL−1) | 100 ± 0.007 | 100 ± 0.003 | 100 ± 0.005 |
| Ciprofloxacin (25 µg mL−1) | 45 ± 0.004 | 51 ± 0.002 | 34 ± 0.006 |
| Ciprofloxacin (0.2 µg mL−1) | 0 | 0 | 0 |
| Ciprofloxacin (0.02 µg mL−1) | 0 | 0 | 0 |
| Mo-CBP3-PepI (1000 µg mL−1) | 0 | 0 | 0 |
| Mo-CBP3-PepIII (1000 µg mL−1) | 0 | 0 | 0 |
| RcAlb-PepI (1000 µg mL−1) | 0 | 0 | 0 |
| RcAlb-PepII (1000 µg mL−1) | 0 | 0 | 0 |
| Mo-CBP3-PepI (0.2 µg mL−1) and ciprofloxacin (6.2 µg mL−1) | 0 | 0 | 0 |
| Mo-CBP3-PepIII (6.2 µg mL−1) and ciprofloxacin (0.2 µg mL−1) | 0 | 0 | 0 |
| RcAlb-PepI (0.04 µg mL−1) and ciprofloxacin (25 µg mL−1) | 0 | 0 | 0 |
| RcAlb-PepII (50 µg mL−1) and ciprofloxacin (0.02 µg mL−1) | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, N.A.S.; Oliveira, J.T.A.; Aguiar, T.K.B.; Bezerra, L.P.; Branco, L.A.C.; Mesquita, F.P.; Freitas, C.D.T.; Souza, P.F.N. Synergistic Antibiofilm Activity between Synthetic Peptides and Ciprofloxacin against Staphylococcus aureus. Pathogens 2022, 11, 995. https://doi.org/10.3390/pathogens11090995
Neto NAS, Oliveira JTA, Aguiar TKB, Bezerra LP, Branco LAC, Mesquita FP, Freitas CDT, Souza PFN. Synergistic Antibiofilm Activity between Synthetic Peptides and Ciprofloxacin against Staphylococcus aureus. Pathogens. 2022; 11(9):995. https://doi.org/10.3390/pathogens11090995
Chicago/Turabian StyleNeto, Nilton A. S., Jose T. A. Oliveira, Tawanny K. B. Aguiar, Leandro P. Bezerra, Levi A. C. Branco, Felipe P. Mesquita, Cleverson D. T. Freitas, and Pedro F. N. Souza. 2022. "Synergistic Antibiofilm Activity between Synthetic Peptides and Ciprofloxacin against Staphylococcus aureus" Pathogens 11, no. 9: 995. https://doi.org/10.3390/pathogens11090995
APA StyleNeto, N. A. S., Oliveira, J. T. A., Aguiar, T. K. B., Bezerra, L. P., Branco, L. A. C., Mesquita, F. P., Freitas, C. D. T., & Souza, P. F. N. (2022). Synergistic Antibiofilm Activity between Synthetic Peptides and Ciprofloxacin against Staphylococcus aureus. Pathogens, 11(9), 995. https://doi.org/10.3390/pathogens11090995