An Inventory of Anthelmintic Plants across the Globe
Abstract
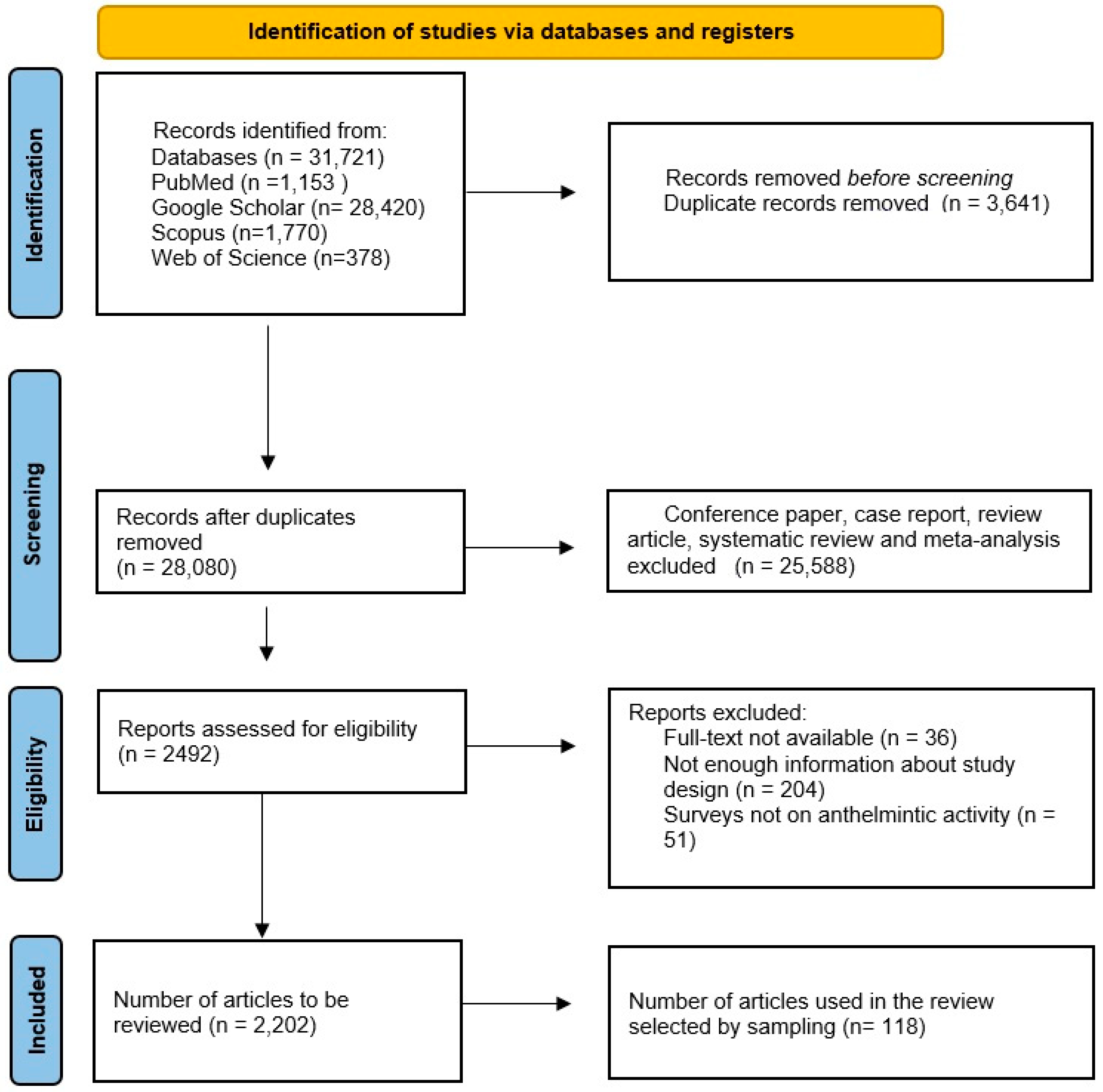
:1. Introduction
2. Chemical Compounds
3. Effect of Plant Extracts in Drug-Resistant Helminths
4. Advantages and Disadvantages of Using Plants for Helminth Parasite Control
5. Recommendations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nirmal, S.A.; Malwadkar, G.; Laware, R. Anthelmintic activity of Pongamia glabra. Songklanakarin J. Sci. Technol. 2007, 29, 755–757. [Google Scholar]
- Onyeyili, P.; Nwosu, C.; Amin, J.; Jibike, J. Anthelmintic activity of crude aqueous extract of Nauclea latifolia stem bark against ovine nematodes. Fitoterapia 2001, 72, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, H.; Kheirandish, F.; Ghasemi Kia, M.; Tavakoli Kareshk, A.; Yarahmadi, M. Chemical composition, protoscolicidal effects and acute toxicity of Pistacia atlantica Desf. fruit extract. Nat. Prod. Res. 2016, 30, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.M. Ca2+ signalling, voltage-gated Ca2+ channels and praziquantel in flatworm neuromusculature. Parasitology 2005, 131, S97–S108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, L.; Bevilaqua, C.; Costa, C.; Macedo, I.; Barros, R.; Rodrigues, A.; Camurça-Vasconcelos, A.; Morais, S.; Lima, Y.; Vieira, L. Anthelmintic activity of Cocos nucifera L. against sheep gastrointestinal nematodes. Vet. Parasitol. 2009, 159, 55–59. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Minsakorn, S.; Nuplod, K.; Puttarak, P.; Chawengkirttikul, R.; Panyarachun, B.; Ngamniyom, A.; Charoenkul, T.; Jaisa-Aad, M.; Panyarachun, P.; Anuracpreeda, P. The anthelmintic effects of medicinal plant extracts against paramphistome parasites, Carmyerius spatiosus. Acta Parasitol. 2019, 64, 566–574. [Google Scholar] [CrossRef]
- Thomsen, H.; Reider, K.; Franke, K.; Wessjohann, L.; Keiser, J.; Dagne, E.; Arnold, N. Characterization of constituents and anthelmintic properties of Hagenia abyssinica. Sci. Pharm. 2012, 80, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Zibaei, M.; Rostamipour, R.; Nayebzadeh, H. Effect of Pistacia atlantica fruit and leaf extracts on hydatid cyst protoscolices. Recent Pat. Antiinfect. Drug Discov. 2016, 11, 53–58. [Google Scholar] [CrossRef]
- Yones, D.A.; Taher, G.A.; Ibraheim, Z.Z. In vitro effects of some herbs used in Egyptian traditional medicine on viability of protoscolices of hydatid cysts. Korean J. Parasitol. 2011, 49, 255. [Google Scholar] [CrossRef]
- Al-Daoody, A.A.; Shareef, A.Y.; Hammoshi, M.H. In Vitro Effects of Ethanolic Extract and Crude Alkaloids of Prosopis farcta Leaves on the Viability of Echinococcus granulosus Protoscolices in Comparison to Mebendazole. RJS Rafidain J. Sci. 2006, 17, 43–52. [Google Scholar]
- Mahmoudvand, H.; Dezaki, E.S.; Kheirandish, F.; Ezatpour, B.; Jahanbakhsh, S.; Harandi, M.F. Scolicidal effects of black cumin seed (Nigella sativa) essential oil on hydatid cysts. Korean J. Parasitol. 2014, 52, 653. [Google Scholar] [CrossRef] [PubMed]
- Barzinji, R.; Kadir, A.; Mothana, R.A.; Nasher, A.K. Effect of leaf extracts of Dendrosicyos socotrana and Jatropha unicostata on the viability of Echinococcus granulosus protoscoleces. EurAsian J. Biosci. 2009, 3, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Rouhani, S.; Salehi, N.; Kamalinejad, M.; Zayeri, F. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J. Investig. Surg. 2013, 26, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, M.; Verma, V.C.; Singh, T.D.; Singh, S.K.; Goel, R.; Nath, G. In-vitro scolicidal activity of Mallotus philippinensis (Lam.) Muell Arg. fruit glandular hair extract against hydatid cyst Echinococcus granulosus. Asian Pac. J. Trop. Dis. 2013, 6, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.C.; Gangwar, M.; Yashpal, M.; Nath, G. Anticestodal activity of endophytic Pestalotiopsis sp. on protoscoleces of hydatid cyst Echinococcus granulosus. Biomed Res. Int. 2013, 2013, 308515. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Mercado, J.M.; Ibarra-Velarde, F.; Alonso-Díaz, M.Á.; Vera-Montenegro, Y.; Avila-Acevedo, J.G.; García-Bores, A.M. In vitro antihelmintic effect of fifteen tropical plant extracts on excysted flukes of Fasciola hepatica. BMC Vet. Res. 2015, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; SUNITA, K.; Singh, R.; Singh, D. Fasciola larvae: Anthelmintic activity of medicinal plant Potentilla fulgens against sporocyst, redia and cercaria. Asian J. Adv. Agric. Res. 2020, 3, 24–30. [Google Scholar]
- Vishwakarma, A.; Kumar, P. In vivo Anthelmintic Activity of Medicinal plant Asparagus racemosus against Larva of Fasciola gigantica. Res. J. Agri. Sci. 2021, 12, 675–680. [Google Scholar]
- Onocha, P.; Olusanya, T. Antimicrobial and anthelmintic evaluation of Nigerian Euphorbiaceae plants 3: Acalypha wilkesiana. Afr. Sci. 2021, 11, 85–89. [Google Scholar]
- Roy, B.; Swargiary, A.; Giri, B.R. Alpinia nigra (Family Zingiberaceae): An anthelmintic medicinal plant of north-east India. Adv. Life Sci. 2012, 2, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Soren, A.D.; Yadav, A.K. In Vitro Anthelmintic Efficacy of Sesbania sesban var. bicolor, Cyperus compressus and Asparagus racemosus against Gastrothylax crumenifer (Trematoda). Proc. Zool. Soc. 2021, 74, 262–267. [Google Scholar] [CrossRef]
- Soren, A.D.; Yadav, A.K. Studies on the anthelmintic potentials of the roots of Asparagus racemosus willd.(Asparagaceae). Clin. Phytoscience 2021, 7, 32. [Google Scholar] [CrossRef]
- Soren, A.D.; Yadav, A.K. Evaluation of in vitro and in vivo anthelmintic efficacy of Cyperus compressus Linn., a traditionally used anthelmintic plant in parasite-animal models. Future J. Pharm. Sci. 2020, 6, 126. [Google Scholar] [CrossRef]
- Soren, A.D.; Chen, R.P.; Yadav, A.K. In vitro and in vivo anthelmintic study of Sesbania sesban var. bicolor, a traditionally used medicinal plant of Santhal tribe in Assam, India. J. Parasit. Dis. 2021, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Lyndem, L.M. An in vitro confirmation of the ethonopharmacological use of Senna plants as anthelmintic against rumen fluke Paramphistomum gracile. BMC Vet. Res. 2019, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, H.; Soad, N.; Farag, T. Tegumental effects of methanolic extract of Balanites aegyptiaca fruits on adult Paramphistomum microbothrium (Fischoeder 1901) under laboratory conditions. Iran. J. Parasitol. 2016, 11, 396. [Google Scholar] [PubMed]
- Lalthanpuii, P.; Zokimi, Z.; Lalchhandama, K. The toothache plant (Acmella oleracea) exhibits anthelmintic activity on both parasitic tapeworms and roundworms. Pharmacogn. Mag. 2020, 16, 193. [Google Scholar]
- Kosalge, S.B.; Fursule, R.A. Investigation of in vitro anthelmintic activity of Thespesia lampas (Cav.). Asian J. Pharm. Clin. Res. 2009, 2, 69–71. [Google Scholar]
- Rabiu, H.; Subhasish, M. Investigation of in vitro anthelmintic activity of Azadirachta indica leaves. Int. J. Drug Dev. Res. 2011, 3, 94–100. [Google Scholar]
- Ali, N.; Ali Shah, S.W.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I.; Ali, W. Anthelmintic and relaxant activities of Verbascum Thapsus Mullein. BMC Complement. Altern. Med. 2012, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.; Shah, S.W.A.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii, C. Koch and Teucrium Stocksianum boiss. BMC Complement. Altern. Med. 2011, 11, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabiul, H.; Subhasish, M. Investigation of in vitro anthelmintic activity of Cinnamomum camphor leaves. Int. J. Drug Dev. Res. 2011, 3, 295–300. [Google Scholar]
- Subhasish, M.; Rabiul, H.; Parag, G.; Debasish, D. Investigation of in vitro anthelmintic activity of Clerodendron inerme. Int. J. Drug Dev. Res. 2010, 2, 826–829. [Google Scholar]
- Lalthanpuii, P.B.; Lalchhandama, K. Phytochemical analysis and in vitro anthelmintic activity of Imperata cylindrica underground parts. BMC Complement. Med. Ther. 2020, 20, 332. [Google Scholar] [CrossRef]
- Tekwu, E.M.; Bosompem, K.M.; Anyan, W.K.; Appiah-Opong, R.; Owusu, K.B.-A.; Tettey, M.D.; Kissi, F.A.; Appiah, A.A.; Penlap Beng, V.; Nyarko, A.K. In vitro assessment of anthelmintic activities of Rauwolfia vomitoria (Apocynaceae) stem bark and roots against parasitic stages of Schistosoma mansoni and cytotoxic study. J. Parasitol. Res. 2017. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, R.N.; Rehder, V.L.G.; Oliveira, A.S.S.; Jeraldo, V.d.L.S.; Linhares, A.X.; Allegretti, S.M. Anthelmintic activity in vitro and in vivo of Baccharis trimera (Less) DC against immature and adult worms of Schistosoma mansoni. Exp. Parasitol. 2014, 139, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Godinho, L.S.; de Carvalho, A.; Soares, L.; Barbosa de Castro, C.C.; Dias, M.M.; Pinto, P.d.F.; Crotti, A.E.M.; Pinto, P.L.S.; de Moraes, J.; Da Silva Filho, A.A. Anthelmintic activity of crude extract and essential oil of Tanacetum vulgare (Asteraceae) against adult worms of Schistosoma mansoni. Sci. World J. 2014. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.F.; de Azevedo, D.P.; dos Santos Mata, R.d.C.; de Mendonça, D.I.D.; Sant’Ana, A.E.G. The lethality of Euphorbia conspicua to adults of Biomphalaria glabrata, cercaria of Schistosoma mansoni and larvae of Artemia salina. Bioresour. Technol. 2007, 98, 135–139. [Google Scholar] [CrossRef]
- Atjanasuppat, K.; Wongkham, W.; Meepowpan, P.; Kittakoop, P.; Sobhon, P.; Bartlett, A.; Whitfield, P.J. In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. J. Ethnopharmacol. 2009, 123, 475–482. [Google Scholar] [CrossRef]
- Kumar, V.K.; Kumar, P.; Venkatachalam, T. Investigation of anthelmintic activity of Pergularia daemia leaves. Pharmacophore 2014, 5, 44–48. [Google Scholar]
- Lalthanpuii, P.B.; Lalchhandama, K. Chemical composition and broad-spectrum anthelmintic activity of a cultivar of toothache plant, Acmella oleracea, from Mizoram, India. Pharm. Biol. 2020, 58, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Zreiq, R.; Hafiz, T.A.; Mubaraki, M.A.; Sulaiman, S.; Algahtani, F.; Abdel-Gaber, R.; Al-Shaebi, E.M.; Al-Quraishy, S. Anthelmintic and antimicrobial activity of Indigofera oblongifolia leaf extracts. Saudi J. Biol. Sci. 2020, 27, 594–598. [Google Scholar] [CrossRef]
- Flota-Burgos, G.J.; Rosado-Aguilar, J.A.; Rodríguez-Vivas, R.I.; Borges-Argáez, R.; Martínez-Ortiz-de-Montellano, C.; Gamboa-Angulo, M. Anthelmintic activity of extracts and active compounds from Diospyros anisandra on Ancylostoma caninum, Haemonchus placei and Cyathostomins. Front. Vet. Sci. 2020, 7, 565103. [Google Scholar] [CrossRef] [PubMed]
- Lalchhandama, K.; Roy, B.; Dutta, B.K. Anthelmintic activity of Acacia oxyphylla stem bark against Ascaridia galli. Pharm. Biol. 2009, 47, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Patil, S.; Dharanguttikar, V.; Mohite, S. Anthelmintic Activity of Malvastrum Coromandelianum Leaf Extracts against Pheretima Posthuma and Ascardia Galli. Int. J. Sci. Res. Chemi. 2020, 5, 18–24. [Google Scholar]
- Ezea, B.O.; Ogbole, O.O.; Ajaiyeoba, E.O. In vitro anthelmintic properties of root extracts of three Musa species. J. Pharm. Bioresour. 2019, 16, 145–151. [Google Scholar] [CrossRef]
- Williams, A.R.; Soelberg, J.; Jäger, A.K. Anthelmintic properties of traditional African and Caribbean medicinal plants: Identification of extracts with potent activity against Ascaris suum in vitro. Parasite 2016, 23, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.R.; Fryganas, C.; Ramsay, A.; Mueller-Harvey, I.; Thamsborg, S.M. Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum. PLoS ONE 2014, 9, e97053. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Shakya, S.; Soni, V.K.; Dangi, A.; Kumar, N.; Bhattacharya, S.-M. The n-hexane and chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi. Int. Immunopharmacol. 2009, 9, 716–728. [Google Scholar] [CrossRef]
- Mathew, N.; Misra-Bhattacharya, S.; Perumal, V.; Muthuswamy, K. Antifilarial lead molecules isolated from Trachyspermum ammi. Molecules 2008, 13, 2156–2168. [Google Scholar] [CrossRef] [Green Version]
- Gaur, R.; Sahoo, M.; Dixit, S.; Fatma, N.; Rastogi, S.; Kulshreshtha, D.; Chatterjee, R.; Murthy, P. Antifilarial activity of Caesalpinia bonducella against experimental filarial infections. Indian J. Med. Res. 2008, 128, 65. [Google Scholar] [PubMed]
- Misra, N.; Sharma, M.; Raj, K.; Dangi, A.; Srivastava, S.; Misra-Bhattacharya, S. Chemical constituents and antifilarial activity of Lantana camara against human lymphatic filariid Brugia malayi and rodent filariid Acanthocheilonema viteae maintained in rodent hosts. Parasitol. Res. 2007, 100, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, Y.; Kamachi, T.; Yanagi, T.; Caceres, A.; Maki, J.; Aoki, Y. Macrofilaricidal and microfilaricidal effects of Neurolaena lobata, a Guatemalan medicinal plant, on Brugia pahangi. J. Helminthol. 2005, 79, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Okem, A.; Finnie, J.; Van Staden, J. Pharmacological, genotoxic and phytochemical properties of selected South African medicinal plants used in treating stomach-related ailments. J. Ethnopharmacol. 2012, 139, 712–720. [Google Scholar] [CrossRef]
- Ndjonka, D.; Agyare, C.; Lüersen, K.; Djafsia, B.; Achukwi, D.; Nukenine, E.; Hensel, A.; Liebau, E. In vitro activity of Cameroonian and Ghanaian medicinal plants on parasitic (Onchocerca ochengi) and free-living (Caenorhabditis elegans) nematodes. J. Helminthol. 2011, 85, 304–312. [Google Scholar] [CrossRef]
- Katiki, L.M.; Ferreira, J.F.; Gonzalez, J.M.; Zajac, A.M.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol. 2013, 192, 218–227. [Google Scholar] [CrossRef]
- von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Mendoza-de Gives, P.; Valles-de la Mora, B.; González-Cortazar, M.; Zamilpa, A.; Gallegos, E.C. Elucidation of Leucaena leucocephala anthelmintic-like phytochemicals and the ultrastructural damage generated to eggs of Cooperia spp. Vet. Parasitol. 2015, 214, 89–95. [Google Scholar] [CrossRef]
- Osei Akoto, C.; Acheampong, A.; Boakye, Y.D.; Naazo, A.A.; Adomah, D.H. Anti-inflammatory, antioxidant, and anthelmintic activities of Ocimum basilicum (Sweet Basil) fruits. J. Chemistry 2020. [Google Scholar] [CrossRef]
- A Yousef, B.; M Hassan, H.; Zhang, L.-Y.; Jiang, Z.-Z. Anticancer potential and molecular targets of pristimerin: A mini-review. Curr. Cancer Drug Targets. 2017, 17, 100–108. [Google Scholar] [CrossRef]
- Kaiaty, A.M.; Salib, F.A.; El-Gameel, S.M.; Hussien, A.M.; Kamel, M.S. Anthelmintic activity of pomegranate peel extract (Punica granatum) and synthetic anthelmintics against gastrointestinal nematodes in cattle, sheep, goats, and buffalos: In vivo study. Parasitol. Res. 2021, 120, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, M.L.S.; de Moura, M.C.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Bezerra, A.C.D.S.; da Silva, M.D.C. Nematicidal activity of a water soluble lectin from seeds of Moringa oleifera. Int. J. Biol. Macromol. 2018, 108, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Alowanou, G.; Olounladé, P.; Akouèdegni, G.; Faihun, A.; Koudandé, D.; Hounzangbé-Adoté, S. In vitro anthelmintic effects of Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis leaf extracts on Haemonchus contortus, an abomasal nematode of small ruminants. Parasitol. Res. 2019, 118, 1215–1223. [Google Scholar] [CrossRef]
- Ndlela, S.; Mkwanazi, M.; Chimonyo, M. In vitro efficacy of plant extracts against gastrointestinal nematodes in goats. Trop. Anim. Health Prod. 2021, 53, 295. [Google Scholar] [CrossRef] [PubMed]
- Castagna, F.; Piras, C.; Palma, E.; Musolino, V.; Lupia, C.; Bosco, A.; Rinaldi, L.; Cringoli, G.; Musella, V.; Britti, D. Green veterinary pharmacology applied to parasite control: Evaluation of Punica granatum, Artemisia campestris, Salix caprea aqueous macerates against gastrointestinal nematodes of sheep. Vet. Sci. 2021, 8, 237. [Google Scholar] [CrossRef]
- Piza, M.; Féboli, A.; Augusto, J.; Anjos, L.; Laurentiz, A.; Royo, V.; Alvarenga, F.; Laurentiz, R. In vitro ovicidal and larvicidal activity of Psidium cattleianum Sabine leaves against gastrointestinal nematodes of naturally infected sheep. Bol. Ind. Anim. 2019, 76, 1–8. [Google Scholar] [CrossRef]
- Castagna, F.; Britti, D.; Oliverio, M.; Bosco, A.; Bonacci, S.; Iriti, G.; Ragusa, M.; Musolino, V.; Rinaldi, L.; Palma, E. In vitro anthelminthic efficacy of aqueous pomegranate (Punica granatum L.) extracts against gastrointestinal nematodes of sheep. Pathogens 2020, 9, 1063. [Google Scholar] [CrossRef]
- Jogpal, B.; Swarnakar, G.; Chouhan, H.S.; Roat, K. In vitro anthelmintic activity of medicinal plant Tinospora cordifolia extract on amphistome Gastrothylax crumenifer. Ecol. Environ. Conserv. 2021, 27, 231–234. [Google Scholar]
- Irum, S.; Ahmed, H.; Mukhtar, M.; Mushtaq, M.; Mirza, B.; Donskow-Łysoniewska, K.; Qayyum, M.; Simsek, S. Anthelmintic activity of Artemisia vestita Wall ex DC. and Artemisia maritima L. against Haemonchus contortus from sheep. Vet. Parasitol. 2015, 212, 451–455. [Google Scholar] [CrossRef]
- Acharya, J.; Hildreth, M.B.; Reese, R.N. In vitro screening of forty medicinal plant extracts from the United States Northern Great Plains for anthelmintic activity against Haemonchus contortus. Vet. Parasitol. 2014, 201, 75–81. [Google Scholar] [CrossRef]
- Tariq, K.; Chishti, M.; Ahmad, F.; Shawl, A. Anthelmintic efficacy of Achillea millifolium against gastrointestinal nematodes of sheep: In vitro and in vivo studies. J. Helminthol. 2008, 82, 227–233. [Google Scholar] [CrossRef]
- Ferreira, L.; Castro, P.; Chagas, A.; França, S.; Beleboni, R. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L.(Annonaceae) against Haemonchus contortus from sheep. Exp. Parasitol. 2013, 134, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.M.; Oliveira, J.T.; Rocha, C.Q.; Ferreira, A.T.; Perales, J.; Zanatta, A.C.; Vilegas, W.; Silva, C.R.; Costa-Junior, L.M. Myracrodruon urundeuva seed exudates proteome and anthelmintic activity against Haemonchus contortus. PLoS ONE 2018, 13, e0200848. [Google Scholar] [CrossRef]
- Ahmed, M.; Laing, M.; Nsahlai, I. In vitro anthelmintic activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J. Helminthol. 2013, 87, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Alawa, C.; Adamu, A.; Gefu, J.; Ajanusi, O.; Abdu, P.; Chiezey, N.; Alawa, J.; Bowman, D. In vitro screening of two Nigerian medicinal plants (Vernonia amygdalina and Annona senegalensis) for anthelmintic activity. Vet. Parasitol. 2003, 113, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kahiya, C.; Mukaratirwa, S.; Thamsborg, S.M. Effects of Acacia nilotica and Acacia karoo diets on Haemonchus contortus infection in goats. Vet. Parasitol. 2003, 115, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zamilpa, A.; García-Alanís, C.; López-Arellano, M.; Hernández-Velázquez, V.; Valladares-Cisneros, M.; Salinas-Sánchez, D.; Mendoza-de Gives, P. In vitro nematicidal effect of Chenopodium ambrosioides and Castela tortuosa n-hexane extracts against Haemonchus contortus (Nematoda) and their anthelmintic effect in gerbils. J. Helminthol. 2019, 93, 434–439. [Google Scholar] [CrossRef]
- Sebai, E.; Serairi, R.; Saratsi, K.; Abidi, A.; Sendi, N.; Darghouth, M.A.; Wilson, M.S.; Sotiraki, S.; Akkari, H. Hydro-ethanolic extract of Mentha pulegium exhibit anthelmintic and antioxidant proprieties in vitro and in vivo. Acta Parasitol. 2020, 65, 375–387. [Google Scholar] [CrossRef]
- Dhadde Gurunath, S.; Mali Hanmant, S.; Sapate Rohit, B.; Vakhariya Rohan, R.; Raut Indrayani, D.; Nitalikar Manoj, M. Investigation of In-Vitro Anthelmintic Activity of Methanolic Extract of Tylophora Indica Leaves against Haemonchus contortus. J. Univ. Shanghai Sci. Technol. 2021, 23, 37–42. [Google Scholar]
- Oliveira, A.F.; Junior, L.M.C.; Lima, A.S.; Silva, C.R.; Ribeiro, M.N.; Mesquista, J.W.; Rocha, C.Q.; Tangerina, M.M.; Vilegas, W. Anthelmintic activity of plant extracts from Brazilian savanna. Vet. Parasitol. 2017, 236, 121–127. [Google Scholar] [CrossRef]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mravčáková, D.; Komáromyová, M.; Babják, M.; Urda Dolinská, M.; Königová, A.; Petrič, D.; Čobanová, K.; Ślusarczyk, S.; Cieslak, A.; Várady, M. Anthelmintic activity of wormwood (Artemisia absinthium L.) and mallow (Malva sylvestris L.) against Haemonchus contortus in sheep. Animals 2020, 10, 219. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.H.; Ejo, M.; Feyera, T.; Regassa, D.; Mummed, B.; Huluka, S.A. In vitro anthelmintic activity of crude extracts of Artemisia herba-alba and Punica granatum against Haemonchus contortus. J. Parasitol. Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.A.; Islam, M.R.; Lovelu, M.A.; Nahar, S.F.; Dutta, P.K.; Talukder, M.H. In vitro evaluation of anthelmintic activity of tannin-containing plant Artemisia extracts against Haemonchus contortus from goat: Anthelmintic activity of tannin-containing plants Artemisia. J. Bangladesh Agric. Univ. 2019, 17, 363–368. [Google Scholar] [CrossRef]
- Hoste, H.; Brunet, S.; Paolini, V.; Bahuaud, D.; Chauveau, S.; Fouraste, I.; Lefrileux, Y. Compared in vitro anthelmintic effects of eight tannin-rich plants browsed by goats in the southern part of France. Option Méditerrenéennes 2009, 85, 431–436. [Google Scholar]
- Iqbal, Z.; Lateef, M.; Ashraf, M.; Jabbar, A. Anthelmintic activity of Artemisia brevifolia in sheep. J. Ethnopharmacol. 2004, 93, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Chishti, M.; Ahmad, F.; Shawl, A. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Parasitol. 2009, 160, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Manolaraki, F.; Sotiraki, S.; Stefanakis, A.; Skampardonis, V.; Volanis, M.; Hoste, H. Anthelmintic activity of some Mediterranean browse plants against parasitic nematodes. Parasitology 2010, 137, 685–696. [Google Scholar] [CrossRef]
- Busari, I.; Soetan, K.; Aiyelaagbe, O.; Babayemi, O. Phytochemical screening and in vitro anthelmintic activity of methanolic extract of Terminalia glaucescens leaf on Haemonchus contortus eggs. Acta Trop. 2021, 223, 106091. [Google Scholar] [CrossRef]
- Eguale, T.; Tadesse, D.; Giday, M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. J. Ethnopharmacol. 2011, 137, 108–113. [Google Scholar] [CrossRef]
- Zarza-Albarrán, M.; Olmedo-Juárez, A.; Rojo-Rubio, R.; Mendoza-de Gives, P.; González-Cortazar, M.; Tapia-Maruri, D.; Mondragón-Ancelmo, J.; García-Hernández, C.; Blé-González, E.A.; Zamilpa, A. Galloyl flavonoids from Acacia farnesiana pods possess potent anthelmintic activity against Haemonchus contortus eggs and infective larvae. J. Ethnopharmacol. 2020, 249, 112402. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; de Jesús Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Borges-Argáez, R.; Cáceres-Farfán, M.; Mancilla-Montelongo, G.; Mathieu, C. Bio-guided fractionation to identify Senegalia gaumeri leaf extract compounds with anthelmintic activity against Haemonchus contortus eggs and larvae. Vet. Parasitol. 2019, 270, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, F.C.; Gordon, I.J.; Wright, A.; Benvenutti, M.A.; Saumell, C. Efecto antihelmíntico in vitro de extractos de plantas sobre larvas infectantes de nematodos gastrointestinales de rumiantes. Arch. Med. Vet. 2010, 42, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Nyasse, B.; Ngantchou, I.; Nono, J.-J.; Schneider, B. Antifilarial activity in vitro of polycarpol and 3-O-acetyl aleuritolic acid from cameroonian medicinal plants against Onchocerca gutturosa. Nat. Prod. Res. 2006, 20, 391–397. [Google Scholar] [CrossRef]
- Ndjonka, D.; Rapado, L.; Silber, A.; Liebau, E.; Wrenger, C. Natural products as a source for treating neglected parasitic diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef]
- Rakhshandehroo, E.; Asadpour, M.; Malekpour, S.; Jafari, A. The anthelmintic effects of five plant extracts on the viability of Parascaris equorum larvae. Equine Vet. Educ. 2017, 29, 219–224. [Google Scholar] [CrossRef]
- GS, R.I.D.; Yadav, J.; Sapate, R.; Mali, H. In vitro Anthelmintic Activity of crude extract of flowers of Bougainvillea Spectabilis Wild against Pheretima Posthuma. Int. J. Pharm. Pharm. Sci. 2020, 17, 1–18. [Google Scholar]
- Chander, P.A.; Sri, H.Y.; Sravanthi, N.B.; Susmitha, U.V. In vitro anthelmintic activity of Barleria buxifolia on Indian adult earthworms and estimation of total flavonoid content. Asian Pac. J. Trop. Dis. 2014, 4, S233–S235. [Google Scholar] [CrossRef]
- Desai, H.; Kapadia, M.; Kharat, A. Evaluation of anthelmintic activity of Plumbago zeylanica Linn. Int. J. Pharm. Sci. 2012, 3, 4281. [Google Scholar]
- Carvalho, V.; Ramos, L.d.A.; da Silva, C.; Nebo, L.; Moraes, D.; Da Silva, F.; Da Costa, N.; Junior, R.d.O.R.; De Souza, L.; Rodrigues, R. In vitro anthelmintic activity of Siparuna guianensis extract and essential oil against Strongyloides venezuelensis. J. Helminthol. 2020, 94, e50. [Google Scholar] [CrossRef]
- Shalaby, H.A.; El Namaky, A.H.; Khalil, F.A.; Kandil, O.M. Efficacy of methanolic extract of Balanites aegyptiaca fruits on Toxocara vitulorum. Vet. Parasitol. 2012, 183, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ríos-de Álvarez, L.; Jackson, F.; Greer, A.; Grant, G.; Jackson, E.; Morrison, A.; Huntley, J. Direct anthelmintic and immunostimulatory effects of oral dosing semi-purified phytohaemagglutinin lectin in sheep infected with Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2012, 187, 267–274. [Google Scholar] [CrossRef]
- dos Santos, A.F.; Fonseca, S.A.; César, F.A.; de Azevedo Albuquerque, M.C.P.; Santana, J.V.; Santana, A.E.G. A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol. Res. 2014, 113, 1077–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.; Sharifi-Rad, M.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, R.; Raeisi, S. Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L. Molecules 2015, 20, 7034–7047. [Google Scholar] [CrossRef] [Green Version]
- Akkari, H.; Ezzine, O.; Dhahri, S.; B’chir, F.; Rekik, M.; Hajaji, S.; Darghouth, M.A.; Jamâa, M.L.B.; Gharbi, M. Chemical composition, insecticidal and in vitro anthelmintic activities of Ruta chalepensis (Rutaceae) essential oil. Ind. Crops. Prod. 2015, 74, 745–751. [Google Scholar] [CrossRef]
- Moreno, F.C.; Gordon, I.J.; Knox, M.; Summer, P.; Skerrat, L.; Benvenutti, M.A.; Saumell, C. Anthelmintic efficacy of five tropical native Australian plants against Haemonchus contortus and Trichostrongylus colubriformis in experimentally infected goats (Capra hircus). Vet. Parasitol. 2012, 187, 237–243. [Google Scholar] [CrossRef]
- Mansur, F.; Luoga, W.; Buttle, D.; Duce, I.; Lowe, A.; Behnke, J. The anthelmintic efficacy of natural plant cysteine proteinases against two rodent cestodes Hymenolepis diminuta and Hymenolepis microstoma in vitro. Vet. Parasitol. 2014, 201, 48–58. [Google Scholar] [CrossRef]
- Kalani, K.; Kushwaha, V.; Sharma, P.; Verma, R.; Srivastava, M.; Khan, F.; Murthy, P.; Srivastava, S.K. In vitro, in silico and in vivo studies of ursolic acid as an anti-filarial agent. PLoS ONE 2014, 9, e111244. [Google Scholar]
- Kushwaha, S.; Roy, S.; Maity, R.; Mallick, A.; Soni, V.K.; Singh, P.K.; Chaurasiya, N.D.; Sangwan, R.S.; Misra-Bhattacharya, S.; Mandal, C. Chemotypical variations in Withania somnifera lead to differentially modulated immune response in BALB/c mice. Vaccine 2012, 30, 1083–1093. [Google Scholar] [CrossRef]
- Kushwaha, S.; Soni, V.K.; Singh, P.K.; Bano, N.; Kumar, A.; Sangwan, R.S.; Bhattacharya, S.M. Withania somnifera chemotypes NMITLI 101R, NMITLI 118R, NMITLI 128R and withaferin A protect Mastomys coucha from Brugia malayi infection. Parasite Immunol. 2011, 34, 199–209. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Misra, S.; Gupta, J.; Misra-Bhattacharya, S. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite, Brugia malayi. Eur. J. Med. Chem. 2012, 50, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.G.; Machado, C.B.; Morais, E.R.; de Carvalho Moreira, É.B.; Soares, C.S.; da Silva, S.H.; Da Silva Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009, 104, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Nishino, K.; Roberts, M.C.; Tolmasky, M.; Aminov, R.I.; Zhang, L. Mechanisms of antibiotic resistance. Front. Microbiol. 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestrini, M.; Tava, A.; Mancini, S.; Salari, F.; Perrucci, S. In vitro anthelmintic activity of saponins derived from Medicago spp. plants against donkey gastrointestinal nematodes. Vet. Sci. 2019, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Maestrini, M.; Tava, A.; Mancini, S.; Tedesco, D.; Perrucci, S. In vitro anthelmintic activity of saponins from Medicago spp. against sheep gastrointestinal nematodes. Molecules 2020, 25, 242. [Google Scholar] [CrossRef] [Green Version]
- Buza, V.; Cătană, L.; Andrei, S.; Ștefănuț, L.; Răileanu, Ș.; Matei, M.; Vlasiuc, I.; Cernea, M. In vitro anthelmintic activity assessment of six medicinal plant aqueous extracts against donkey strongyles. J. Helminthol. 2020, 94, E147. [Google Scholar] [CrossRef]
- Aremu, A.; Finnie, J.; Van Staden, J. Potential of South African medicinal plants used as anthelmintics–Their efficacy, safety concerns and reappraisal of current screening methods. S. Afr. J. Bot. 2012, 82, 134–150. [Google Scholar] [CrossRef] [Green Version]
- Irum, S.; Ahmed, H.; Mirza, B.; Donskow-Łysoniewska, K.; Muhammad, A.; Qayyum, M.; Simsek, S. In vitro and in vivo anthelmintic activity of extracts from Artemisia parviflora and A. sieversiana. Helminthologia 2017, 54, 218–224. [Google Scholar] [CrossRef]
- Zenebe, S.; Feyera, T.; Assefa, S. In vitro anthelmintic activity of crude extracts of aerial parts of Cissus quadrangularis L. and leaves of Schinus molle L. against Haemonchus contortus. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [Green Version]
- Spiegler, V.; Hensel, A.; Seggewiß, J.; Lubisch, M.; Liebau, E. Transcriptome analysis reveals molecular anthelmintic effects of procyanidins in C. elegans. PLoS ONE 2017, 12, e0184656. [Google Scholar] [CrossRef] [Green Version]
- Sambodo, P.; Prastowo, J.; Kurniasih, K.; Indarjulianto, S. In vitro potential anthelmintic activity of Biophytum petersianum on Haemonchus contortus. Vet. World. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Thooyavan, G.; Kathikeyan, J.; Govindarajalu, B. Anthelmintic activity of Abutilon indicum leaf extract on sheep tapeworm Moniezia expansa in vitro. J. Pharmacogn. Phytochem. 2018, 7, 317–321. [Google Scholar]
- Zangueu, C.B.; Olounlade, A.P.; Ossokomack, M.; Djouatsa, Y.N.N.; Alowanou, G.G.; Azebaze, A.G.B.; Llorent-Martínez, E.J.; de Córdova, M.L.F.; Dongmo, A.B.; Hounzangbe-Adote, M.S. In vitro effects of aqueous extract from Maytenus senegalensis (Lam.) Exell stem bark on egg hatching, larval migration and adult worms of Haemonchus contortus. BMC Vet. Res. 2018, 14, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga de Oliveira, M.I.; Rodrigues Brandão, F.; Rocha da Silva, M.J.; Carvalho Rosa, M.; Santana Farias, C.F.; Silva dos Santos, D.; Majolo, C.; Oliveira, M.R.d.; Chaves, F.C.M.; Bizzo, H.R. In vitro anthelmintic efficacy of essential oils in the control of Neoechinorhynchus buttnerae, an endoparasite of Colossoma macropomum. J. Essent. Oil Res. 2021, 33, 509–522. [Google Scholar] [CrossRef]
- Zaman, M.A.; Qamar, W.; Yousaf, S.; Mehreen, U.; Shahid, Z.; Khan, M.K.; Qamar, M.F.; Kamran, M. In vitro experiments revealed the anthelmintic potential of herbal complex against Haemonchus contortus. Pak. Vet. J. 2019, 40, 271–273. [Google Scholar]
- Salgado, J.A.; Santos, C.d.P. Overview of anthelmintic resistance of gastrointestinal nematodes of small ruminants in Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 3–17. [Google Scholar] [CrossRef] [Green Version]
- von Samson-Himmelstjerna, G. Molecular diagnosis of anthelmintic resistance. Vet. Parasitol. 2006, 136, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Demeler, J.; Küttler, U.; El-Abdellati, A.; Stafford, K.; Rydzik, A.; Varady, M.; Kenyon, F.; Coles, G.; Höglund, J.; Jackson, F. Standardization of the larval migration inhibition test for the detection of resistance to ivermectin in gastro intestinal nematodes of ruminants. Vet. Parasitol. 2010, 174, 58–64. [Google Scholar] [CrossRef]
- Beech, R.; Skuce, P.; Bartley, D.; Martin, R.; Prichard, R.; Gilleard, J. Anthelmintic resistance: Markers for resistance, or susceptibility? Parasitology 2011, 138, 160–174. [Google Scholar] [CrossRef] [Green Version]
- Demeler, J.; Küttler, U.; von Samson-Himmelstjerna, G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Vet. Parasitol. 2010, 170, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; P Sinha Babu, S. Phenolics and terpenoids; the promising new search for anthelmintics: A critical review. Mini Rev. Med. Chem. 2016, 16, 1415–1441. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, B.; Bickle, Q.; Yousif, F.; Fakorede, F.; Mouries, M.-A.; Nwaka, S. Schistosomes: Challenges in compound screening. Expert Opin. Drug Discov. 2007, 2, S53–S61. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.G.; Sakanari, J.A.; Caffrey, C.R. Anthelmintic drug discovery: Into the future. J. Parasitol. 2015, 101, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.V.; Lodeyro, A.F.; Malheiros, A.; Zacchino, S.A.; Roveri, O.A. Inhibition of the mitochondrial ATP synthesis by polygodial, a naturally occurring dialdehyde unsaturated sesquiterpene. Biochem. Pharmacol. 2005, 70, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tritten, L.; Braissant, O.; Keiser, J. Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro. Parasitol. 2012, 139, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M.; Kingston, D.G. Natural products as pharmaceuticals and sources for lead structures. Pract. Med. Chem. 2008, 159–186. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasit Vectors. 2019, 12, 306. [Google Scholar] [CrossRef]
| Parasite | Study Model | Plant Family | Plant Name | Plant Tissue | Extract | Effective Concentration and Mortality Rate (%) | Reference |
|---|---|---|---|---|---|---|---|
| Carmyerius spatiosus | In vitro | Leguminosae | Cassia siamea | Leaves and heartwood | Ethyl acetate extracts | Highest anthelminthic effect | [7] |
| Plumbaginaceae | Plumbago zeylanica | Roots | n-butanol extract | ||||
| Plumbaginaceae | Plumbago indica | Roots | hexane, ethyl acetate, and n-butanol extract | ||||
| Combretaceae | Terminalia catappa | Leaves | n-butanol and water extract | ||||
| Clonorchis sinensis | In vitro | Rosaceae | Hagenia abyssinica | Female flowers | Crude extract | 5 h (100 µg/mL) | [8] |
| Echinococcus granulosus (protoscolex) | In vitro | Anacardiaceae | Pistacia atlantica | Fruits and leaves | Hydroalcoholic extracts | 100%; killed protoscoleces (50 mg/mL in 10 min) | [3] |
| Leaves and fruits | Hydroalcoholic extracts | 0.1% concentration of fresh fruit extract (99.09 ± 1.27 mg/mL) and leaf extract (89.25 ± 18.42 mg/mL) had strong scolicidal effects in 360 min | [9] | ||||
| In vitro | Lamiaceae | Salvia officinalis | Aerial parts | Ethanolic extract | 100% (6–8 days) | [10] | |
| Fabaceae | Prosopis farcta | Leaves | Ethanolic extract Crude alkaloids | 25% scolicidal activity with a 500 mg/mL dose after 24 h | [11] | ||
| 57% scolicidal activity with a 500 mg/mL dose after 24 h | |||||||
| Ranunculaceae | Nigella sativa | Seeds | Essential oil (Thymoquinone) | 100% scolicidal activity with a 1 mg/mL dose after 10 min | [12] | ||
| Cucurbitaceae | Dendrosicyos socotrana | Leaves | Aqueous and methanolic extracts | 100% scolicidal activity with a 5000 μg/mL dose after 360 h (methanolic extract) and 408 h (aqueous extract) | [13] | ||
| Euphorbiaceae | Jatropha unicostata | Aqueous and methanolic extracts | 100% scolicidal activity with a 1000 μg/mL dose after 288 h (both extracts) | ||||
| Berberidaceae | Berberis vulgaris | Fruits | Aqueous extracts | 98.7% scolicidal activity with a 2 mg/mL dose after 30 min | [14] | ||
| Euphorbiaceae | Mallotus philippinensis | Fruits | Methanolic extracts | 99% scolicidal activity with a 20 mg/mL dose after 60 min | [15] | ||
| Echinococcus granulosus protoscolex | In vitro | Meliaceae | Azadirachta indica | Whole plant | Ethanolic extracts | Up to 97% mortality with 30 min of incubation | [16] |
| Echinostoma caproni | In vitro | Rosaceae | Hagenia abyssinica | Female flowers | Crude extract | 51 h (100 µg/mL) | [8] |
| Fasciola hepatica | In vitro | Fabaceae | Acacia farnesiana | Leaves | Hexane, ethyl acetate, and methanolic extracts | 0% (500 mg/L) | [17] |
| Asteraceae | Artemisia absinthium | 0% (500 mg/L) | |||||
| Artemisia mexicana | 100% (500 mg/L) | ||||||
| Papaveraceae | Bocconia frutescens | 100% (500 mg/L) | |||||
| Fabaceae | Cajanus cajan | 100% (500 mg/L) | |||||
| Boraginaceae | Cordia spp. | 0% (500 mg/L) | |||||
| Malvaceae | Hibiscus rosa sinensis | 0% (500 mg/L) | |||||
| Verbenaceae | Lantana camara | 100% (500 mg/L) | |||||
| Fabaceae | Leucaena diversifolia | 0% (500 mg/L) | |||||
| Meliaceae | Melia azedarach | 13% (500 mg/L) | |||||
| Lamiaceae | Mentha sp. | 0% (500 mg/L) | |||||
| Ocimum basilicum | 0% (500 mg/L) | ||||||
| Piperaceae | Piper auritum | 100% (500 mg/L) | |||||
| Dysphania | Teloxys ambrosioides | 0% (500 mg/L) | |||||
| Fasciola larvae (sporocyst, redia, and cercaria) | In vitro | Rosaceae | Potentilla fulgens | Dried root powder | Ether, chloroform, methanolic, acetone, and ethanolic extracts | 8 h LC50 was 54.20 mg/L for sporocysts, 49.37 mg/L for redia, and 38.13 mg/L for cercaria | [18] |
| Fasciola gigantica larvae (sporocysts, redia, and cerceria) | In vivo | Asparagaceae | Asparagus racemosus | Dried root powder | Ether, chloroform, methanolic, acetone, and ethanolic extracts | 2 h LC50 was 79.93% | [19] |
| Fasciola gigantica and Taenia solium | In vitro | Euphorbiaceae | Acalypha wilkesiana | Extracts | Methanolic extracts of leaves, stems, and roots | All extracts exhibited anthelmintic activity in vitro | [20] |
| Fasciola hepatica | In vitro | Rosaceae | Hagenia abyssinica | Female flowers | Crude extract | 1 h (100 µg/mL) | [8] |
| Fasciolopsis buski | In vitro | Zingiberaceae | Alpinia nigra | Shoot | Crude alcoholic extract | 3.94 ± 0.06 h death time (20 mg/mL concentration) | [21] |
| Gastrothylax crumenifer | In vitro | Fabaceae | Sesbania sesban var. bicolor | Fresh leaves | Methanolic extracts of dried plants | Better than praziquantel | [22] |
| Cyperaceae | Cyperus compressus | Roots | |||||
| Asparagaceae | Asparagus racemosus | Roots | |||||
| Hymenolepis diminuta and Syphacia obvelata | In vitro In vivo | Asparagaceae | Asparagus racemosus | Roots | Methanolic extract | 53.88% and 24% reduction in EPG * and worm counts, respectively (30 mg/mL concentration) | [23] |
| Hymenolepis diminuta | In vitro | Cyperaceae | Cyperus compressus | Roots | Methanolic extract | 61.74% reduction in the EPG and 24% reduction in worm counts (30 mg/mL concentration) | [24] |
| Hymenolepis diminuta | In vitro | Fabaceae | Sesbania sesban | Fresh Leaves | Methanolic extract | 65.10% reduction in EPG counts, 56% reduction in worm counts (30 mg/mL concentration) | [25] |
| Paramphistomum gracile | In vitro | Fabaceae | Senna alata, S. alexandrina, and S. occidentalis | Leaf extract | Ethanolic extracts | Dose-dependent effects on motility and mortality | [26] |
| Paramphistomum microbothrium | In vitro | Zygophyllaceae | Balanites aegyptiaca | Fruits | Methanolic extract | 200 µg/ml, at which distinct damage to the whole body surface of the trematodes | [27] |
| Raillietina echinobothrida | In vitro | Asteraceae | Acmella oleracea | Leaves | Methanolic extract | 18.42 ± 0.95 h survival time (20 mg/mL concentration) | [28] |
| Raillietina spiralis | In vitro | Malvaceae | Thespesia lampas | Roots | Aqueous extracts | 51 ± 0.33 min death time (20 mg/mL concentration) | [29] |
| Raillietina spiralis | In vitro | Meliaceae | Azadirachta Indica | Leaves | Aqueous extract | 46 ± 0.53 min death time (20 mg/mL concentration) | [30] |
| Raillietina spiralis | In vitro | Scrophulariaceae | Verbascum Thapsus | Fresh Leaves | Methanolic extract | 86 ± 5 min death time (20 mg/mL concentration) | [31] |
| Raillietina spiralis | In vitro | Asteraceae | Achillea wilhelmsii | Fresh Leaves | Methanolic extract | 40 min death time (20 mg/mL concentration) | [32] |
| Raillietina spiralis | In vitro | Lauraceae | Cinnamomum camphora | Leaves | Aqueous extracts | 47 ± 0.54 min death time (20 mg/mL concentration) | [33] |
| Raillietina spiralis | In vitro | Verbenaceae | Clerodendron inerme | Leaves | Aqueous extracts | 45 ± 0.52 min death time (20 mg/mL concentration) | [34] |
| Raillietina tetragona | In vitro | Poaceae | Imperata cylindrica | Underground parts (rhizomes and roots) | Chloroform (medium polar solvent) | Dose-dependent anthelmintic activity | [35] |
| Schistosoma mansoni | In vitro | Apocynaceae | Rauwolfia vomitoria | Stem bark and roots | Ethanolic extract | High activity against cercariae and adult worms | [36] |
| Syphacia obvelata | In vitro | Cyperaceae | Cyperus compressus | Roots | Methanolic extract | 28.92% reduction in the EPG and 33.85% reduction in worm counts (30 mg/mL concentration) | [24] |
| Syphacia obvelata | In vitro | Fabaceae | Sesbania sesban | Fresh leaves | Methanolic extract | EPG and worm counts reduced by 34.32% and 47.08%, respectively (30 mg/mL concentration) | [25] |
| Schistosoma mansoni | In vivo | Asteraceae | Baccharis trimera | Leaves | Crude dichloromethane extract (DE) and aqueous fraction (AF) | 98% (AF) 97% (DE) | [37] |
| Tanacetum vulgare | Aerial parts | Crude extract and Essential oil | 100% | [38] | |||
| Schistosoma mansoni | In vitro | Rosaceae | Hagenia abyssinica | Female flowers | Crude extract | 3 h (100 µg/mL) | [8] |
| Schistosoma mansoni | In vitro | Euphorbiaceae | Euphorbia conspicua | Leaves | Leaf extract | 100% (100 µg/mL) | [39] |
| Piperaceae | Piper chaba | Fruits | Methylene chloride extract | Strongest activity | [40] | ||
| Taenia solium | In vitro | Asclepiadaceae | Pergularia daemia | Leaves | Ethanolic extract | 210.00 ± 0.52 min death time (25 mg/mL concentration) | [41] |
| Aqueous extract | 221.12 ± 0.61 | ||||||
| Taenia tetragona | In vitro | Asteraceae | Acmella oleracea | Leaves | Hexane extract | The lethal concentration (LC50) of the plant extract was 5128.61 ppm on T. tetragona and 8921.50 ppm on A. perspicillum | [42] |
| Parasite | Study Model | Plant Family | Plant Name | Plant Part Used | Extract/Compound | LC50 * | References |
|---|---|---|---|---|---|---|---|
| Allolobophora caliginosa | In vitro | Fabaceae | Indigofera oblongifolia | Leaves | Leaf extracts | 15 ± 2 and 8.6 ± 1 h survival time with leaf extracts at 200 mg/mL and 300 mg/mL, respectively | [43] |
| Ancylostoma caninum, Haemonchus placei, andCyathostomins | In vivo | Ebenaceae | Diospyros anisandra | Leaves and bark | Extracts and active compounds | Wide-spectrum anthelmintic activity | [44] |
| Ascardia galli | In vitro | Malvaceae | Thespesia lampas | Roots | Aqueous extracts | 43 ± 0.86 min death time (20 mg/mL concentration) | [29] |
| Ascardia galli | In vitro | Mimosaceae | Acacia oxyphylla | Fresh stems | Ethanolic extracts | 55.17 h ± 1.04 h death time (0. 5 mg/ mL concentration) | [45] |
| Ascardia galli | In vitro | Meliaceae | Azadirachta Indica | Leaves | Aqueous extract | 46 ± 0.26 min death time (20 mg/mL concentration) | [30] |
| Ascardia galli | In vitro | Scrophulariaceae | Verbascum Thapsus | Fresh Leaves | Methanolic extract | 81 ± 4 min death time (20 mg/mL concentration) | [31] |
| Ascardia galli | In vitro | Asteraceae | Achillea wilhelmsii | Fresh Leaves | Methanolic extract | 40 min death time (20 mg/mL concentration) | [32] |
| Ascardia galli | In vitro | Lauraceae | Cinnamomum camphora | Leaves | Aqueous extracts | 52 ± 0.43 min death time (20 mg/mL concentration) | [33] |
| Ascardia galli | In vitro | Verbenaceae | Clerodendron inerme | Leaves | Aqueous extracts | 50 ± 0.31 min death time (20 mg/mL concentration) | [34] |
| Ascardia galli and Pheretima posthuma | In vitro | Malvaceae | Malvastrum coromandelianum | Leaves | Methanolic and ethyl acetate extracts | Significant anthelmintic activity | [46] |
| Ascaris lumbricoides | In vitro | Musaceae | Musa paradisiaca, M. sapientum, and M. nana | Roots | Methanol root extracts | Death time 151.39 ± 0.1 min at 200 mg/mL | [47] |
| Ascaris lumbricoides | In vitro | Asclepiadaceae | Pergularia daemia | Leaves | Ethanolic Extract | 98.42 ± 0.57 min death time (25 mg/mL concentration) | [41] |
| Aqueous Extract | 109.91 ± 0.49 min death time (25 mg/mL concentration) | ||||||
| Ascaris suum L3 larvae | In vitro | Lythraceae | Punica granatum | Fruit Peel | Ethanolic extracts | EC50 values 164% | [48] |
| Rutaceae | Zanthoxylum zanthoxyloides | Roots | EC50 values 97% | ||||
| Rutaceae | Clausena anisata | Roots | EC50 values 74% | ||||
| Ascaris suum L3 larvae | In vitro | Acetone/water extracts | Ascaris suum L3 migratory inhibition activity EC50 ** values | [49] | |||
| Pinaceae | Pinus sylvestris | Bark | 48.2% | ||||
| Fabaceae | Onobrychis viciifolia | Whole plant | 41.9% | ||||
| Fabaceae | Trifolium repens | Flowers | 98.4% | ||||
| Grossulariaceae | Ribes nigrum | Leaves | 91.8% | ||||
| Ribes rubrum | Leaves | 86% | |||||
| Brugia malayi | In vivo | Piperaceae | Piper betle | Leaves | Methanolic extracts | Moderate activity | [50] |
| Brugia malayi | In vitro/ In vivo | Apiaceae | Trachyspermum ammi | Dried fruits | Methanolic extracts | 58.93% | [51] |
| Brugia malayi | In vivo | Caesalpiniaceae | Caesalpinia bonducella | Seed kernels | Ethanolic extracts | 96.0% microfilaricidal and 100% sterilization in females | [52] |
| Butanolic extracts | |||||||
| Aqueous fraction | |||||||
| Brugia malayi | In vivo/ In vitro | Verbenaceae | Lantana camara | Stem | Ethanolic extracts | 43.05% adulticidal activity; sterilization of 76% of surviving females | [53] |
| Brugia pahangi | In vitro | Asteraceae | Neurolaena lobata | Leaves | Ethanolic extracts | Completely immotile after 24 h incubation at 500 μg/mL concentration | [54] |
| Caenorhabditis elegans | In vitro | Laminaceae | Tetradenia riparia | Leaves | Ethyl acetate extracts | Most effective minimum lethal concentration value was 0.004 mg/mL | [55] |
| Caenorhabditis elegans | In vitro | Combretaceae | Anogeissus leiocarpus | Stem bark | Ethanolic extracts | 72 h LC50 was between 0.38 and 4.00 mg/mL | [56] |
| Meliaceae | Khaya senegalensis | Leaves | |||||
| Euphorbiaceae | Euphorbia hirta | ||||||
| Annonaceae | Annona senegalensis | Aqueous extracts | |||||
| Apocynaceae | Parquetina nigrescens | ||||||
| Caenorhabditis elegans | In vitro | Sapindaceae | Acer rubrum | Leaves | Ethanolic extracts | Killed 50% (LC50) or 90% (LC90) of the nematodes in 24 h | [57] |
| Fagaceae | Quercus alba | ||||||
| Rosaceae | Rosa multiflora | ||||||
| Anarcardiaceae | Rhus typhina | ||||||
| Fabaceae | Robinia pseudoacacia | ||||||
| Lespedeza cuneata | Leaves and stems | ||||||
| Caenorhabditis elegans | In vitro | Meliaceae | Khaya senegalensis | Stem bark | Ethanolic and aqueous extracts | 72 h LC50 was between 0.38 and 4.00 mg/mL | [56] |
| Combretaceae | Anogeissus leiocarpus | Leaves | |||||
| Euphorbiaceae | Euphorbia hirta | ||||||
| Annonaceae | Annona senegalensis | ||||||
| Apocynaceae | Parquetina nigrescens | ||||||
| Fabaceae | Senna petersiana | ||||||
| Caenorhabditis elegans | In vitro | Plumbaginaceae | Plumbago indica | Root | Methylene chloride | Strongest activity | [40] |
| Cooperia spp. | In vitro | Fabaceae | Leucaena leucocephala | Fresh leaves | Aqueous extract | 52.02 ± 12.39 of egg hatching within 48 h of exposure | [58] |
| Eudrilus eugeniae | In vitro | Lamiaceae | Ocimum basilicum | Fruits | Ethanol and hexane extracts | 213.39 ± 1.05 and 362.98 ± 1.54 death time of ethanolic extract and hexane extract, respectively, at 250 μg/mL concentration | [59] |
| Gastrointestinal nematodes | In vitro/ In vivo | Lamiaceae | Prunella vulgaris | Whole plant | Phenolic compounds | Highest nematode motility (100%) with higher concentrations of methanolic extracts (50 mg/ mL) | [60] |
| Gastrointestinal nematodes | In vivo | Lythraceae | Punica granatum | Fruit peel | Pomegranate peel extract | 7 days after the first and second doses, 85–97% decrease in fecal egg count (FEC) | [61] |
| Gastrointestinal nematodes | In vitro | Moringaceae | Moringa oleifera lectin | Seeds | Distilled water homogenization | 40.4% of eggs unhatched at 250 μg/mL dose | [62] |
| Gastrointestinal nematodes | In vitro | Phyllanthaceae | Bridelia ferruginea | Leaves | Methanolic and acetone extracts | The number of eggs that hatched was reduced in a concentration-dependent manner (p < 0.01) upon treatment | [63] |
| Combretaceae | Combretum glutinosum | ||||||
| Rubiaceae | Mitragyna inermis | ||||||
| Gastrointestinal nematodes of goats | In vitro | Vitaceae | Cissus quadrangularis | Aerial parts | Aqueous (cold and boiled) and methanolic extracts | Statistically significant effect | [64] |
| Asphodelaceae | Aloe marlothii | Leaves | |||||
| Mimosoideae | Albizia anthelmintica | Bark | |||||
| Vitaceae | Cissus rotundifolia | Bark | |||||
| Anacardiaceae | Sclerocarya birrea | Bark | |||||
| Fabaceae | Vachellia xanthophloea | Bark | |||||
| Gastrointestinal nematodes of sheep | In vivo | Punicaceae | Punica granatum | Fruit (seeds and peel) | Boiled extracts | 8–40% (21st day) | [65] |
| Asteraceae | Artemisia campestris | Whole plant | 3–36% (21st day) | ||||
| Salicaceae | Salix caprea | Bark and leaves | 7–40% (21st day) | ||||
| Gastrointestinal nematodes of sheep | In vitro | Myrtaceae | Psidium cattleianum | Fruits | Hydroalcoholic extract | 80% in the inhibition of larval migration | [66] |
| Gastrointestinal nematodes of sheep | In vitro | Punicaceae | Aqueous Pomegranate | Fruit pulp | Methanolic and gallic acid extracts | Significant inhibition of egg hatching within 48 h of exposure, highlighting a high (>82%) efficacy in vitro at all tested doses | [67] |
| Gastrothylax crumenifer | In vitro | Menispermaceae | Tinospora cordifolia | Plant stems | Alcoholic and aqueous extracts | mortality rate of 100% at concentration of 100 mg/mL | [68] |
| Haemonchus contortus | In vitro | Asteraceae | Artemisia maritima | Whole plants | Methanolic extracts | 84.5% | [69] |
| Artemisia vestita | 87.2% | ||||||
| Haemonchus contortus | In vitro | Ericaceae | Arctostaphylos uva-ursi | Leaves | Methanolic extracts | 95.8 ± 0.5% inhibition in DMSO | [70] |
| Anacardiaceae | Rhus glabra | 90.2 ± 0.9% inhibition in DMSO | |||||
| Asteraceae | Balsamorhiza sagittata | 88.1 ± 1.2% inhibition in DMSO | |||||
| Ranunculaceae | Caltha palustris | 86.5 ± 1.2% inhibition in DMSO | |||||
| Boraginaceae | Cynoglossum officinale | 84.7 ± 1.0% inhibition in DMSO | |||||
| Asteraceae | Solidago mollis | 82.8 ± 1.4% inhibition in DMSO | |||||
| Asteraceae | Centaurea stoebe | 78.1 ± 1.5% inhibition in DMSO | |||||
| Fabaceae | Glycyrrhiza lepidota | 77.6 ± 2.3% inhibition in DMSO | |||||
| Anacardiaceae | Rhus aromatica | 100% inhibition in DMSO | |||||
| Asteraceae | Ericameria nauseosa | 100% inhibition in DMSO | |||||
| Apiaceae | Perideridia gairdneri | 100% inhibition in DMSO | |||||
| Geraniaceae | Geranium viscosissimum | 100% inhibition in DMSO | |||||
| Asteraceae | Chrysothamnus viscidiflora | 100% inhibition in DMSO | |||||
| Asteraceae | Liatris punctata | Roots | 100% inhibition in DMSO | ||||
| Fabaceae | Melilotus alba | Leaves | 100% inhibition in DMSO | ||||
| Fabaceae | Melilotus officinalis | 100% inhibition in DMSO | |||||
| Papaveraceae | Sanguinaria canadensis | Roots | 98.5 ± 0.3% inhibition in DMSO | ||||
| Orobanchaceae | Pedicularis racemosa | Leaves | 74.2 ± 0.9% inhibition in DMSO | ||||
| Lamiaceae | Stachys palustris | 72.9 ± 1.8% inhibition in DMSO | |||||
| Lamiaceae | Agastache foeniculum | 70.05 ± 0.7% inhibition in DMSO | |||||
| Lamiaceae | Monarda fistulosa | 69.5 ± 1.5% inhibition in DMSO | |||||
| Fabaceae | Pediomelum argophyllum | 69.7 ± 1.8% inhibition in DMSO | |||||
| Lamiaceae | Lycopus americanus | 76.0 ± 2.3% inhibition in DMSO | |||||
| Ranunculaceae | Clematis ligusticifolia | 68.7 ± 2.0% inhibition in DMSO | |||||
| Amaryllidaceae | Allium cernuum | 68.4 ± 1.3% inhibition in DMSO | |||||
| Asteraceae | Conyza canadensis | 76.8 ± 2.1% Inhibition in MOPS | |||||
| Cornaceae | Cornus sericea | 57.4 ± 3.1% inhibition in DMSO | |||||
| Rosaceae | Rubus idaeus | 51.9 ± 1.6% inhibition in DMSO | |||||
| Ranunculaceae | Actaea rubra | 45.2 ± 1.5% Inhibition in DMSO | |||||
| Caprifoliaceae | Symphoricarpos occidentalis | 43.1 ± 3.3% Inhibition in DMSO | |||||
| Asteraceae | Artemisia ludoviciana | 40.8 ± 2.0% inhibition in DMSO | |||||
| Asteraceae | Artemisia frigida | 36.2 ± 1.65% inhibition in DMSO | |||||
| Asteraceae | Tanacetum vulgare | 33.5 ± 2.0% inhibition in DMSO | |||||
| Cleomaceae | Cleome serrulata | 23.9 ± 1.7% Inhibition in DMSO | |||||
| Onagraceae | Epilobium angustifolium | 23.2 ± 3.5% inhibition in DMSO | |||||
| Fagaceae | Quercus macrocarpa | 18.3 ± 2.2% Inhibition in DMSO | |||||
| Salicaceae | Salix exigua | 5.9 ± 0.7% Inhibition in DMSO | |||||
| Haemonchus contortus | In vitro | Asteraceae | Artemisia absinthium | Leaves | Crude aqueous and ethanolic extracts | Aqueous extracts exhibited greater anthelmintic activity | [71] |
| Haemonchus contortus | In vitro | Rutaceae | Citrus aurantifolia | Essential oils from fruit peel | Oil extracts | Oil has limonene (56.37%), β-pinene (11.86%) and γ-terpinene (11.42%) | [72] |
| Annonaceae | Annona muricata | Leaves | Aqueous extracts | Aqueous extract of A. muricata leaves at serial dilutions of 50%, 25%, 12.5% and 6.25% inhibited the motility of L3 by 83.29%, 89.08%, 74.62% and 30.47% respectively | |||
| Haemonchus contortus | In vitro | Anacardiaceae | Myracrodruon urundeuva | Seeds | Ethanolic and hexane extracts | Inhibition of larval development (LC50 = 0.29 mg mL−1) | [73] |
| Haemonchus contortus | In vitro | Liliaceae | Allium sativum | Bulbs | Ethanolic extracts | 84.0 ± 4.3 | [74] |
| Asphodelaceae | Aloe ferox | Leaves | 86.9 ± 2.9 | ||||
| Bromeliaceae | Ananas comosus | 100 ± 1.0 | |||||
| Caricaceae | Carica papaya | 76.0 ± 5.1 | |||||
| Moraceae | Ficus benjamina | 78.1 ± 3.5 | |||||
| Moraceae | Ficus ingens | 78.1 ± 5.7 | |||||
| Moraceae | Ficus carica (brown) | 56.3 ± 2.8 | |||||
| Moraceae | Ficus carica (white) | 74.1 ± 7.9 | |||||
| Moraceae | Ficus indica | 44.5 ± 7.0 | |||||
| Moraceae | Ficus lutea | 60.0 ± 6.3 | |||||
| Moraceae | Ficus elastica | 77.8 ± 6.6 | |||||
| Moraceae | Ficus natalensis | 68.8 ± 7.2 | |||||
| Moraceae | Ficus sur | 81.3 ± 5.6 | |||||
| Moraceae | Ficus sycomorus | 6.3 ± 4.3 | |||||
| Moraceae | Ficus ornamental thai | 60.0 ± 1.7 | |||||
| Lamiaceae | Leonotis leonurus | 56.5 ± 6.1 | |||||
| Moraceae | Melia azedarach | 66.7 ± 4.4 | |||||
| Fabaceae | Peltophorum africanum | 65.2 ± 4.0 | |||||
| Amaryllidaceae | Scadoxus puniceus | 59.4 ± 8.2 | |||||
| Fabaceae | Lespedeza cuneata | 100 ± 1.6 | |||||
| Leguminosae | Tephrosia inandensis | 64.0 ± 7.8 | |||||
| Canellaceae | Warburgia ugandensis | 81.5 ± 3.5 | |||||
| Canellaceae | Warburgia salutaris | 80.8 ± 3.4 | |||||
| Cucurbitaceae | Cucumis myriocarpus | 60.0 ± 5.7 | |||||
| Zingiberaceae | Zingiber officinale | Rhizomes | 72.0 ± 2.5 | ||||
| Haemonchus contortus | In vitro | Asteraceae | Vernonia amygdalina | Leaves | Hot water extracts | Ineffective | [75] |
| Annonaceae | Annona senegalensis | Stem barks | 88.5% | ||||
| Haemonchus contortus | In vivo | Fabaceae | Acacia nilotica | Leaves | Without extraction | 10% reduction in worm | [76] |
| Acacia karroo | 34% reduction in worm | ||||||
| Haemonchus contortus | In vitro and In vivo | Amaranthaceae | Chenopodium ambrosioides | Leaves and stems | Organic maceration | 96.3% (invitro), 45.8% (in vivo) at 40 mg/mL dose | [77] |
| Simaroubaceae | Castela tortuosa | 78.9% (in vitro) 27.1% (in vivo) at 20 mg/mL dose | |||||
| Haemonchus contortus | In vivo and In vitro | Lamiaceae | Mentha pulegium | Aerial parts | Hydroethanolic extract | 91.58% inhibition in the egg hatch assay at 8 mg/mL after 48 h. 65.2% inhibition at 8 mg/mL after 8 h in adult worm motility | [78] |
| Haemonchus contortus | In vitro | Apocynaceae | Tylophora Indica | Leaves | Methanolic extract | 100% mortality after 6 h exposure at 50 mg/mL of concentration | [79] |
| Haemonchus contortus | In vitro | Passifloraceae | Turnera ulmifolia | Leaves and roots | Hydroacetonic and hydroalcoholic extracts | The highest egg hatching inhibition with the lowest LC50 value of 430 μg/mL (95%, CI 400–460 μg/mL) | [80] |
| Fabaceae | Parkia platycephala | Leaves and seeds | LC50 1340, 95% CI 1170-1550 μg/mL | ||||
| Fabaceae | Dimorphandra gardneriana | Leaves and bark | Ineffective | ||||
| Haemonchus contortus | In vitro | Lauraceae | Persea americana | Dried seeds | Hot water extracts | 76.9 ± 7.2% effective in 500 μg/mL dose | [81] |
| Haemonchus contortus | In vitro and In vivo | Asteraceae | Artemisia absinthium | Whole plant | Crude methanolic extracts | Strong anthelmintic effect | [82] |
| Malvaceae | Malva sylvestris | ||||||
| Haemonchus contortus | In vitro | Asteraceae | Artemisia herba-alba | Stems and leaves | Crude methanolic extracts | 98.67% inhibition of egg hatching at 1 mg/mL concentration | [83] |
| Punicaceae | Punica granatum | Peel and roots | Eggs unhatched at the end of the observation period | ||||
| Haemonchus contortus | In vitro | Asteraceae | Artemisia vulgaris | Leaves | Aqueous and ethanolic extracts | %100 | [84] |
| Haemonchus contortus | In vitro | Fagaceae | Castanea sativa | Stems and leaves | Ethanolic extracts | All plants showed some anthelmintic activity on both L3 larvae and adult worms) | [85] |
| Fabaceae | Sarothamnus scoparius | Stems and leaves | |||||
| Pinaceae | Pinus sylvestris | Stems and leaves | |||||
| Fagaceae | Quercus robur | Leaves | |||||
| Oleaceae | Fraxinus excelsior | Leaves | |||||
| Betulaceae | Corylus avellana | Leaves | |||||
| Ericaceae | Erica erigena | Stems and leaves | |||||
| Fabaceae | Acacia holosericea | ||||||
| Acacia salicina | |||||||
| Cupressaceae | Callitris endlicheri | ||||||
| Casuarina cunninghamiana | |||||||
| Lauraceae | Neolitsea dealbata | ||||||
| Haemonchus contortus | In vivo | Asteraceae | Artemisia absinthium | Whole plant | Aqueous and methanolic extracts | 4.3–67.2% reduction in EPG | [86] |
| Haemonchus contortus | In vitro | Asteraceae | Artemisia absinthium | Aerial parts | Crude aqueous extracts | Worm motility inhibition was 73.6% | [87] |
| Crude ethanolic extracts | Worm motility inhibition was 94.7% | ||||||
| Haemonchus contortus | In vivo | Anacardiaceae | Pistacia lentiscus | Leaves | Acetone extracts | Significant decreases in egg excretion | [88] |
| Fagaceae | Quercus coccifera | ||||||
| Onobrychis viciifolia | |||||||
| Ceratonia siliqua | |||||||
| Medicago sativa | |||||||
| Haemonchus contortus eggs | In vitro | Combretaceae | Terminalia glaucescens | Leaves | Methanolic extracts | 87.55% inhibition of egg hatching at the 100 µg/mL dose | [89] |
| Haemonchus contortus eggs | In vitro | Lamiaceae | Leucas martinicensis | Stems and bark | Crude aqueous and hydroalcoholic extracts | Complete inhibition of egg hatching at the 1 mg/mL dose | [90] |
| Leonotis ocymifolia | Aerial parts | ||||||
| Fabaceae | Senna occidentalis | Leaves | |||||
| Polygonaceae | Rumex abyssinicus | Stems and bark | |||||
| Leguminosae | Albizia schimperiana | ||||||
| Haemonchus contortus eggs and larvae | In vitro | Fabaceae | Acacia farnesiana | Dried pods | Hydroalcoholic extracts | 100% ovicidal and 75.2% larvicidal activity at the 50 mg/mL dose | [91] |
| Haemonchus contortus eggs and larvae | In vitro | Fabaceae | Senegalia gaumeri | Leaves | Methanolic extracts | Ovicidal effect in the morula stage | [92] |
| Haemonchus spp. | In vitro | Casuarinaceae | Allocasuarina torulosa | Fresh leaves | Methanolic extracts | 64.14–89.83% exposure at the 30 mg/mL concentration | [93] |
| Fabaceae | Acacia holosericea | ||||||
| Acacia salicina | |||||||
| Cupressaceae | Callitris endlicheri | ||||||
| Casuarinaceae | Casuarina cunninghamiana | ||||||
| Lauraceae | Neolitsea dealbata | ||||||
| Onchocerca gutturosa | In vitro | Annonaceae | Polyalthia suaveolens | Bark | Hexane extracts | Significant inhibitory effect on the vitality of adult male worms | [94] |
| Euphorbiaceae | Discoglypremna caloneura | ||||||
| Onchocerca ochengi | In vitro | Salicaceae | Homalium africanum | Leaves | Hexane methylene chloride extracts | Significant effect | [95] |
| Parascaris equorum | In vitro | Asteraceae | Artemisia dracunculus | Leaves | Methanolic extracts | 90% inhibition of egg hatching and high larvicidal effect at concentrations ≥100 mg/mL | [96] |
| Myrtaceae | Eucalyptus camadulensis | Leaves | |||||
| Lamiaceae | Mentha pulegium | Aerial parts | |||||
| Lamiaceae | Zataria multiflora | Aerial parts | |||||
| Liliaceae | Allium sativum | Bulbs | |||||
| Pheretima posthuma | In vitro | Nyctaginaceae | Bougainvillea spectabilis | Crude extract of flowers | Ethanolic and aqueous extracts | 39 min (time of death) at a concentration of 50 mg/mL | [97] |
| Pheretima posthuma | In vitro | Acanthaceae | Barleria buxifolia | Leaves | Ethanolic extract | 89.00 ± 1.82 min for death time at a concentration of 100 mg/mL | [98] |
| Pheretima posthuma | In vitro | Plumbaginaceae | Plumbagozeylanica | Leaves | Methanolic Extract | 81 ± 1.5 min death time (concentration of 20 mg/mL) | [99] |
| Water Extract | 228 ± 1.2 min death time (concentration of 20 mg/mL | ||||||
| Strongyloides venezuelensis | In vitro | Siparunaceae | Siparuna guianensis | Leaves | Hexane extracts | Significant inhibitory effect on the vitality of adult male worms | [100] |
| Toxocara vitulorum | In vitro | Zygophyllaceae | Balanites aegyptiaca | Fruits | Methanolic extract | 120 μg/ml after 24 h complete disruption of the muscle cells | [101] |
| Teladorsagia circumcincta L1 larvae | In vivo | Fabaceae | Phaseolus vulgaris | Seeds | Lectin purification | Worm burden 4416 ± 878 (control) 3475 ± 792 (treated) | [102] |
| Trichostrongylus colubriformis L1 larvae | Worm burden 6708 ± 414 (control) 6500 ± 295.5 (treated) | ||||||
| Trichostrongylus colubriformis | In vivo | Moraceae | Artocarpus integrifolia | Whole plant | Ethanolic extracts | Reduced concentration of nematode eggs (2.3 mg semi-purified PHA lectin/kg LW/day) | [102] |
| Fabaceae | Canavalia ensiformis | ||||||
| Fabaceae | Phaseolus vulgaris | ||||||
| Fabaceae | Maackia murensis | ||||||
| Fabaceae | Robinia pseudoacacia | ||||||
| Moraceae | Maclura pomifera | ||||||
| Fabaceae | Dolichos biflorus | ||||||
| Poaceae | Triticum vulgare | ||||||
| Amaryllidaceae | Galanthus nivalis | ||||||
| Rosaceae | Rosa multiflora |
| Compound | Parasite Species | Study Model | Reported Mortality | Reference |
|---|---|---|---|---|
| A penta-substituted pyridine alkaloid | Schistosoma mansoni | In vitro | 100% | [103] |
| Essential oil | Echinococcus granulosus (protoscolex) | In vitro | 79.22% scolicidal activity with the 20 mg/mL dose during 60 min | [104] |
| Essential oil (Thymoquinone) | Echinococcus granulosus(protoscolex) | In vitro | 100% scolicidal activity with the 1 mg/mL dose after 10 min | [12] |
| Essential oil | Haemonchus contortus | In vitro and in vivo | 33.3% and 87.5% inhibition motility for flower essential oil | [105] |
| 29.1% and 75% for leaf essential oil | ||||
| 87.2% | ||||
| Lectin purification | Teladorsagia circumcincta (L1) | In vivo | Worm burden 4416 ± 878 (control) 3475 ± 792 (treated) | [102] |
| Trichostrongylus colubriformis (L1) | Worm burden 6708 ± 414 (control) 6500 ± 295.5 (treated) | |||
| Tannin | Cooperia spp. | In vivo | Higher activity | [106] |
| Cysteine proteinases (CP) | Hymenolepis diminuta | In vitro | CP extracts exhibited anthelmintic activity in vitro | [107] |
| Pristimerin | Anticestodal | Invitro In vivo | EPG by 94 ± 5%, 8 ± 4%, 6 ± 3%, and 97 ± 4%, respectively | [60] |
| Ursolic acid | Brugia malayi | Invitro In vivo | 86% inhibition | [108] |
| Withaferin A | Brugia malayi | In vivo | 4.3% reduced parasite load using 8 μg/mL within 24 h | [109,110] |
| Galactolipid-1 Galactolipid-2 Galactolipid-3 Galactolipid-4 | Brugia malayi | In vitro In vivo | Fraction F1: 80%; Fraction F2: 30%; Fraction F3: 40%; Fraction F4: 100% (31.25 μg/mL) | [111] |
| Curcumin | Schistosoma mansoni | In vitro | 100% mortality in male and female | [112] |
| Aporphine | Anisakis simplex and Hymenolepis nana | In vitro | No cestocidal and nematocidal effects against H. nana and A. simplex | [113] |
| Derived saponins | Donkey Gastrointestinal Nematodes | In vitro | Significant (p < 0.05) inhibition of nematode egg hatching (>80%) | [114] |
| Maclura pomifera agglutinin | Teladorsagia circumcincta | In vivo | Direct anthelmintic effect on nematode fecundity and an indirect effect by enhancing local immune responses in the host | [102] |
| Tannins | Teladorsagia circumcincta, Haemonchus contortus, and Trichostrongylus colubriformis | In vitro | Larval migration inhibition assay on third-stage larvae (L3) and adult worm motility inhibition assay | [85] |
| Essential oil | Gastrointestinal nematodes | In vitro | 33.30% inhibition motility | [105] |
| 87.50% inhibition motility | ||||
| Saponins | Gastrointestinal nematodes | In vitro | Strong anthelmintic activity | [115] |
| Donkey strongyles | In vitro | Strong anthelmintic activity | [116] | |
| Tannins | Trichostrongylus colubriformis | In vitro | Larval migration inhibition assay on third-stage larvae (L3) and adult worms | [85] |
| Condensed and hydrolyzable tannins | Caenorhabditis elegans | In vitro | Killed 50% (LC50) or 90% (LC90) of nematodes in 24 h | [57] |
| Tannins | Trichostrongylus colubriformis | In vitro | Larval migration inhibition assay on third-stage larvae (L3) and adult worms | [85] |
| Flavonoids, condensed tannins, and gallotannin | Caenorhabditis elegans | In vitro | Minimum lethal concentration was 0.13–0.52 mg/mL | [117] |
| Methylene chloride | Caenorhabditis elegans | In vitro | Strongest effect | [40] |
| Tannins, phenolic compounds, and steroids | Haemonchus contortus | In vitro, In vivo | 100% inhibition of egg hatching, highest activity for adult motility, and larvicidal assay | [118] |
| Antimicrobial agents, alkaloids, flavonoids, tannins, and phenols | Haemonchus contortus | In vitro | High activity for adulticidal and egg hatching inhibition | [119] |
| Polyphenols | Caenorhabditis elegans | In vitro and in vivo | Inhibition of larval migration | [120] |
| Phenolic compounds | Gastrointestinal nematodes | In vitro In vivo | Highest nematode motility (100%) in the higher concentrations of methanolic extract (50 mg/mL) | [60] |
| Presence of saponin, alkaloids, flavonoids, and tannins | Haemonchus contortus | In vitro | High mortality rate | [121] |
| Presence of eugenol and asarone | Moniezia expansa | In vitro | 100 mg/mL concentration and the time taken for the paralysis of the parasite amounts to 66.3 ± 0.03 min and death was recorded after 93.2 ± 0.09 min | [122] |
| Proanthocyanidins and flavonoids | Haemonchus contortus | In vitro | Larval migration inhibition and adult worms’ motility inhibition | [123] |
| Essential oils | Neoechinorhynchus buttnerae, endoparasite of Colossoma macropomum | In vitro | All essential oils showed 100% anthelmintic efficacy within 24 h | [124] |
| 100% mortality was observed in the group treated with 100 mg/mL of herbal complex | Haemonchus contortus | In vitro | Anthelmintic potential | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; Kilinc, S.G.; Celik, F.; Kesik, H.K.; Simsek, S.; Ahmad, K.S.; Afzal, M.S.; Farrakh, S.; Safdar, W.; Pervaiz, F.; et al. An Inventory of Anthelmintic Plants across the Globe. Pathogens 2023, 12, 131. https://doi.org/10.3390/pathogens12010131
Ahmed H, Kilinc SG, Celik F, Kesik HK, Simsek S, Ahmad KS, Afzal MS, Farrakh S, Safdar W, Pervaiz F, et al. An Inventory of Anthelmintic Plants across the Globe. Pathogens. 2023; 12(1):131. https://doi.org/10.3390/pathogens12010131
Chicago/Turabian StyleAhmed, Haroon, Seyma Gunyakti Kilinc, Figen Celik, Harun Kaya Kesik, Sami Simsek, Khawaja Shafique Ahmad, Muhammad Sohail Afzal, Sumaira Farrakh, Waseem Safdar, Fahad Pervaiz, and et al. 2023. "An Inventory of Anthelmintic Plants across the Globe" Pathogens 12, no. 1: 131. https://doi.org/10.3390/pathogens12010131
APA StyleAhmed, H., Kilinc, S. G., Celik, F., Kesik, H. K., Simsek, S., Ahmad, K. S., Afzal, M. S., Farrakh, S., Safdar, W., Pervaiz, F., Liaqat, S., Zhang, J., & Cao, J. (2023). An Inventory of Anthelmintic Plants across the Globe. Pathogens, 12(1), 131. https://doi.org/10.3390/pathogens12010131







