Orientia tsutsugamushi Infection Stimulates Syk-Dependent Responses and Innate Cytosolic Defenses in Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Infection and Tissue Collection
2.2. Infection of Mouse Bone Marrow-Derived Macrophages (MΦ)
2.3. In Vitro Viability Staining
2.4. Quantitative Reverse Transcription PCR (qRT-PCR) and RNA Sequencing
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. Syk Inhibition Reduces Expression of Mincle and Clec5a during O. tsutsugamushi Infection
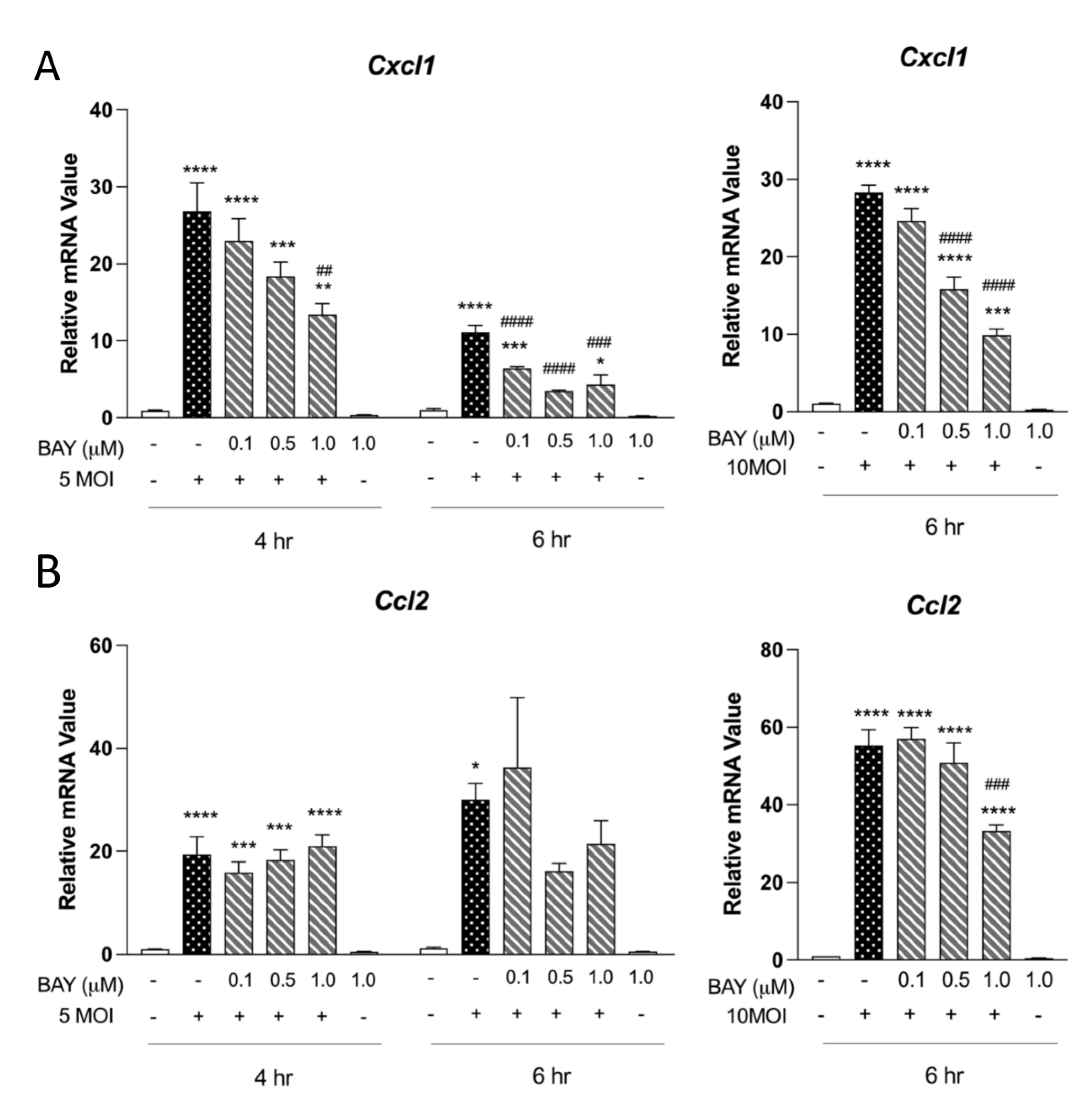
3.2. Syk Is Critical for Neutrophil-Chemotactic Chemokines in MΦ
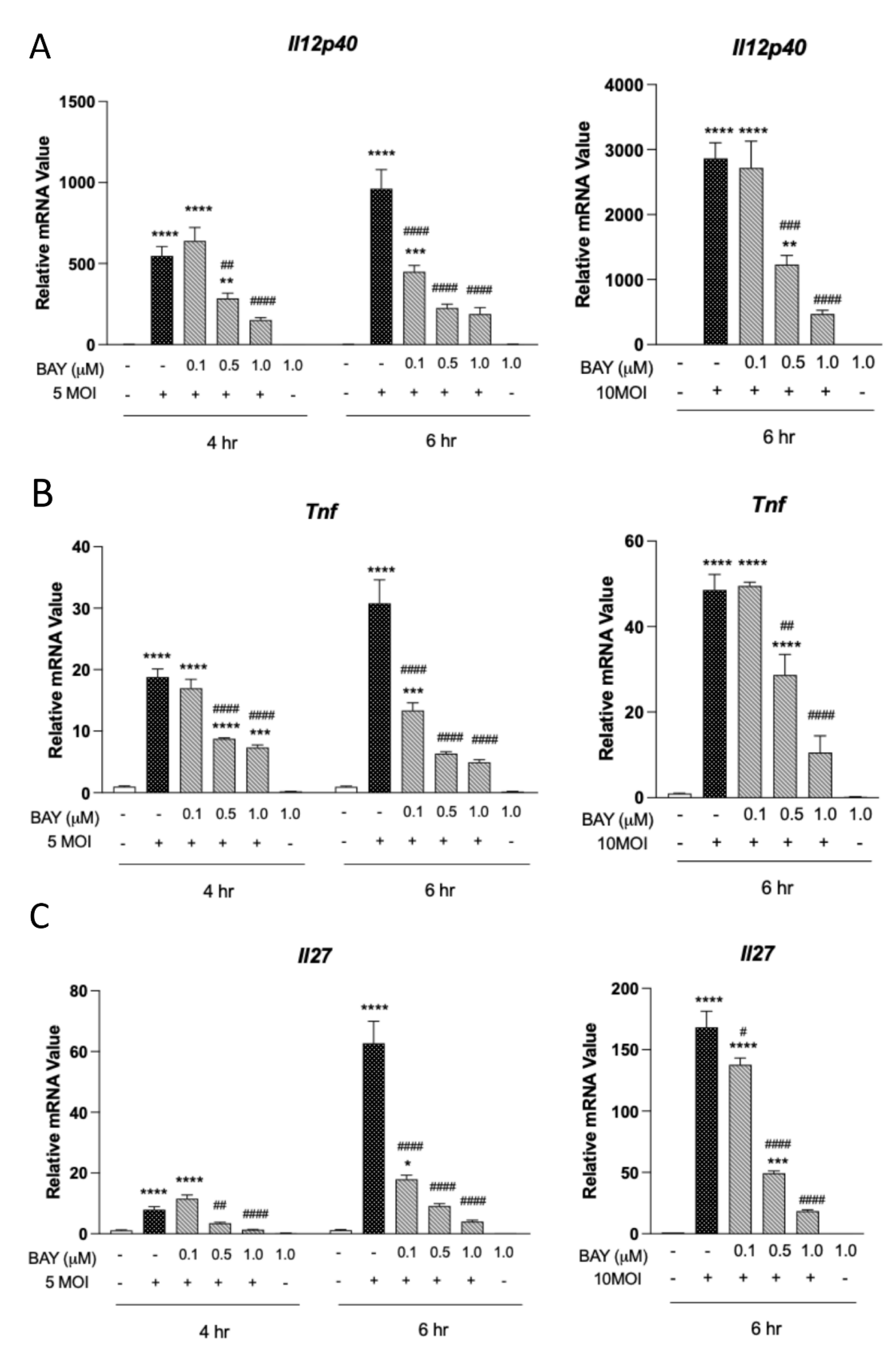
3.3. Type 1 Cytokine Production Is Reliant on Syk Signaling in MΦ
3.4. Expression of Innate Antiviral-like Genes Is Syk Dependent
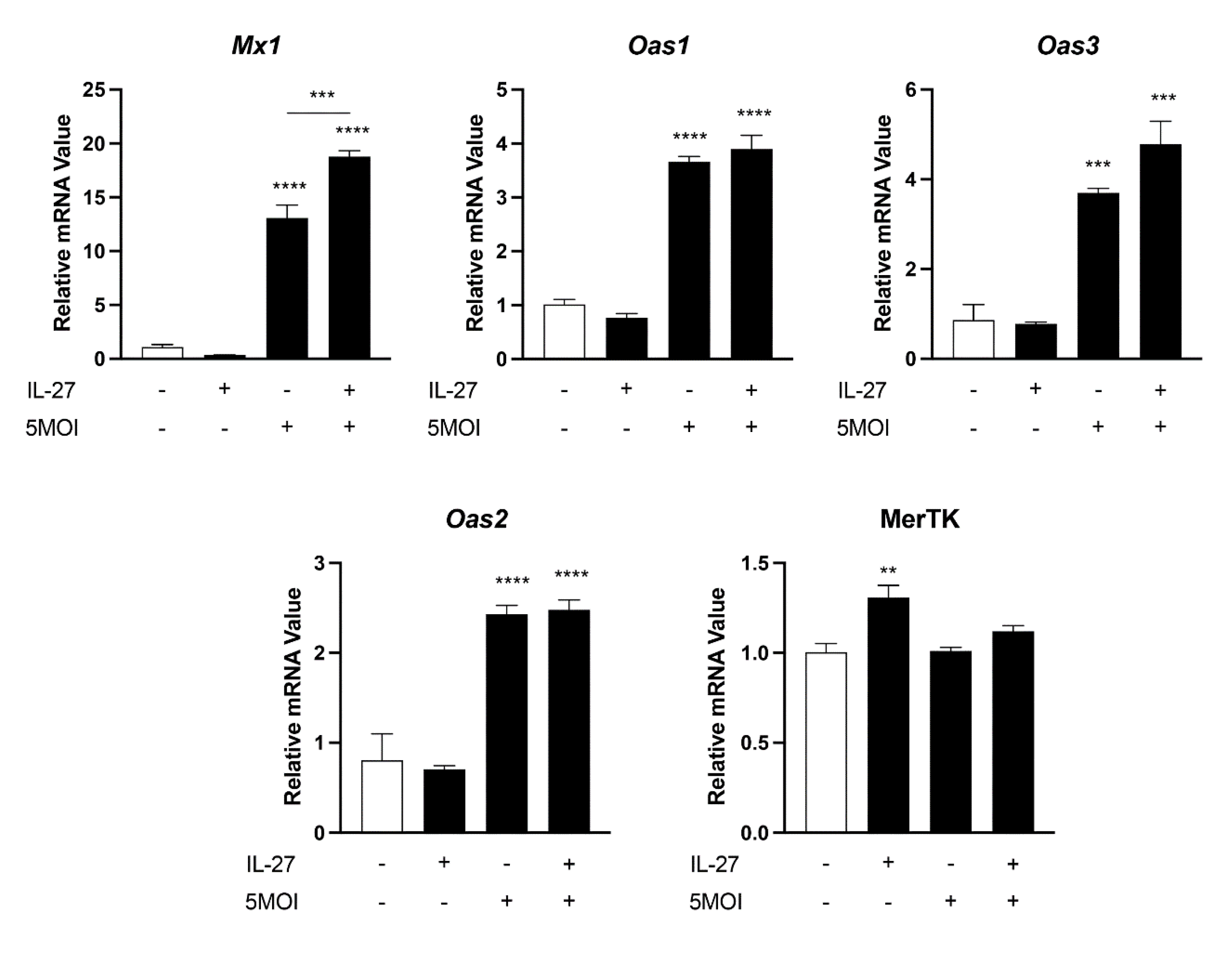
3.5. Innate Antiviral-like Responses Are Amplified by Recombinant IL-27
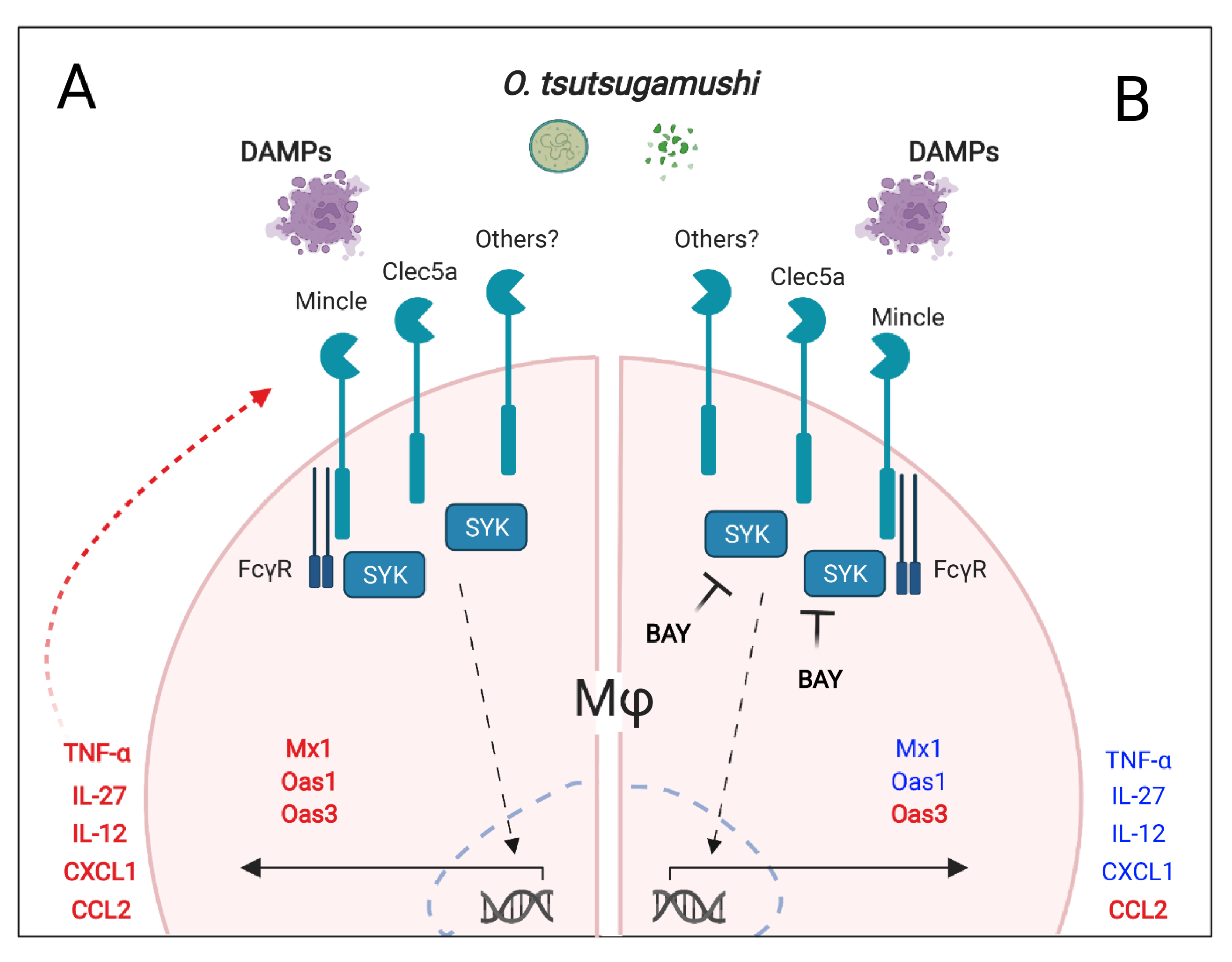
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Dittrich, S.; López, J.; Phuklia, W.; Martinez-Valdebenito, C.; Velásquez, K.; Blacksell, S.D.; Paris, D.H.; Abarca, K. Endemic Scrub Typhus in South America. N. Engl. J. Med. 2016, 375, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Richards, A.L. Scrub typhus: No longer restricted to the Tsutsugamushi triangle. Trop. Med. Infect. Dis. 2018, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Liu, S.F.; Liu, J.W.; Chung, Y.H.; Su, M.C.; Lin, M.C. Acute respiratory distress syndrome in scrub typhus. Am. J. Trop. Med. Hyg. 2007, 76, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Salje, J. Orientia tsutsugamushi: A neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017, 13, e1006657. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Chroust, Z.; Onyoni, F.; Soong, L. Pattern Recognition Receptors in Innate Immunity to Obligate Intracellular Bacteria. Zoonoses 2021, 1, 10. [Google Scholar] [CrossRef]
- Dittrich, S.; Card, E.; Phuklia, W.; Rudgard, W.E.; Silousok, J.; Phoumin, P.; Bouthasavong, L.; Azarian, S.; Davong, V.; Dance, D.A.; et al. Survival and Growth of Orientia tsutsugamushi in Conventional Hemocultures. Emerg. Infect. Dis. 2016, 22, 1460–1463. [Google Scholar] [CrossRef]
- Evans, S.M.; Rodino, K.G.; Adcox, H.E.; Carlyon, J.A. Orientia tsutsugamushi uses two Ank effectors to modulate NF-kappaB p65 nuclear transport and inhibit NF-kappaB transcriptional activation. PLoS Pathog. 2018, 14, e1007023. [Google Scholar] [CrossRef]
- Gharaibeh, M.; Hagedorn, M.; Lilla, S.; Hauptmann, M.; Heine, H.; Fleischer, B.; Keller, C. Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus. Infect. Immun. 2016, 84, 3379–3387. [Google Scholar] [CrossRef]
- Min, C.K.; Kim, H.I.; Ha, N.Y.; Kim, Y.; Kwon, E.K.; Yen, N.T.H.; Youn, J.I.; Jeon, Y.K.; Inn, K.S.; Choi, M.S.; et al. A Type I Interferon and IL-10 Induced by Orientia tsutsugamushi Infection Suppresses Antigen-Specific T Cells and Their Memory Responses. Front. Immunol. 2018, 9, 2022. [Google Scholar] [CrossRef]
- Rodino, K.G.; Adcox, H.E.; Martin, R.K.; Patel, V.; Conrad, D.H.; Carlyon, J.A. The Obligate Intracellular Bacterium Orientia tsutsugamushi Targets NLRC5 To Modulate the Major Histocompatibility Complex Class I Pathway. Infect. Immun. 2019, 87, e00876-18. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Jun, Y.H.; Suh, J.W.; Kang, J.S.; Choi, H.J.; Woo, S.Y. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb. Pathog. 2010, 49, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.E.; Hong, H.J.; Dearth, A.; Kobayashi, K.S.; Koh, Y.S. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in asc-dependent manner. PLoS ONE 2012, 7, e39042. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Card, G.; Liang, Y.; Trent, B.; Rosenzweig, H.; Soong, L. Orientia tsutsugamushi selectively stimulates the C-type lectin receptor Mincle and type 1-skewed proinflammatory immune responses. PLoS Pathog. 2021, 17, e1009782. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, A.M.; Brown, G.D. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011, 32, 151–156. [Google Scholar] [CrossRef]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Ostrop, J.; Lang, R. Contact, Collaboration, and Conflict: Signal Integration of Syk-Coupled C-Type Lectin Receptors. J. Immunol. 2017, 198, 1403–1414. [Google Scholar] [CrossRef]
- Muller, B.J.; Westheider, A.; Birkner, K.; Seelig, B.; Kirschnek, S.; Bogdan, C.; von Loewenich, F.D. Anaplasma phagocytophilum Induces TLR- and MyD88-Dependent Signaling in In Vitro Generated Murine Neutrophils. Front. Cell. Infect. Microbiol. 2021, 11, 627630. [Google Scholar] [CrossRef]
- Sun, S.; Xue, D.; Chen, Z.; Ou-Yang, Y.; Zhang, J.; Mai, J.; Gu, J.; Lu, W.; Liu, X.; Liu, W.; et al. R406 elicits anti-Warburg effect via Syk-dependent and -independent mechanisms to trigger apoptosis in glioma stem cells. Cell Death Dis. 2019, 10, 358. [Google Scholar] [CrossRef]
- Iizasa, E.; Chuma, Y.; Uematsu, T.; Kubota, M.; Kawaguchi, H.; Umemura, M.; Toyonaga, K.; Kiyohara, H.; Yano, I.; Colonna, M.; et al. TREM2 is a receptor for non-glycosylated mycolic acids of mycobacteria that limits anti-mycobacterial macrophage activation. Nat. Commun. 2021, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Davidson, D.; Li, R.; Zhong, M.C.; Qian, J.; Chen, J.; Veillette, A. Inflammatory macrophages exploit unconventional pro-phagocytic integrins for phagocytosis and anti-tumor immunity. Cell Rep. 2021, 37, 110111. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Li, Y.; Hanafusa, Y.; Yeh, Y.S.; Maruki-Uchida, H.; Kawakami, S.; Sai, M.; Goto, T.; Ito, T.; Kawada, T. Piceatannol exhibits anti-inflammatory effects on macrophages interacting with adipocytes. Food Sci. Nutr. 2017, 5, 76–85. [Google Scholar] [CrossRef]
- Shelite, T.R.; Saito, T.B.; Mendell, N.L.; Gong, B.; Xu, G.; Soong, L.; Valbuena, G.; Bouyer, D.H.; Walker, D.H. Hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus [corrected]. PLoS Negl. Trop. Dis. 2014, 8, e2966. [Google Scholar] [CrossRef] [PubMed]
- Soong, L.; Wang, H.; Shelite, T.R.; Liang, Y.; Mendell, N.L.; Sun, J.; Gong, B.; Valbuena, G.A.; Bouyer, D.H.; Walker, D.H. Strong type 1, but impaired type 2, immune responses contribute to Orientia tsutsugamushi-induced pathology in mice. PLoS Negl. Trop. Dis. 2014, 8, e3191. [Google Scholar] [CrossRef] [PubMed]
- Kwock, J.T.; Handfield, C.; Suwanpradid, J.; Hoang, P.; McFadden, M.J.; Labagnara, K.F.; Floyd, L.; Shannon, J.; Uppala, R.; Sarkar, M.K.; et al. IL-27 signaling activates skin cells to induce innate antiviral proteins and protects against Zika virus infection. Sci. Adv. 2020, 6, eaay3245. [Google Scholar] [CrossRef] [PubMed]
- Trent, B.; Liang, Y.; Xing, Y.; Esqueda, M.; Wei, Y.; Cho, N.H.; Kim, H.I.; Kim, Y.S.; Shelite, T.R.; Cai, J.; et al. Polarized lung inflammation and Tie2/angiopoietin-mediated endothelial dysfunction during severe Orientia tsutsugamushi infection. PLoS Negl. Trop. Dis. 2020, 14, e0007675. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Han, S.; Wang, Z.; Zhang, Y.; Li, X.; Xia, N.; Yu, W.; Jia, C.; Ni, Y.; et al. Targeting SYK of monocyte-derived macrophages regulates liver fibrosis via crosstalking with Erk/Hif1alpha and remodeling liver inflammatory environment. Cell Death Dis. 2021, 12, 1123. [Google Scholar] [CrossRef]
- Wang, J.; Wakeham, J.; Harkness, R.; Xing, Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J. Clin. Investig. 1999, 103, 1023–1029. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Mika-Gospodorz, B.; Giengkam, S.; Westermann, A.J.; Wongsantichon, J.; Kion-Crosby, W.; Chuenklin, S.; Wang, L.C.; Sunyakumthorn, P.; Sobota, R.M.; Subbian, S.; et al. Dual RNA-seq of Orientia tsutsugamushi informs on host-pathogen interactions for this neglected intracellular human pathogen. Nat. Commun. 2020, 11, 3363. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, Y.; Chen, B.; Chen, Y.; Yu, Z.; Wei, H.; Zhang, L.; Huang, S.; Rothman, P.B.; Gao, G.F.; et al. Critical role of Syk-dependent STAT1 activation in innate antiviral immunity. Cell Rep. 2021, 34, 108627. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S.; Hsieh, S.L. CLEC2 and CLEC5A: Pathogenic Host Factors in Acute Viral Infections. Front. Immunol. 2019, 10, 2867. [Google Scholar] [CrossRef] [PubMed]
- Schick, J.; Schäfer, J.; Alexander, C.; Dichtl, S.; Murray, P.J.; Christensen, D.; Sorg, U.; Pfeffer, K.; Schleicher, U.; Lang, R. Cutting Edge: TNF Is Essential for Mycobacteria-Induced MINCLE Expression, Macrophage Activation, and Th17 Adjuvanticity. J. Immunol. 2020, 205, 323–328. [Google Scholar] [CrossRef]
- Geahlen, R.L.; McLaughlin, J.L. Piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochem. Biophys. Res. Commun. 1989, 165, 241–245. [Google Scholar] [CrossRef]
- He, Y.; Xu, L.; Li, B.; Guo, Z.N.; Hu, Q.; Guo, Z.; Tang, J.; Chen, Y.; Zhang, Y.; Tang, J.; et al. Macrophage-Inducible C-Type Lectin/Spleen Tyrosine Kinase Signaling Pathway Contributes to Neuroinflammation After Subarachnoid Hemorrhage in Rats. Stroke 2015, 46, 2277–2286. [Google Scholar] [CrossRef]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M.A.; Jain, D.; et al. Correction: Dectin-1 Activation by a Natural Product beta-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. J. Immunol. 2016, 196, 3968. [Google Scholar] [CrossRef]
- Lv, L.L.; Tang, P.M.; Li, C.J.; You, Y.K.; Li, J.; Huang, X.R.; Ni, J.; Feng, M.; Liu, B.C.; Lan, H.Y. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 2017, 91, 587–602. [Google Scholar] [CrossRef]
- Keller, C.A.; Hauptmann, M.; Kolbaum, J.; Gharaibeh, M.; Neumann, M.; Glatzel, M.; Fleischer, B. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl. Trop. Dis. 2014, 8, e3064. [Google Scholar] [CrossRef]
- Curto, P.; Simões, I.; Riley, S.P.; Martinez, J.J. Differences in Intracellular Fate of Two Spotted Fever Group Rickettsia in Macrophage-Like Cells. Front. Cell. Infect. Microbiol. 2016, 6, 80. [Google Scholar] [CrossRef]
- Radulovic, S.; Price, P.W.; Beier, M.S.; Gaywee, J.; Macaluso, J.A.; Azad, A. Rickettsia-macrophage interactions: Host cell responses to Rickettsia akari and Rickettsia typhi. Infect. Immun. 2002, 70, 2576–2582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curto, P.; Riley, S.P.; Simões, I.; Martinez, J.J. Macrophages Infected by a Pathogen and a Non-pathogen Spotted Fever Group. Front. Cell. Infect. Microbiol. 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Koo, J.E.; Koh, Y.S. Mitogen-activated protein kinases are involved in tumor necrosis factor alpha production in macrophages infected with Orientia tsutsugamushi. Microbiol. Immunol. 2009, 53, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Tantibhedhyangkul, W.; Prachason, T.; Waywa, D.; El Filali, A.; Ghigo, E.; Thongnoppakhun, W.; Raoult, D.; Suputtamongkol, Y.; Capo, C.; Limwongse, C.; et al. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: Relationship with gene expression in patients with scrub typhus. PLoS Negl. Trop. Dis. 2011, 5, e1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kim, S.; Kim, H.K.; Lee, Y.; Shin, C.; Lee, C.S.; Jeong, B.H. Genome-Wide Association Study Identifies Eight Novel Loci for Susceptibility of Scrub Typhus and Highlights Immune-Related Signaling Pathways in Its Pathogenesis. Cells 2021, 10, 570. [Google Scholar] [CrossRef]
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.P. OAS proteins and cGAS: Unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 2014, 14, 521–528. [Google Scholar] [CrossRef]
- Leisching, G.; Cole, V.; Ali, A.T.; Baker, B. OAS1, OAS2 and OAS3 restrict intracellular M. tb replication and enhance cytokine secretion. Int. J. Infect. Dis. 2019, 80S, S77–S84. [Google Scholar] [CrossRef]
- Soong, L.; Shelite, T.R.; Xing, Y.; Kodakandla, H.; Liang, Y.; Trent, B.J.; Horton, P.; Smith, K.C.; Zhao, Z.; Sun, J.; et al. Type 1-skewed neuroinflammation and vascular damage associated with Orientia tsutsugamushi infection in mice. PLoS Negl. Trop. Dis. 2017, 11, e0005765. [Google Scholar] [CrossRef]
- Shelite, T.R.; Liang, Y.; Wang, H.; Mendell, N.L.; Trent, B.J.; Sun, J.; Gong, B.; Xu, G.; Hu, H.; Bouyer, D.H.; et al. IL-33-Dependent Endothelial Activation Contributes to Apoptosis and Renal Injury in Orientia tsutsugamushi-Infected Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004467. [Google Scholar] [CrossRef]

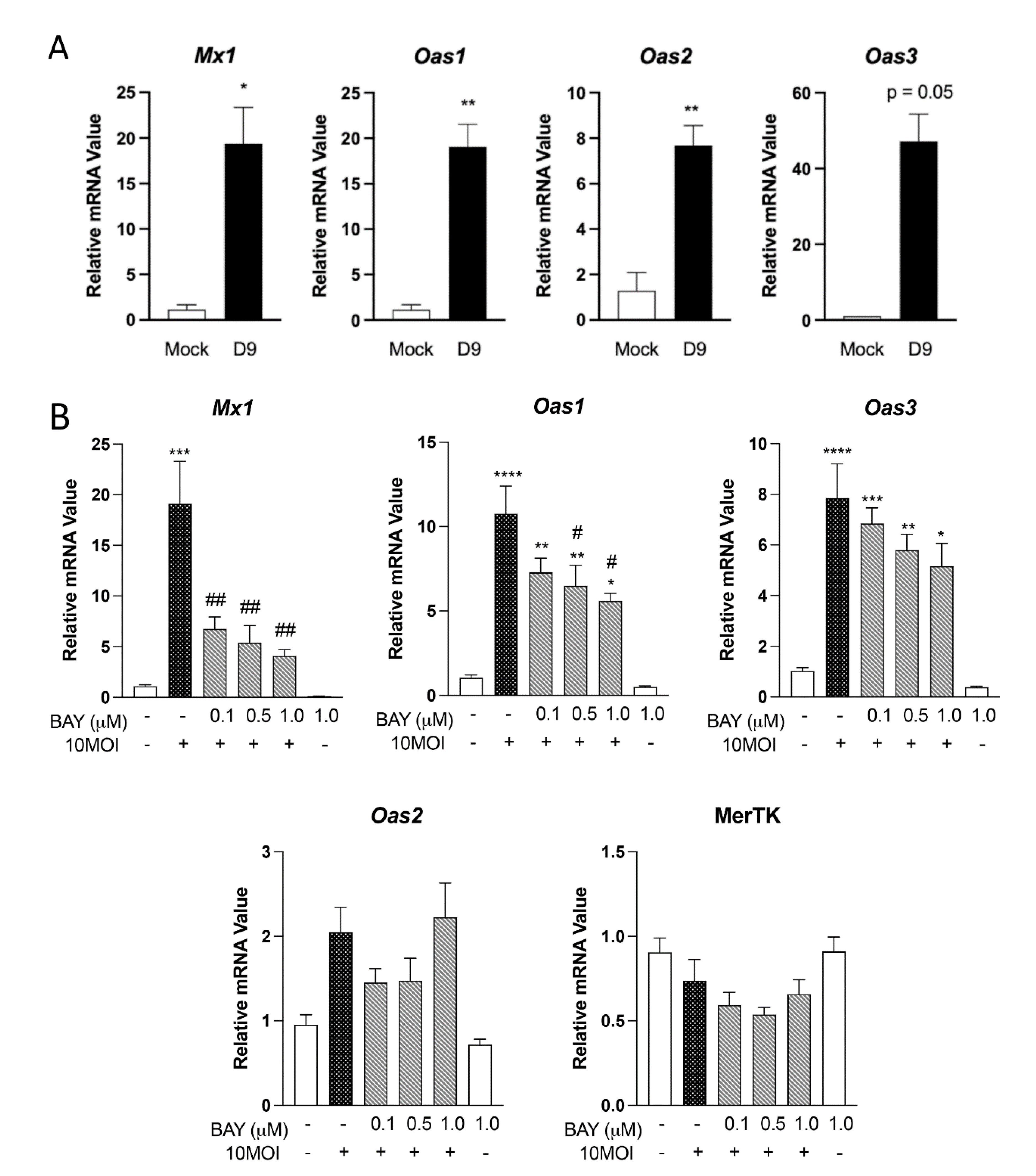
| Gene | Fold Change |
|---|---|
| Oas3 | 35.99 * |
| Mx1 | 33.03 * |
| Oas1b | 14.16 * |
| Oas2 | 7.90 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, J.; Gonzales, C.; Chroust, Z.; Liang, Y.; Soong, L. Orientia tsutsugamushi Infection Stimulates Syk-Dependent Responses and Innate Cytosolic Defenses in Macrophages. Pathogens 2023, 12, 53. https://doi.org/10.3390/pathogens12010053
Fisher J, Gonzales C, Chroust Z, Liang Y, Soong L. Orientia tsutsugamushi Infection Stimulates Syk-Dependent Responses and Innate Cytosolic Defenses in Macrophages. Pathogens. 2023; 12(1):53. https://doi.org/10.3390/pathogens12010053
Chicago/Turabian StyleFisher, James, Casey Gonzales, Zachary Chroust, Yuejin Liang, and Lynn Soong. 2023. "Orientia tsutsugamushi Infection Stimulates Syk-Dependent Responses and Innate Cytosolic Defenses in Macrophages" Pathogens 12, no. 1: 53. https://doi.org/10.3390/pathogens12010053
APA StyleFisher, J., Gonzales, C., Chroust, Z., Liang, Y., & Soong, L. (2023). Orientia tsutsugamushi Infection Stimulates Syk-Dependent Responses and Innate Cytosolic Defenses in Macrophages. Pathogens, 12(1), 53. https://doi.org/10.3390/pathogens12010053








