Assessment of Polydopamine to Reduce Streptococcus mutans Adhesion to a Dental Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Master Model and Soft Lithography
2.3. PDA Preparation
2.4. Surface Preparation
2.5. S. mutans Inoculum Preparation
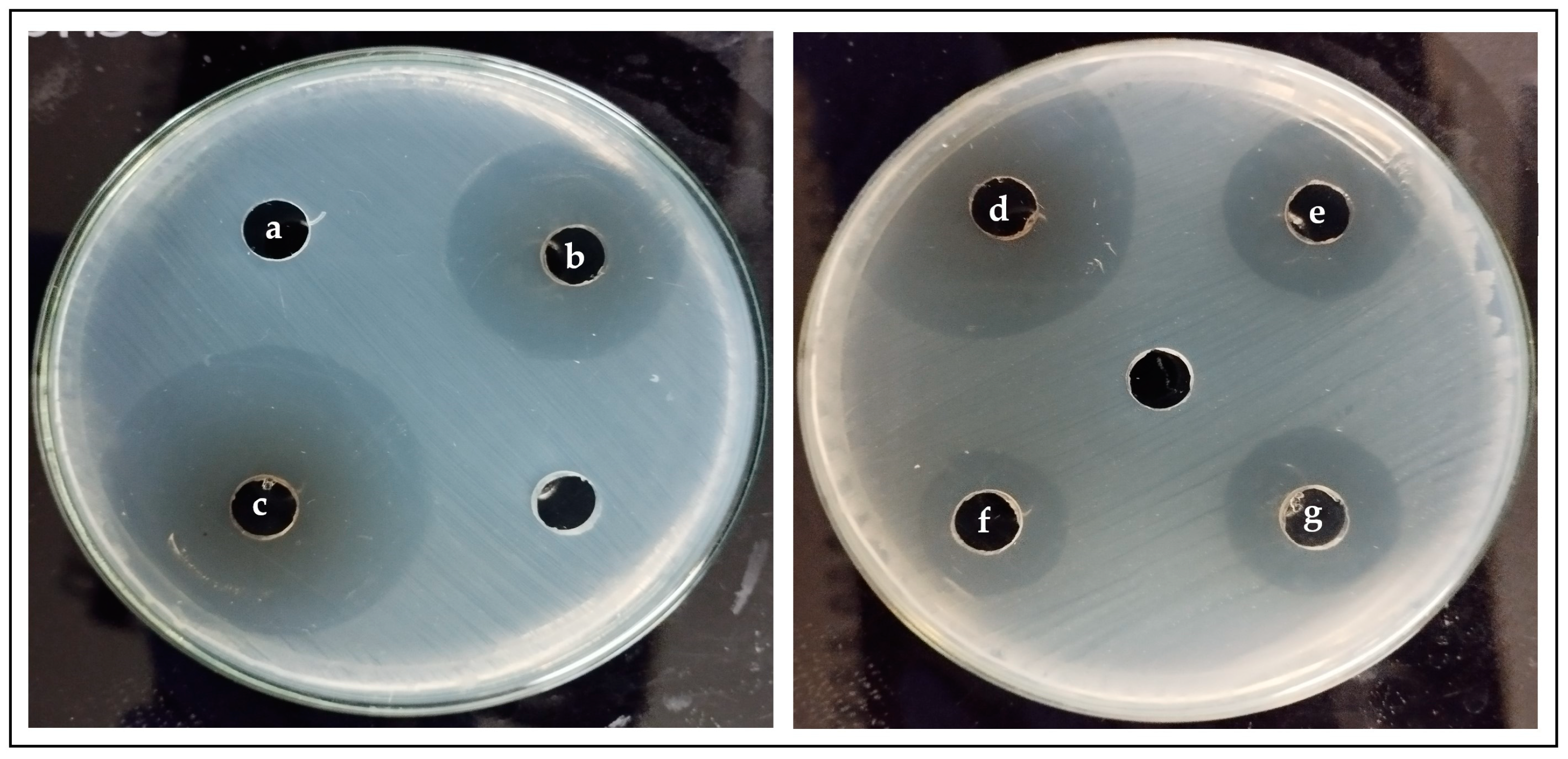
2.6. Antibacterial Effect of PDA on S. mutans
2.7. Surface Characterization
2.8. Spectroscopic Analysis
2.9. S. mutans Adhesion Testing
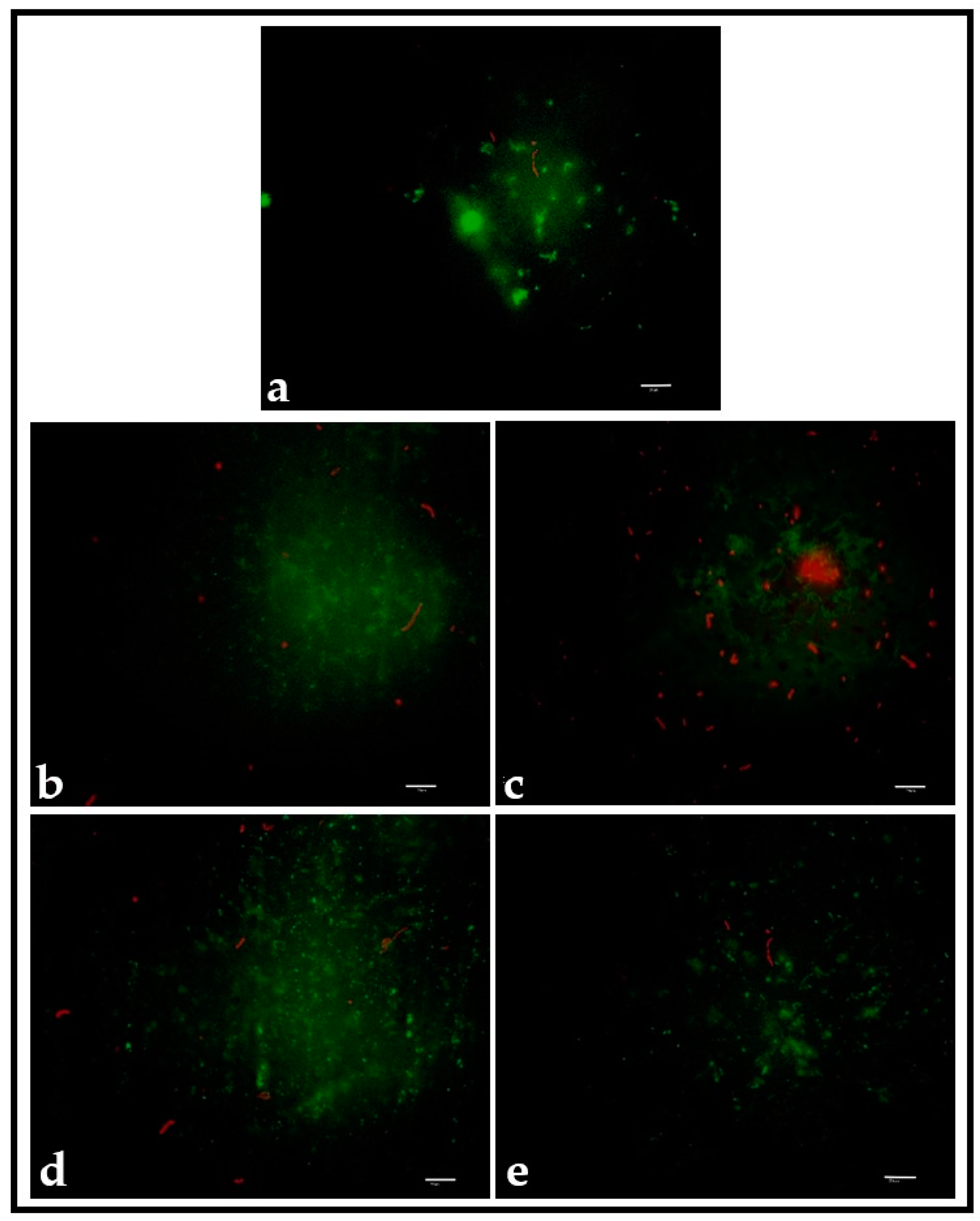
2.10. Staining of S. mutans Adhered to PMMA Discs
2.11. Statistical Analysis
3. Results
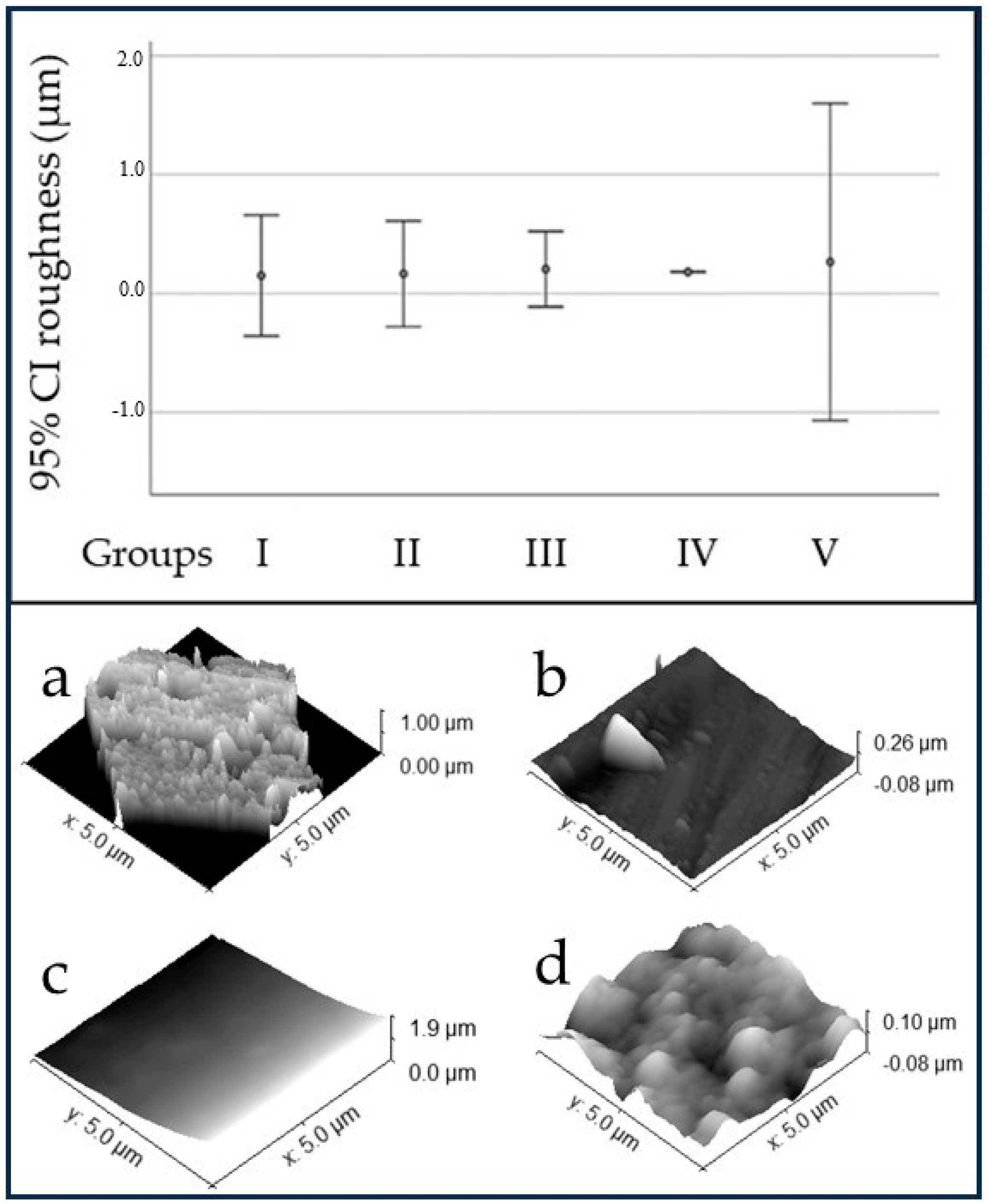
3.1. Surface Characterization
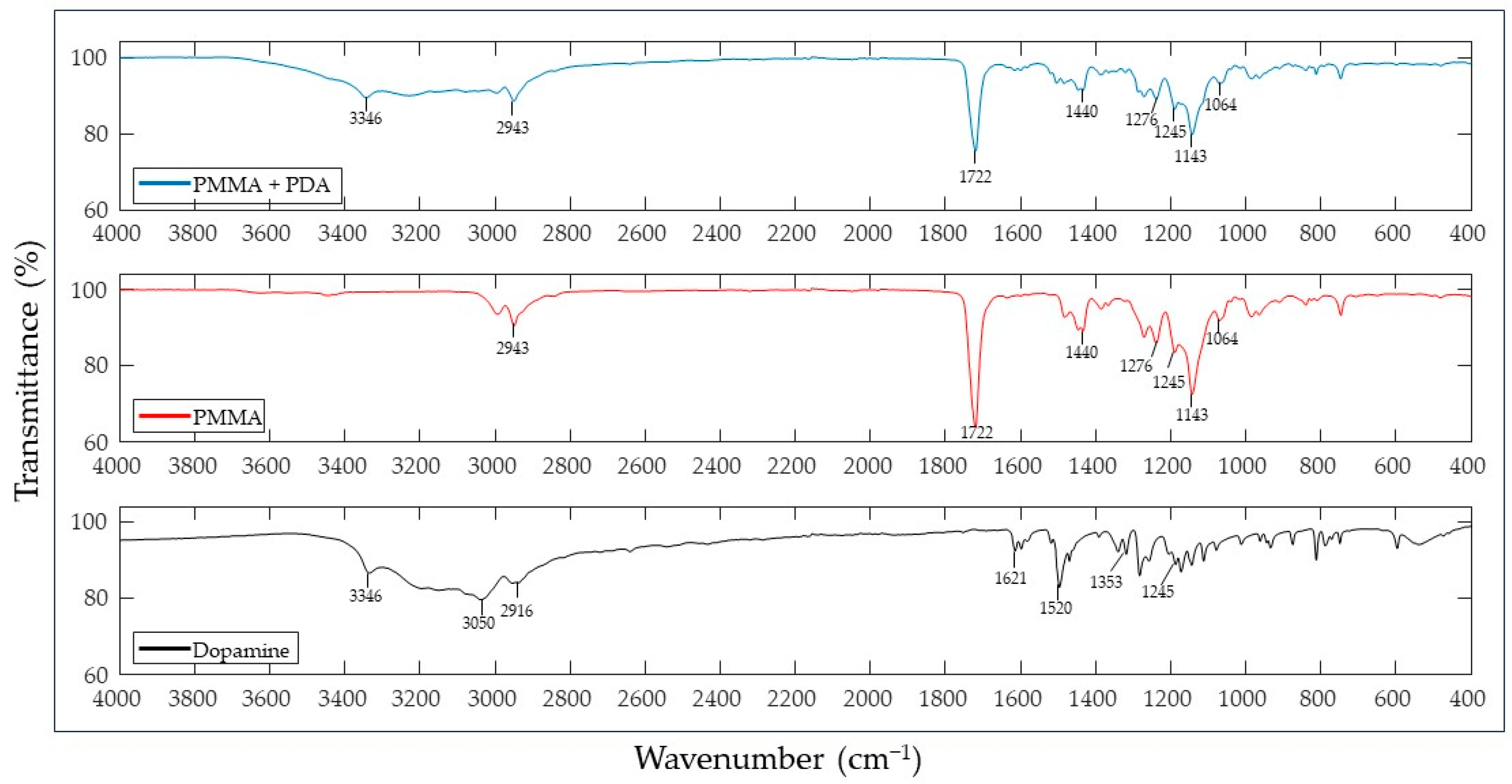
3.2. Spectroscopic Analysis
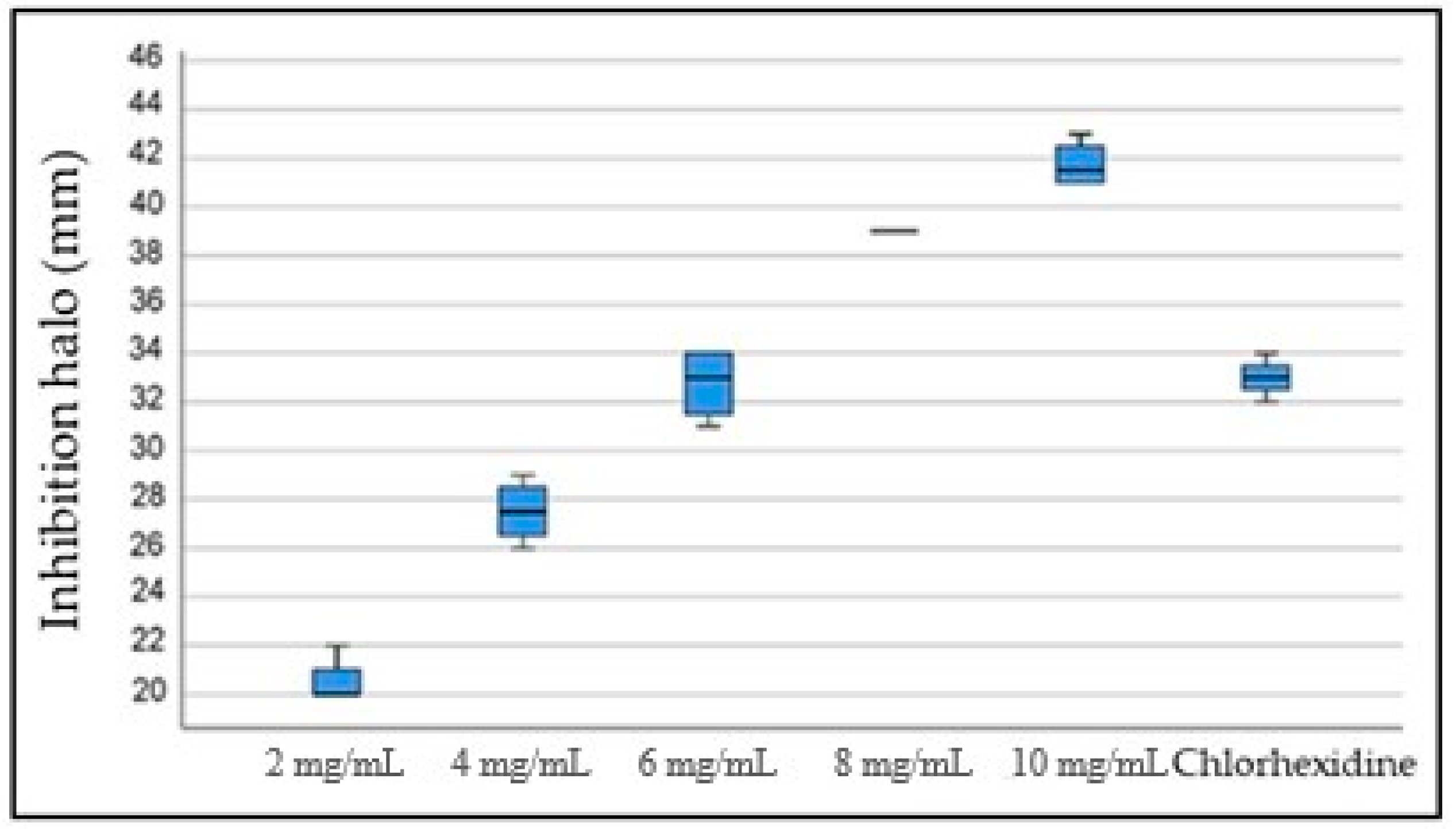
3.3. Antibacterial Effect of PDA against S. mutans
3.4. Adhesion of S. mutans to the Surface of PMMA Discs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.; Tamayo, L.; Gulppi, M.; Rabagliati, F.; Flores, M.; Urzúa, M.; Azócar, M.; Zagal, J.H.; Encinas, M.V.; Zhou, X.; et al. Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles. Molecules 2018, 23, 2747. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yuan, W.; Guo, Y.; Wu, Q.; Wang, F.; Xuan, H. Anti-biofilm activities of Chinese Poplar Propolis essential oil against Streptococcus mutans. Nutrients 2022, 14, 3290. [Google Scholar] [CrossRef] [PubMed]
- O´Brien, E.; Mondal, K.; Chen, C.; Hanley, L.; Drummond, J.; Rockne, K. Relationships between composite roughness and Streptococcus mutans biofilm depth under shear in vitro. J. Dent. 2023, 134, 104535. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Rosentritt, M.; Bürgers, R.; Handel, G. Adhesion of Streptococcus mutans NCTC 10449 to artificial teeth: An in vitro study. J. Prost. Dent. 2008, 100, 309–315. [Google Scholar] [CrossRef]
- Buergers, R.; Rosentritt, M.; Handel, G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J. Prosthet. Dent. 2007, 98, 461–469. [Google Scholar] [CrossRef]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Gomes, A.; Sampaio-Maia, B.; Vasconcelos, M.; Fonseca, P.; Figueiral, M. In Situ Evaluation of the Microbial Adhesion on a Hard Acrylic Resin and a Soft Liner Used in Removable Prostheses. Int. J. Prosthodont. 2015, 28, 65–71. [Google Scholar] [CrossRef]
- Aguayo, S.; Marshall, H.; Pratten, J.; Bradshaw, D.; Brown, J.S.; Porter, S.R.; Spratt, D.; Bozec, L. Early adhesion of Candida albicans onto dental acrylic surfaces. J. Dent. Res. 2017, 96, 917–923. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Wang, Y. Antibacterial orthodontic cement to combat biofilm and white spot lesions. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Rodil, S.E. Modificación Superficial De Biomateriales Metálicos. Materiales 2009, 29, 67–83. [Google Scholar]
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef]
- Hanawa, T. In vivo metallic biomaterials and surface modification. Mater. Sci. Engine A 1999, 267, 260–266. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.Y.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Arango-Santander, S.; Freitas, S.; Pelaez-Vargas, A.; Garcia, C. Silica Sol-Gel Patterned Surfaces Based on Dip-Pen Nanolithography and Microstamping: A Comparison in Resolution and Throughput. Key Eng. Mater. 2016, 720, 264–268. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Weibel, D.B.; DiLuzio, W.R.; Whitesides, G.M. Microfabrication meets microbiology. Nat. Rev. Microbiol. 2007, 5, 209–218. [Google Scholar] [CrossRef]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid. Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef]
- Han, Z.; Mu, Z.; Yin, W.; Li, W.; Niu, S.; Zhang, J.; Ren, L. Biomimetic multifunctional surfaces inspired from animals. Adv. Colloid. Interface Sci. 2016, 234, 27–50. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Lessons from nature--an overview. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 1445–1486. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, J.; Yang, Y.; Zheng, M.; Yang, J.; Tan, J. Surface modification of zirconia with polydopamine to enhance fibroblast response and decrease bacterial activity in vitro: A potential technique for soft tissue engineering applications. Colloid Surf. B Biointerfaces 2015, 136, 74–83. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, X.; Zhou, Q.; Zou, J.; Li, P.; Breukink, E.; Gu, Q. Plantaricin NC8 from Lactobacillus plantarum causes cell membrane disruption to Micrococcus luteus without targeting lipid II. Appl. Microbiol. Biotechnol. 2018, 102, 7465–7473. [Google Scholar] [CrossRef]
- Airen, B.; Sarkar, P.A.; Tomar, U.; Bishen, K.A. Antibacterial effect of propolis derived from tribal region on Streptococcus mutans and Lactobacillus acidophilus: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 48–52. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Trans. 2019, 17, 82–95. [Google Scholar] [CrossRef]
- Choi, S.H.; Jang, Y.S.; Jang, J.H.; Bae, T.S.; Lee, S.J.; Lee, M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 7067. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, S.; Kang, W.; Lin, H.; Hu, Y. Surface modification of Ti6Al4V alloy by polydopamine grafted GO/ZnO nanocomposite coating. Surf. Coat. Tech. 2021, 422, 127534. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Aizenberg, J. Bacteria Pattern Spontaneously on Periodic Nanostructure Arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Hasan, J.; Chatterjee, K. Recent advances in engineering topography mediated antibacterial surfaces. Nanoscale 2015, 7, 15568–15575. [Google Scholar] [CrossRef]
- Xu, L.; Siedlecki, C.A. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater. 2012, 8, 72–81. [Google Scholar] [CrossRef]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Thilak Kumar, R.; Ponnusamy, S. Vibrational spectra and normal coordinate analysis of adrenaline and dopamine. Indian J. Pure Appl. Phys. 2007, 45, 884–892. [Google Scholar]
- Xu, Y.; Den, Z.; Chen, Y.; Wu, F.; Huang, C.; Hu, Y. Preparation and characterization of mussel-inspired hydrogels based on methacrylated cathecol chitosan and dopamine methacrylamide. Int. J. Biol. Macromol. 2023, 229, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.B.; Daily, Z.A.; Alsharbaty, M.H.; Abullais, S.S.; Arora, S.; Lafta, A.H.; Jalil, A.T.; Almulla, A.F.; Ramírez-Coronel, A.A.; Aravindhan, S.; et al. Effect of PMMA sealing treatment on the corrosion behavior of plasma electrolytic oxidized titanium dental implant in fluoride-containing saliva solution. Mater. Res. Express 2022, 9, 125401. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S. Waterproof and Water Repellent Textiles and Clothing, 1st ed.; Elsevier: London, UK, 2018; Chapter 11; pp. 267–297. [Google Scholar]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W.; Yohe, S.T. Superhydrophobic Materials for Biomedical Applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling Resistance of Reverse Osmosis Membrane Modified with Polydopamine. Desalination 2014, 336, 87–96. [Google Scholar] [CrossRef]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking-assisted Method. Nat. Publ. Gr. 2016, 6, 24420. [Google Scholar] [CrossRef]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Memb. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Satou, J.; Fukunaga, A.; Satou, N.; Shintani, H.; Okuda, K. Streptococcal adherence on various restorative materials. J. Dent. Res. 1988, 67, 588–591. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Burton, Z.; Bhushan, B. Surface characterization and adhesion and friction properties of hydrophobic leaf surfaces. Ultramicroscopy 2006, 106, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.S.; Cho, I.; Yoon, E. The role of bio-inspired hierarchical structures in wetting. Bioinspir. Biomim. 2015, 10, 26009. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 1–11. [Google Scholar] [CrossRef]
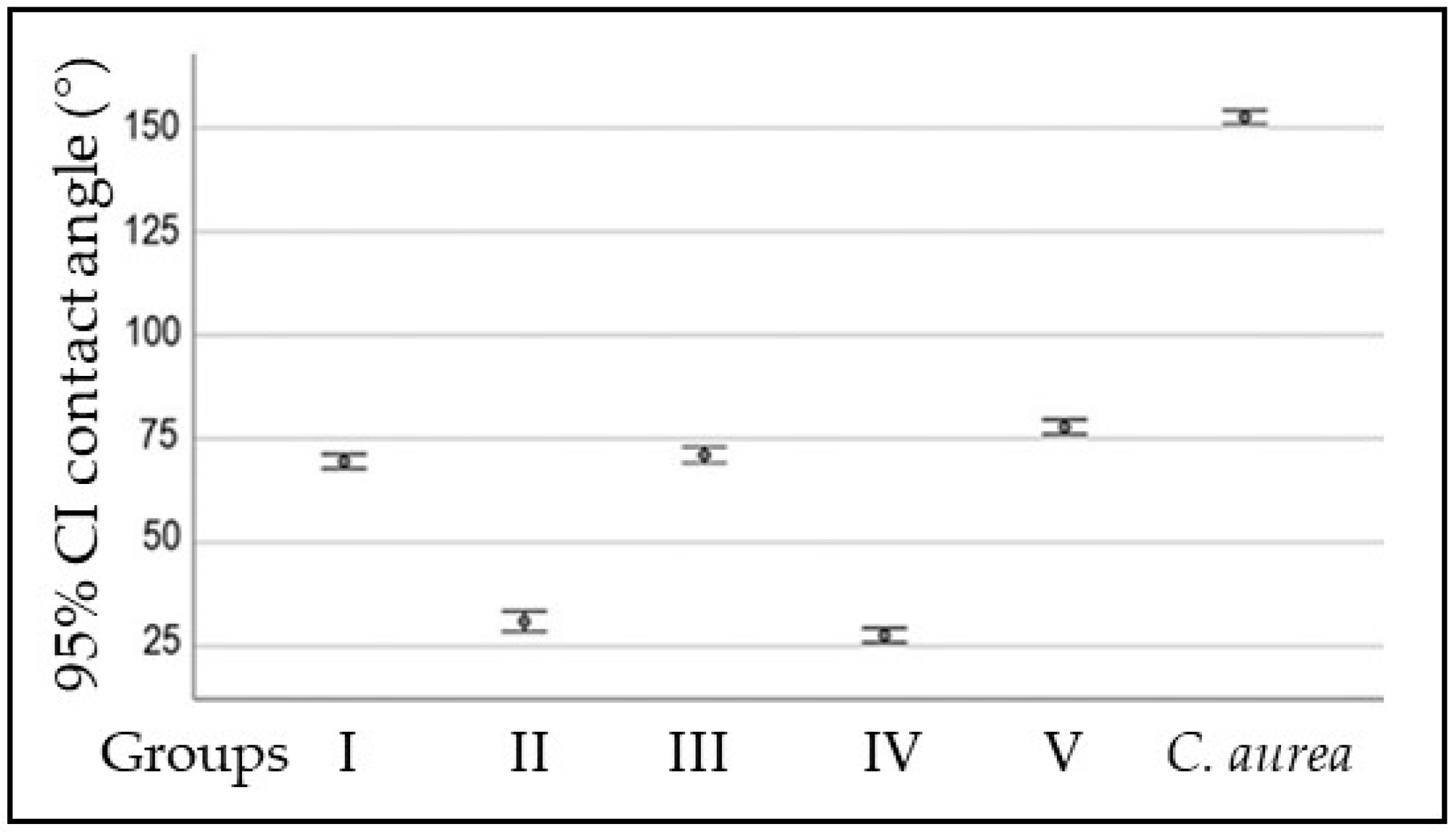

| Surface | Contact Angle (°) | p Value |
|---|---|---|
| PMMA | (Median ± SD) | ˂0.001 |
| Group I | 69.56 ± 2.46 | |
| Group II | 30.96 ± 3.44 | |
| Group III | 71.11 ± 2.71 | |
| Group IV | 27.61 ± 2.44 | |
| Group V | 77.88 ± 2.46 | |
| C. aurea | 152.59 ± 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arango-Santander, S.; Martinez, C.; Bedoya-Correa, C.; Sanchez-Garzon, J.; Franco, J. Assessment of Polydopamine to Reduce Streptococcus mutans Adhesion to a Dental Polymer. Pathogens 2023, 12, 1223. https://doi.org/10.3390/pathogens12101223
Arango-Santander S, Martinez C, Bedoya-Correa C, Sanchez-Garzon J, Franco J. Assessment of Polydopamine to Reduce Streptococcus mutans Adhesion to a Dental Polymer. Pathogens. 2023; 12(10):1223. https://doi.org/10.3390/pathogens12101223
Chicago/Turabian StyleArango-Santander, Santiago, Carlos Martinez, Claudia Bedoya-Correa, Juliana Sanchez-Garzon, and John Franco. 2023. "Assessment of Polydopamine to Reduce Streptococcus mutans Adhesion to a Dental Polymer" Pathogens 12, no. 10: 1223. https://doi.org/10.3390/pathogens12101223
APA StyleArango-Santander, S., Martinez, C., Bedoya-Correa, C., Sanchez-Garzon, J., & Franco, J. (2023). Assessment of Polydopamine to Reduce Streptococcus mutans Adhesion to a Dental Polymer. Pathogens, 12(10), 1223. https://doi.org/10.3390/pathogens12101223







