Oregano Essential Oil versus Conventional Disinfectants against Salmonella Typhimurium and Escherichia coli O157:H7 Biofilms and Damage to Stainless-Steel Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibacterial Compounds
2.2. Minimum Inhibitory (MIC) and Bactericidal Concentrations (MBC) of OEO, NaClO, H2O2, and Benz against Planktonic S. Typhimurium and E. coli O157:H7
2.3. Cellular Adhesion of S. Typhimurium and E. coli O157:H7 on Stainless Steel during Incubation Time
2.4. Minimum Biofilm Inhibitory (MBIC) and Minimum Biofilm Eradication Concentrations (MBEC) of OEO, NaClO, H2O2, and Benz against S. Typhimurium and E. coli O157:H7
2.5. Swimming and Swarming Motility of Treated S. Typhimurium and E. coli O157:H7
2.6. Weight Loss and Apparent Damage to Stainless Steel in Contact with Antibacterial Compounds
2.7. Experimental Designs and Statistical Analysis
3. Results
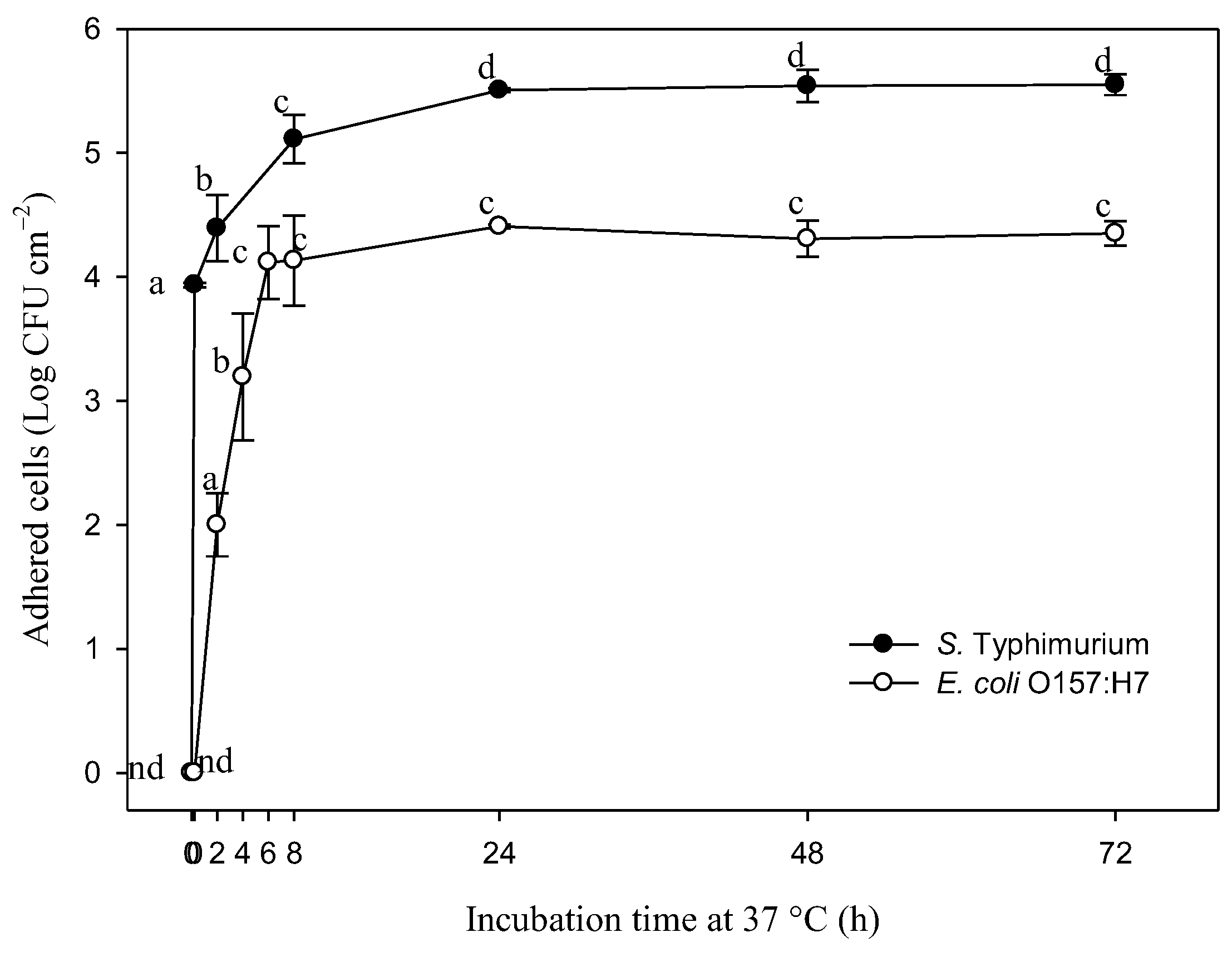
3.1. Adhesion of S. Typhimurium and E. coli O157:H7 on Stainless-Steel
3.2. OEO, NaClO, H2O2, and Benz against Planktonic S. Typhimurium and E. coli O157:H7
3.3. Swimming and Swarming Motility of S. Typhimurium and E. coli O157:H7
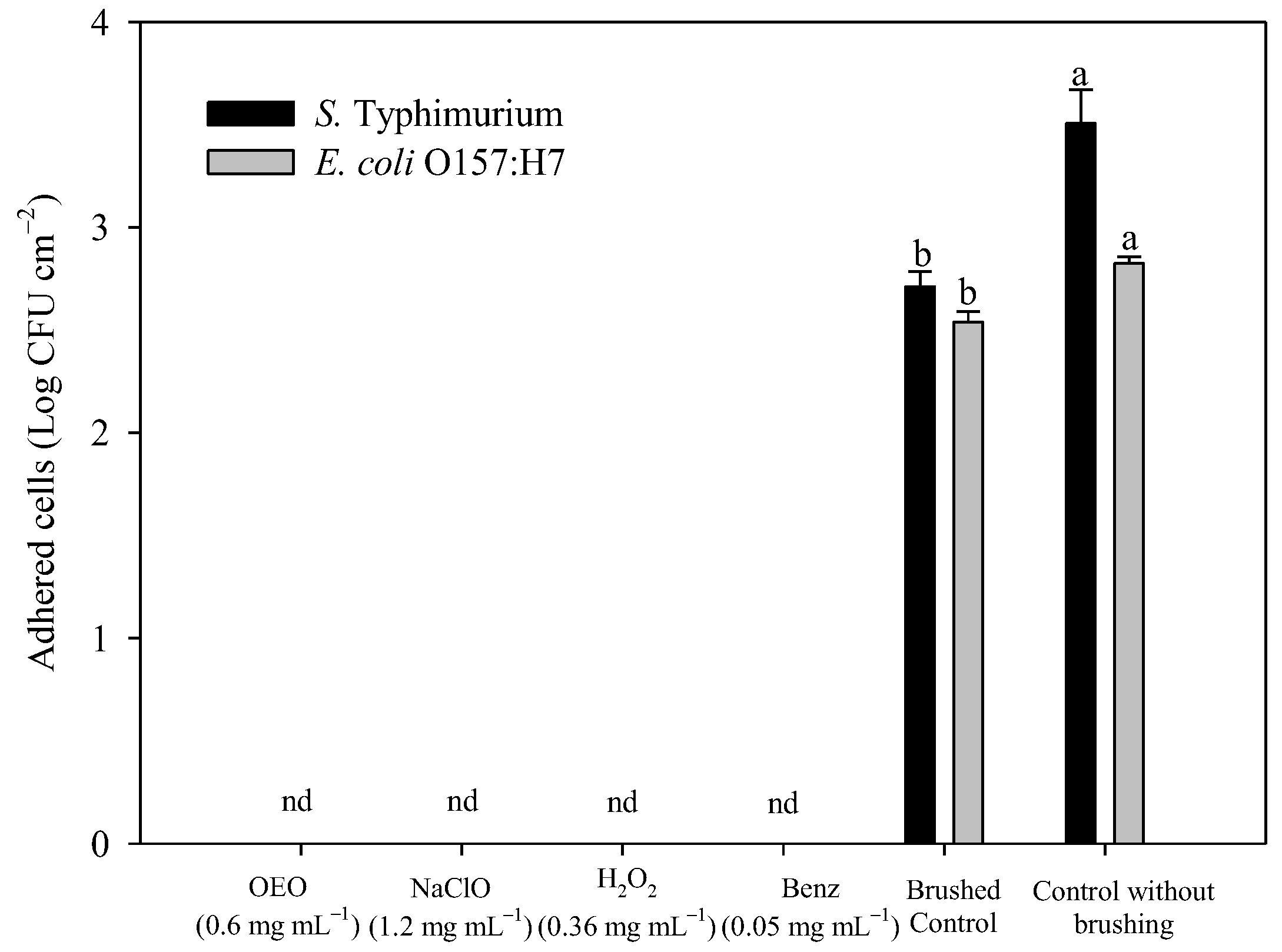
3.4. Effect of Antibacterial Agents Combined with Brushing on Viable Adhered Bacteria, Weight Loss, and Apparent Damage to Stainless Steel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Hoe, Y.W.; Zheng, Q.; Chung, H.-J.; Yuk, H.-G. Biofilm formation by Salmonella Enteritidis in a simulated liquid egg processing environment and its sensitivity to chlorine and hot water treatment. Food Control 2017, 73, 595–600. [Google Scholar] [CrossRef]
- Krogsgård Nielsen, C.; Kjems, J.; Mygind, T.; Snabe, T.; Schwarz, K.; Serfert, Y.; Meyer, R.L. Antimicrobial effect of emulsion-encapsulated isoeugenol against biofilms of food pathogens and spoilage bacteria. Int. J. Food Microbiol. 2017, 242, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. List of Selected Multistate Foodborne Outbreak Investigations. 2019. Available online: https://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html (accessed on 16 May 2019).
- Ayebah, B.; Hung, Y.-C. Electrolyzed water and its corrosiveness on various surface materials commonly found in food processing facilities. J. Food Process Eng. 2005, 28, 247–264. [Google Scholar] [CrossRef]
- Barish, J.A.; Goddard, J.M. Stability of nonfouling stainless steel heat exchanger plates against commercial cleaning agents. J. Food Eng. 2014, 124, 143–151. [Google Scholar] [CrossRef]
- Gkana, E.N.; Giaouris, E.D.; Doulgeraki, A.I.; Kathariou, S.; Nychas, G.-J.E. Biofilm formation by Salmonella Typhimurium and Staphylococcus aureus on stainless steel under either mono- or dual-species multi-strain conditions and resistance of sessile communities to sub-lethal chemical disinfection. Food Control 2017, 73, 838–846. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Makovcova, J.; Babak, V.; Kulich, P.; Masek, J.; Slany, M.; Cincarova, L. Dynamics of mono-and dual-species biofilm formation and interactions between Staphylococcus aureus and Gram-negative bacteria. Microb. Biotechnol. 2017, 10, 819–832. [Google Scholar] [CrossRef]
- Araújo, P.; Lemos, M.; Mergulhão, F.; Melo, L.; Simões, M. Antimicrobial resistance to disinfectants in biofilms. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. Formatex Badajoz 2011, 3, 826–834. [Google Scholar]
- Meireles, A.; Borges, A.; Giaouris, E.; Simões, M. The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res. Int. 2016, 86, 140–146. [Google Scholar] [CrossRef]
- Almeida, R.N.; Costa, P.R.; de Oliveira, J.A.S.; Ferreira, M.B.; Martins, O.A.; Raghiante, F. Natural alternatives for reducing bacterial biofilms in food industry. Rev. Bras. De Hig. E Sanidade Anim. RBHSA 2017, 11, 144–150. [Google Scholar] [CrossRef]
- Waters, B.W.; Tatum, J.M.; Hung, Y.-C. Effect of chlorine-based sanitizers properties on corrosion of metals commonly found in food processing environment. J. Food Eng. 2014, 121, 159–165. [Google Scholar] [CrossRef]
- Znini, M.; Majidi, L.; Bouyanzer, A.; Paolini, J.; Desjobert, J.M.; Costa, J.; Hammouti, B. Essential oil of Salvia aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5 M H2SO4. Arab. J. Chem. 2012, 5, 467–474. [Google Scholar] [CrossRef]
- Boumhara, K.; Tabyaoui, M.; Jama, C.; Bentiss, F. Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1M HCl solution: Electrochemical and XPS investigations. J. Ind. Eng. Chem. 2015, 29, 146–155. [Google Scholar] [CrossRef]
- Bouyanzer, A.; Hammouti, B. A study of anti-corrosive effects of Artemisia oil on steel. Pigment Resin Technol. 2004, 33, 287–292. [Google Scholar] [CrossRef]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I.J.P. Contribution of essential oils to the fight against microbial biofilms—A review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric 2016, 96, 3772–3778. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.; Bernal-Mercado, A.; Lizardi-Mendoza, J.; Silva-Espinoza, B.; Cruz-Valenzuela, M.; Gonzalez-Aguilar, G.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J. Phenolic extracts from grape stems inhibit Listeria monocytogenes motility and adhesion to food contact surfaces. J. Adhes. Sci. Technol. 2018, 32, 889–907. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Kerekes, E.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Antibiofilm forming and antiquorum sensing activity of selected essential oils and their main components on foodrelated microorganisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Chamdit, S.; Siripermpool, P. Antimicrobial effect of clove and lemongrass oils against planktonic cells and biofilms of Staphylococcus aureus. Mahidol Univ. J. Pharm. Sci. 2012, 39, 28–36. [Google Scholar]
- Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A.A.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Synergistic mode of action of catechin, vanillic and protocatechuic acids to inhibit the adhesion of uropathogenic Escherichia coli on silicone surfaces. J. Appl. Microbiol. 2020, 128, 387–400. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.B.; Acosta, E.J.T.R.; de Rezende Pinto, L.; Graeff, M.; Spolidorio, D.M.P.; Almeida, R.S.; Porto, V.C. Microscopical analysis of Candida albicans biofilms on heat-polymerised acrylic resin after chlorhexidine gluconate and sodium hypochlorite treatments. Mycoses 2011, 54, 712–717. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Perumal, P.; Wand, M.; Sutton, J.; Bock, L. Evaluation of the effectiveness of hydrogen-peroxide-based disinfectants on biofilms formed by Gram-negative pathogens. J. Hosp. Infect. 2014, 87, 227–233. [Google Scholar] [CrossRef]
- Almatroudi, A.; Gosbell, I.B.; Hu, H.; Jensen, S.O.; Espedido, B.; Tahir, S.; Glasbey, T.; Legge, P.; Whiteley, G.; Deva, A. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: Implications for infection control. J. Hosp. Infect. 2016, 93, 263–270. [Google Scholar] [CrossRef]
- Piercey, M.J.; Ells, T.C.; Macintosh, A.J.; Hansen, L.T. Variations in biofilm formation, desiccation resistance and Benzalkonium chloride susceptibility among Listeria monocytogenes strains isolated in Canada. Int. J. Food Microbiol. 2017, 257, 254–261. [Google Scholar] [CrossRef]
- Varga, A.; Kocić-Tanackov, S.; Čabarkapa, I.; Aćimović, M.; Tomičić, Z.; Quality, F. Chemical composition and antibacterial activity of spice essential oils against Escherichia coli and Salmonella Typhimurium. Arch. Fur. Liturg. 2019, 70, 177–185. [Google Scholar]
- Fazlara, A.; Ekhtelat, M. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 23–29. [Google Scholar]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial activity of thyme (Tymus vulgaris) and oregano (Origanum vulgare) essential oils against some food-borne microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]




| Bacteria | Compounds (mg mL−1) | ||||
|---|---|---|---|---|---|
| OEO | NaClO | H2O2 | Benz | ||
| Salmonella Typhimurium | MIC | 0.250 | 0.500 | 0.060 | 0.020 |
| MBC | 0.300 | 0.600 | 0.080 | 0.025 | |
| MBIC | 0.150 | 0.450 | 0.030 | 0.022 | |
| MBEC | 0.300 | 1.200 | 0.160 | 0.050 | |
| Escherichia coliO157:H7 | MIC | 0.220 | 0.400 | 0.140 | 0.020 |
| MBC | 0.260 | 0.500 | 0.160 | 0.025 | |
| MBIC | 0.170 | 0.320 | 0.050 | 0.016 | |
| MBEC | 0.520 | 1.000 | 0.360 | 0.050 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Solorza, J.M.; Ayala-Zavala, J.F.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Silva-Espinoza, B.A. Oregano Essential Oil versus Conventional Disinfectants against Salmonella Typhimurium and Escherichia coli O157:H7 Biofilms and Damage to Stainless-Steel Surfaces. Pathogens 2023, 12, 1245. https://doi.org/10.3390/pathogens12101245
Luna-Solorza JM, Ayala-Zavala JF, Cruz-Valenzuela MR, González-Aguilar GA, Bernal-Mercado AT, Gutierrez-Pacheco MM, Silva-Espinoza BA. Oregano Essential Oil versus Conventional Disinfectants against Salmonella Typhimurium and Escherichia coli O157:H7 Biofilms and Damage to Stainless-Steel Surfaces. Pathogens. 2023; 12(10):1245. https://doi.org/10.3390/pathogens12101245
Chicago/Turabian StyleLuna-Solorza, Jesus M., J. Fernando Ayala-Zavala, M. Reynaldo Cruz-Valenzuela, Gustavo A. González-Aguilar, Ariadna T. Bernal-Mercado, M. Melissa Gutierrez-Pacheco, and Brenda A. Silva-Espinoza. 2023. "Oregano Essential Oil versus Conventional Disinfectants against Salmonella Typhimurium and Escherichia coli O157:H7 Biofilms and Damage to Stainless-Steel Surfaces" Pathogens 12, no. 10: 1245. https://doi.org/10.3390/pathogens12101245
APA StyleLuna-Solorza, J. M., Ayala-Zavala, J. F., Cruz-Valenzuela, M. R., González-Aguilar, G. A., Bernal-Mercado, A. T., Gutierrez-Pacheco, M. M., & Silva-Espinoza, B. A. (2023). Oregano Essential Oil versus Conventional Disinfectants against Salmonella Typhimurium and Escherichia coli O157:H7 Biofilms and Damage to Stainless-Steel Surfaces. Pathogens, 12(10), 1245. https://doi.org/10.3390/pathogens12101245









