Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida
Abstract
:1. Introduction
2. Materials and Methods
3. Results
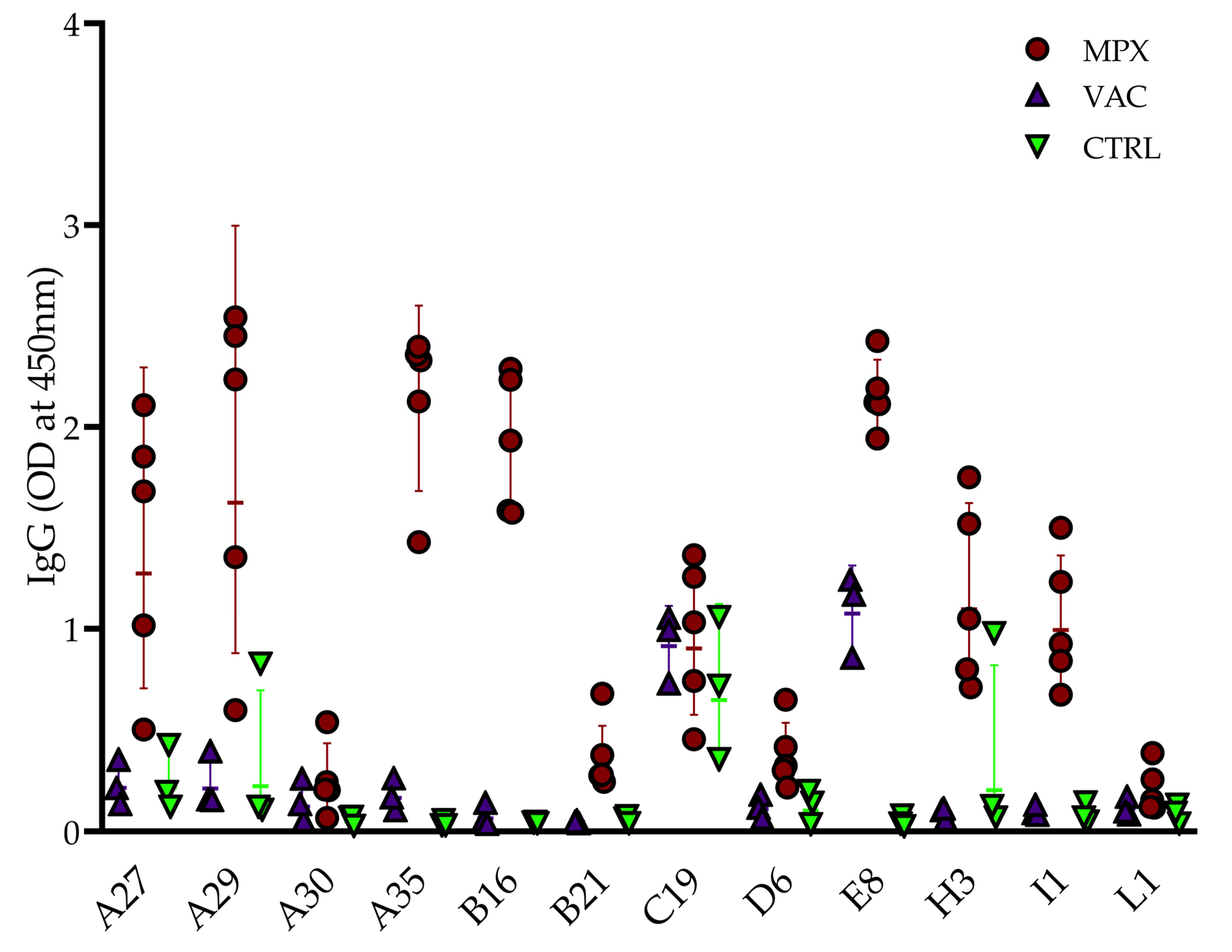
3.1. Preliminary Screen
3.2. Assay Validation
3.3. Antibody Seroprevalence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowley, R.; Greenaway, P.J. Nucleotide sequence comparison of homologous genomic regions from variola, monkeypox, and vaccinia viruses. J. Med. Virol. 1990, 31, 267–271. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef]
- Larkin, H. New Report on Mpox Cases Leading Up to 2022 Global Outbreak. JAMA 2023, 329, 531. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Mpox: 2022 Global Map & Case Count. Available online: Cdc.gov/poxvirus/mpox/response/2022/world-map.html (accessed on 6 August 2023).
- Florida Department of Health. Reportable Diseases Frequency Report. 2023. Available online: https://www.flhealthcharts.gov (accessed on 6 August 2023).
- Butts, S.; Young, B.; Blackmon, J.; Doblecki-Lewis, S. Addressing Disparities in Pre-Exposure Prophylaxis (PrEP) Access: Implementing a Community-Centered Mobile PrEP Program in South Florida. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Florida Department of Health. Persons with HIV. 2023. Available online: https://www.flhealthcharts.gov/charts/SearchResult.aspx (accessed on 6 November 2023).
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April-June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Mpox: Clinicians identify severe form with high mortality in advanced HIV patients. BMJ 2023, 380, 422. [Google Scholar] [CrossRef] [PubMed]
- WHO Health Emergencies Programme (WHE). Surveillance, Case Investigation and Contact Tracing for Mpox (Monkeypox): Interim Guidance, 22 December 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Nsubuga, P.; White, M.E.; Thacker, S.B.; Anderson, M.A.; Blount, S.B.; Broome, C.V.; Chiller, T.M.; Espitia, V.; Imtiaz, R.; Sosin, D.; et al. Public Health Surveillance: A Tool for Targeting and Monitoring Interventions. In Disease Control Priorities in Developing Countries; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA; New York, NY, USA, 2006. [Google Scholar]
- Reda, A.; El-Qushayri, A.E.; Shah, J. Asymptomatic monkeypox infection: A call for greater control of infection and transmission. Lancet Microbe 2023, 4, e15–e16. [Google Scholar] [CrossRef]
- Lederman, E.R.; Reynolds, M.G.; Karem, K.; Braden, Z.; Learned-Orozco, L.A.; Wassa-Wassa, D.; Moundeli, O.; Hughes, C.; Harvey, J.; Regnery, R.; et al. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: Evidence for monkeypox virus exposure. Am. J. Trop. Med. Hyg. 2007, 77, 1150–1156. [Google Scholar] [CrossRef]
- Luciani, L.; Lapidus, N.; Amroun, A.; Falchi, A.; Souksakhone, C.; Mayxay, M.; Dubot-Peres, A.; Villarroel, P.M.S.; Diarra, I.; Koita, O.; et al. Orthopoxvirus Seroprevalence and Infection Susceptibility in France, Bolivia, Laos, and Mali. Emerg. Infect. Dis. 2022, 28, 2463–2471. [Google Scholar] [CrossRef]
- Waddell, C.J.; Filardo, T.D.; Prasad, N.; Pellegrini, G.J., Jr.; Persad, N.; Carson, W.C.; Navarra, T.; Townsend, M.B.; Satheshkumar, P.S.; Lowe, D.; et al. Possible Undetected Mpox Infection Among Persons Accessing Homeless Services and Staying in Encampments—San Francisco, California, October-November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 227–231. [Google Scholar] [CrossRef]
- Lim, C.K.; Roberts, J.; Moso, M.; Liew, K.C.; Taouk, M.L.; Williams, E.; Tran, T.; Steinig, E.; Caly, L.; Williamson, D.A. Mpox diagnostics: Review of current and emerging technologies. J. Med. Virol. 2023, 95, e28429. [Google Scholar] [CrossRef]
- Dubois, M.E.; Slifka, M.K. Retrospective analysis of monkeypox infection. Emerg. Infect. Dis. 2008, 14, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Kennedy, R.B.; Tosh, P.K. Prevention of monkeypox with vaccines: A rapid review. Lancet Infect. Dis. 2022, 22, e349–e358. [Google Scholar] [CrossRef] [PubMed]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Wolff Sagy, Y.; Zucker, R.; Hammerman, A.; Markovits, H.; Arieh, N.G.; Abu Ahmad, W.; Battat, E.; Ramot, N.; Carmeli, G.; Mark-Amir, A.; et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat. Med. 2023, 29, 748–752. [Google Scholar] [CrossRef]
- Malone, S.M.; Mitra, A.K.; Onumah, N.A.; Brown, A.; Jones, L.M.; Tresvant, D.; Brown, C.S.; Onyia, A.U.; Iseguede, F.O. Safety and Efficacy of Post-Eradication Smallpox Vaccine as an Mpox Vaccine: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2963. [Google Scholar] [CrossRef] [PubMed]
- Merad, Y.; Gaymard, A.; Cotte, L.; Perpoint, T.; Alfaiate, D.; Godinot, M.; Becker, A.; Cannesson, O.; Batalla, A.S.; Oria-Yassir, F.; et al. Outcomes of post-exposure vaccination by modified vaccinia Ankara to prevent mpox (formerly monkeypox): A retrospective observational study in Lyon, France, June to August 2022. Eurosurveill 2022, 27, 2200882. [Google Scholar] [CrossRef]
- Thy, M.; Peiffer-Smadja, N.; Mailhe, M.; Kramer, L.; Ferre, V.M.; Houhou, N.; Tarhini, H.; Bertin, C.; Beaumont, A.L.; Gare, M.; et al. Breakthrough Infections after Postexposure Vaccination against Mpox. N. Engl. J. Med. 2022, 387, 2477–2479. [Google Scholar] [CrossRef]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of peptide-based ELISA for serological diagnostics: A retrospective study of human monkeypox infection. Vector Borne Zoonotic Dis. 2012, 12, 400–409. [Google Scholar] [CrossRef]
- Taha, T.Y.; Townsend, M.B.; Pohl, J.; Karem, K.L.; Damon, I.K.; Mbala Kingebeni, P.; Muyembe Tamfum, J.J.; Martin, J.W.; Pittman, P.R.; Huggins, J.W.; et al. Design and Optimization of a Monkeypox virus Specific Serological Assay. Pathogens 2023, 12, 396. [Google Scholar] [CrossRef]
- Hammarlund, E.; Lewis, M.W.; Carter, S.V.; Amanna, I.; Hansen, S.G.; Strelow, L.I.; Wong, S.W.; Yoshihara, P.; Hanifin, J.M.; Slifka, M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005, 11, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.L.; Hunt, D.T.; Kulas, K.E.; Chave, K.; Styer, L.; Chakravarthi, S.T.; Cai, G.Y.; Bermudez-Gonzalez, M.C.; Kleiner, G.; Altman, D.; et al. Development of a Novel Serological Assay for the Detection of Mpox Infection in Vaccinated Populations. J. Med. Virol. 2023, 95, e29134. [Google Scholar] [CrossRef]
- Yefet, R.; Friedel, N.; Tamir, H.; Polonsky, K.; Mor, M.; Cherry-Mimran, L.; Taleb, E.; Hagin, D.; Sprecher, E.; Israely, T.; et al. Monkeypox infection elicits strong antibody and B cell response against A35R and H3L antigens. iScience 2023, 26, 105957. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Gotz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Zhang, Y.; Tarke, A.; Sidney, J.; Rubiro, P.; Reina-Campos, M.; Filaci, G.; Dan, J.M.; Scheuermann, R.H.; Sette, A. Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans. Cell Host Microbe 2022, 30, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; Guivel-Benhassine, F.; Bruel, T.; Porrot, F.; Planas, D.; Vanhomwegen, J.; Wiedemann, A.; Burrel, S.; Marot, S.; Palich, R.; et al. Complement-dependent mpox-virus-neutralizing antibodies in infected and vaccinated individuals. Cell Host Microbe 2023, 31, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the monkeypox virus genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Munoz, I.; Sanchez-dePrada, L.; Sanchez-Martinez, J.; Rojo-Rello, S.; Dominguez-Gil, M.; Hernan-Garcia, C.; Fernandez-Espinilla, V.; de Lejarazu-Leonardo, R.O.; Castrodeza-Sanz, J.; Eiros, J.M. Possible Mpox Protection from Smallpox Vaccine-Generated Antibodies among Older Adults. Emerg. Infect. Dis. 2023, 29, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Ershler, W.B.; Janowski, M.; Artz, A.; Key, M.L.; McKelvey, J.; Muller, D.; Moss, B.; Ferrucci, L.; Duffey, P.L.; et al. Immunity from smallpox vaccine persists for decades: A longitudinal study. Am. J. Med. 2008, 121, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Renia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, J.C.; Cassaniti, I.; Ferrari, A.; Piralla, A.; Bergami, F.; Arena, F.A.; Paolucci, S.; Rovida, F.; Lilleri, D.; Percivalle, E.; et al. Characterization of immune response against monkeypox virus in cohorts of infected patients, historic and newly vaccinated subjects. J. Med. Virol. 2023, 95, e28778. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, pathogenesis, treatment and prevention. Signal. Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Monteiro, V.S.; Renauer, P.A.; Shang, X.; Suzuki, K.; Ling, X.; Bai, M.; Xiang, Y.; Levchenko, A.; Booth, C.J.; et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Hatami, H.; Jamshidi, P.; Arbabi, M.; Safavi-Naini, S.A.A.; Farokh, P.; Izadi-Jorshari, G.; Mohammadzadeh, B.; Nasiri, M.J.; Zandi, M.; Nayebzade, A.; et al. Demographic, Epidemiologic, and Clinical Characteristics of Human Monkeypox Disease Pre- and Post-2022 Outbreaks: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Technical Report 1: Multi-National Mpox Outbreak, United States, 2022; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- de Oliveira, J.S.; Barbosa Costa, G.; Stoffella Dutra, A.G.; Domingos, I.; Costa, P.; Silva, P.; Kroon, E.G.; de Oliveira, D.B.; Trindade, G.S. Low prevalence of anti-Orthopoxvirus neutralizing antibodies in an urban population of Brazil. J. Med. Virol. 2023, 95, e28859. [Google Scholar] [CrossRef] [PubMed]
- Girometti, N.; Ogoina, D.; Tan, D.H.S.; Pozniak, A.; Klein, M.B. Intersecting HIV and mpox epidemics: More questions than answers. J. Int. AIDS Soc. 2022, 25, e26043. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.L.; Lewis, N.M.; Houck, K.; Canning, M.; Fothergill, A.; Payne, A.B.; Cohen, A.L.; Vance, J.; Brassil, B.; Youngkin, E.; et al. Demographic and Clinical Characteristics of Mpox in Persons Who Had Previously Received 1 Dose of JYNNEOS Vaccine and in Unvaccinated Persons—29 U.S. Jurisdictions, May 22-September 3, 2022. Am. J. Transplant. 2023, 23, 298–303. [Google Scholar] [CrossRef]
- Callaby, H.; Gordon, N.C. Mpox: Evidence for strengthening and sustaining global surveillance. Lancet Glob. Health 2023, 11, e983–e984. [Google Scholar] [CrossRef] [PubMed]
- Otter, A.D.; Jones, S.; Hicks, B.; Bailey, D.; Callaby, H.; Houlihan, C.; Rampling, T.; Gordon, N.C.; Selman, H.; Satheshkumar, P.S.; et al. Monkeypox virus-infected individuals mount comparable humoral immune responses as Smallpox-vaccinated individuals. Nat. Commun. 2023, 14, 5948. [Google Scholar] [CrossRef]



| ID | Age | Gender | HIV Status | MVA-BN Dose 1 Date | MVA-BN Dose 2 Date | Mpox Infection (Date Confirmed) | Serum Collection Date |
|---|---|---|---|---|---|---|---|
| V1 | 57 | Male | Pos | 4 October 2022 | ·· | ·· | 18 October 2022 |
| V2 | 40 | Male | Pos | 30 August 2022 | 6 October 2022 | ·· | 6 October 2022 |
| V3 | 58 | Female | Pos | 7 September 2022 | 5 October 2022 | ·· | 31 October 2022 |
| V4 | 52 | Male | Pos | 4 October 2022 | ·· | ·· | 31 October 2022 |
| V5 | 54 | Male | Pos | 5 September 2022 | 5 October 2022 | ·· | 28 October 2022 |
| V6 | 57 | Male | Pos | 4 October 2022 | 4 November 2022 | ·· | 23 November 2022 |
| V7 | 32 | Female | Neg | 1 July 2022 | ·· | ·· | 10 November 2022 |
| V8 | 61 | Male | Pos | 15 October 2022 | ·· | ·· | 12 December 2022 |
| V9 | 65 | Male | Pos | 1 September 2022 | ·· | ·· | 5 January 2023 |
| V10 | 66 | Male | Pos | 21 September 2022 | ·· | ·· | 29 December 2022 |
| V11 | 24 | Male | Neg | 29 July 2022 | 26 August 2022 | ·· | 6 September 2023 |
| V12 | 23 | Male | Neg | 13 August 2022 | 13 September 2022 | ·· | 23 September 2022 |
| M1 | 28 | Male | Neg | 22 June 2022 | ·· | 10 July 2022 | 16 September 2022 |
| M2 | 39 | Male | Neg | ·· | ·· | 5 August 2022 | 16 August 2022 |
| M3 | 35 | Male | Neg | ·· | ·· | 21 July 2022 | 10 August 2022 |
| M4 | 28 | Male | Neg | ·· | ·· | 25 July 2022 | 6 September 2022 |
| M5 | 31 | Male | Neg | ·· | ·· | 6 July 2022 | 28 July 2022 |
| # of Antibodies Seropositive | Total n (%) | Age in Years (Mean ± SD) | n Male (%) | n PWH (%) |
|---|---|---|---|---|
| 0 | 175 (81.8) | 47.2 ± 14.6 | 108 (61.7) | 97 (55.4) |
| 1 | 28 (13.1) | 55.6 ± 14.3 | 11 (39.3) | 12 (42.9) |
| ≥2 | 11 (5.1) | 56.3 ± 10.2 | 10 (91.0) | 8 (72.7) |
| All Samples | 214 (100%) | 48.7 ± 14.7 | 129 (60.3) | 117 (54.7) |
| Demographics | Antibody (IgG) Positive/Negative (+/−) | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | Age | Gender | HIV Status | A35 | B16 | E8 | H3 | I1 |
| S + 1 | 56 | Male | Neg | + | − | − | + | − |
| S + 2 | 60 | Female | Pos | + | − | + | − | − |
| S + 3 | 54 | Male | Pos | + | + | + | − | − |
| S + 4 | 35 | Male | Pos | + | + | + | + | − |
| S + 5 | 61 | Male | Pos | + | + | + | − | − |
| S + 6 | 62 | Male | Neg | + | − | − | + | − |
| S + 7 | 64 | Male | Pos | + | + | − | − | − |
| S + 8 | 39 | Male | Pos | + | + | + | − | − |
| S + 9 | 66 | Male | Pos | + | + | − | − | − |
| S + 10 | 62 | Male | Neg | − | + | + | − | − |
| S + 11 | 61 | Male | Pos | − | + | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupritz, J.; Pahwa, S.; Pallikkuth, S. Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida. Pathogens 2023, 12, 1355. https://doi.org/10.3390/pathogens12111355
Kupritz J, Pahwa S, Pallikkuth S. Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida. Pathogens. 2023; 12(11):1355. https://doi.org/10.3390/pathogens12111355
Chicago/Turabian StyleKupritz, Jonah, Savita Pahwa, and Suresh Pallikkuth. 2023. "Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida" Pathogens 12, no. 11: 1355. https://doi.org/10.3390/pathogens12111355
APA StyleKupritz, J., Pahwa, S., & Pallikkuth, S. (2023). Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida. Pathogens, 12(11), 1355. https://doi.org/10.3390/pathogens12111355






