Abstract
Background: Diarrheagenic Escherichia coli (DEC) is one of the most common etiological agents of moderate-to-severe diarrhea in Low- and Middle-Income Countries (LMICs). Therefore, determining the source(s) of DEC in index cases and exposure environment is important for developing a prevention strategy. The current study aims to investigate the prevalence of DEC among children under 5 years and their exposure environment in Ogun State, Nigeria. Methods: Samples from 228 diarrheic children and their exposure environment were collected and screened for E. coli. Bio-chemically compatible distinct colonies were molecularly characterized using a 7-virulence-gene multiplex PCR with virulence factors (VFs) indicative of four pathotypes of E. coli: enterotoxigenic (ETEC), verotoxigenic (VTEC), enteropathogenic (EPEC), and enteroinvasive (EIEC). Representative pathotypes were subjected to antimicrobial susceptibility and over-expressed efflux pump assays. Results: One or more VFs typical of specific pathotypes were detected in 25.9% (59/228) diarrhea cases consisting of ETEC (21.5%) and EPEC (0.4%), while hetero-pathogenic pathotypes were found in 4.0% of cases. Of the food sources, 27.9% (101/362) were positive for DEC, of which ETEC accounted for 21.0%, VTEC 1.9%, EPEC 0.6%, EIEC 0.6%, and hetero-pathogenic pathotypes were 3.9%. Furthermore, ETEC was the only pathotype detected in the wastewater (4/183). Interestingly, the consumption of street-vended foods was the most significant (p = 0.04) risk factor for DEC infection in the study area. A total of 73.3% of selected DEC pathotypes showed resistance to antimicrobials, while 27.5% demonstrated over-expression of efflux pump activity. Conclusion: The high prevalence of ETEC across all sources and the occurrence of hetero-pathogenic DEC in diarrheic children and food sources emphasizes the importance of establishing a better strategy for the control and prevention of diarrhea among children in low- and medium-income households.
1. Introduction
Diarrhea is one of the leading causes of death in children under 5 years, accounting for an annual 1.7 billion global cases with an estimated mortality of 1.3 million deaths, with approximately 25% of this observed mortality occurring in Low- and Middle-Income Countries (LMICs) [1]. The number of diarrhea-related infant mortality in Nigeria remains high despite concerted global efforts aimed at reducing its incidence [2]. In Nigeria, the prevalence of diarrhea was estimated at 18.8%, with an annual mortality of 150,000 deaths [3]. Common routes of exposure to diarrhea are usually through consumption of contaminated water, unhygienic practices in food preparation, lack of potable drinking water, and improper disposal of sewage [4,5]. Different infectious agents have been implicated in the etiology of diarrheal diseases [4].
Diarrheagenic Escherichia coli (DEC) is one of the most common etiological agents of moderate-to-severe diarrhea in LMICs [6]. Various DEC strains are responsible for 30% to 40% of diarrhea episodes in children in developing countries [7]. The DEC group has recently been classified into nine distinct pathotypes based on pathogenomics, phenotypic classification and essential virulence genes defining each subgroup, such as Shiga toxin-producing E. coli (STEC), enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), diffusely adhering E. coli (DAEC), adherent-invasive E. coli (AIEC), and cell-detaching E. coli (CDEC) [8,9]. The EPEC, ETEC, and EAEC are the most common DEC pathotypes and are known to cause moderate-to-severe diarrhea or even death in children in Asia and Africa [10,11,12]. However, studies have reported DEC strains that harbor combinations of virulence factors that are characteristic of two or more DEC pathotypes. These are called hetero-pathogenic E. coli [13]. Previously reported combinations of virulence factors that could lead to more severe diseases are EAEC/STEC, EPEC/STEC, and EPEC/ETEC [14,15,16]. Acute watery and bloody diarrhea cases with more severe symptoms caused by hetero-pathogenic strains in children under 5 years have been reported in Asia [15]. The prevalence and distribution of DEC as a cause of diarrhea in children under 5 years have been shown to vary within regions and countries [17,18,19,20]. Cases of hetero-pathogenic DEC consisting of EPEC/ETEC have been reported in Africa, specifically in Kenya, Ethiopia, and The Gambia [6,10,21]. However, there is a paucity of information on the different DEC pathotypes, most importantly hetero-pathogenic groups in children under 5 years in Nigeria. This study investigated the molecular epidemiology of hetero-pathogenic and diarrheagenic E. coli pathotypes and associated risk factors that predispose children under 5 years with diarrhea to DEC infection in South-West Nigeria.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria for Study Participation
Children of both sexes under the age of 5 years presenting with diarrhea and without any prolonged illnesses were enrolled for this research. Children with diarrhea, prolonged illnesses (even when under five years old), and older than 5 years were excluded from this study.
2.2. Study Location/Population
This study was a cross-sectional hospital-based study conducted between July 2020 and February 2022 at the paediatric wards of six hospitals: Sacred Heart Hospital (7°10′00.2″ N 3°21′20.2″ E), Mowe Primary Healthcare Centre (PHC) (6°48′28.7″ N 3°26′07.9″ E), Ibafo Health Clinic (6°44′38.2″ N 3°25′16.7″ E), Ofada PHC (6°51′54.7″ N 3°25′39.4″ E), Mokoloki PHC (6°52′55.8″ N 3°22′04.8″ E), and Federal Medical Centre (7°08′44.7″ N 3°22′41.4″ E). These hospitals are known to receive high number of paediatric patients across the state. In addition, the demography for this research was noted for low-middle-income earners and high population density. Diarrheal outpatient children ≤ 5 years of age attending these hospitals and whose parents consented to participation in the research were enrolled.
2.3. Sample Collection
Diarrheal stool samples were collected with the help of trained nursing staff. Briefly, samples were collected by allowing the diarrheal child to pass stool on a nylon wrap placed over the rim of a plastic potty or in a disposable diaper. Then, a sterile plastic spoon was used to transfer the stool to a clean, sterile universal bottle prefilled with Cary Blair transport medium (CM0519B, Oxoid, Basingstoke, UK). Sample collection was carefully performed to avoid contamination with urine, soil, or water. In addition, samples from food sources (left-over foods, drinking water, fruits, and vegetables) were fed to the child, and wastewater samples from the households of the diarrheic child were also collected in a clean, leak-proof container. Fruits and vegetables that could not be obtained from the households were bought from markets in proximity to the index cases. All samples were labelled, packed, sealed, and sent to the laboratory within 24 h for analyses. Interviewer-administered questionnaires were used to obtain information about clinical manifestations and sociodemographic characteristics, including gender, age, occupation (of the mother), and sanitary wares of the diarrheic child from the parent(s). The data obtained were used to explore risk factors for DEC-associated diarrhea in children within the study population.
2.4. Bacteria Isolation
Standard culture methods were performed on all samples immediately after reception in the laboratory. The isolation of a single E. coli colony was performed by plating the samples onto sorbitol MacConkey agar (Oxoid, Basingstoke, UK) and incubating for 24–48 h at 37 ± 1 °C. Determination of E. coli isolates was carried out using the following biochemical tests: carbohydrates fermentation, gas production, hydrogen sulphide production, citrate utilization, urease production, indole, and motility. Isolates positive for lactose–glucose and gas production, non-hydrogen sulphide-producing, negative citrate and urease, positive indole, and motility were ascribed as E. coli. The E. coli strain ATCC25922 was used as a positive control. After identification, pure E. coli isolates were stored in brain heart infusion broth (CM0225, Oxoid, Hampshire, UK) with 20% glycerol and stored at −80 °C before the molecular analyses the next day.
2.5. DNA Extraction
The DNA of a single E. coli colony was extracted following a method previously described by Choo et al. [22] with slight modifications. Briefly, 1 mL of the pure E. coli isolate-BHI culture broth was aliquoted into 2 mL snap vials, centrifuged at 5000× g for 3 min, the supernatant discarded, and the cell pellet resuspended in 1 mL sterile distilled water, pulse-vortexed, centrifuged, and the supernatant discarded. The cell pellets were resuspended in 200 µL double-distilled water and heated at 100 °C for 15 min in a dry heat block (Techne, UK), after which the vials were immediately cooled on ice for 5 min. Then, they were centrifuged at 8000× g for 7 min, and 100 µL of the supernatant was used as template DNA for subsequent PCR.
2.6. Detection of Virulence Factors for DEC
Multiplex PCR for E. coli virulence genes using specific primer pairs for verotoxin genes (vtx1 and vtx2), intimin gene (eaeA), heat-stable enterotoxin A genes (estA-human and estA-porcine), heat-labile enterotoxin A (eltA), and invasion plasmid antigen H gene (ipaH) were used in detection of DEC pathotypes as previously described by Persson et al. [23] as shown in Table 1. The oligonucleotide primer sequences for the virulence factors and PCR product sizes. The PCR was performed using a Thermo-cycler (Eppendorf-Nethel-Hinz GmbH, Hamburg, Germany) with a reaction volume of 20 µL containing 1X of Solis BioDyne 5× FIREPol® Master Mix with 12.5 mM MgCl2, primers mix, and 4 µL DNA template. The amplification condition comprised an initial denaturation at 95 °C for 15 min followed by 35 cycles of denaturation at 95 °C for 50 s, annealing at 57 °C for 40 s, elongation at 72 °C for 50 s, and then final elongation at 72 °C for 3 min. The PCR amplicons were visualized on 1.8% w/v agarose gel electrophoresis stained with ethidium bromide. The E. coli strain ATCC25922 was used as a negative control.

Table 1.
Oligonucleotide primers used for PCR amplification of the targeted virulence genes in isolated DEC [23].
2.7. Antibiotic Susceptibility Test
The DEC isolates were tested for susceptibility to Ciprofloxacin (CPR), Tetracycline (TE), Ceftazidime (CAZ), Cefuroxime (CRX), Gentamicin (GEN), Cefixime (CXM), Ofloxacin (OFL), Augmentin (AUG), Nitrofurantoin (NIT) using Kirby–Bauer disk diffusion technique [24] on Mueller Hinton agar (Oxoid, UK) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. Following standard procedure, inhibition zone diameter breakpoints for each antibiotic were measured, and the susceptibility results were interpreted using CLSI guidelines [25]. E. coli (ATCC25922) was included as quality control organisms in antimicrobial susceptibility determination.
2.8. Over-Expression of Efflux Pump Activity in Multidrug Resistant (MDR) DEC Isolates
The DEC isolates resistant to three or more classes of antibiotics were classified as multidrug-resistant (MDR) [26]. MDR-DEC isolates were screened for phenotypic over-expression of efflux pump activity using the ethidium bromide (EtBr)-agar cartwheel method, as described by Martins et al. [27]. Briefly, over-night cultures of MDR DEC isolates were streaked on Trypticase Soy Agar (TSA) (Oxoid, UK) having 0.0 mg/L, 0.5 mg/L, 1.0 mg/L, 1.5 mg/L, and 2.0 mg/L of EtBr and incubated at 37 °C. The degree of fluorescence for each plate was examined under a UV transilluminator (Cleaver Scientific Ltd., Warwickshire, UK). Isolates without fluorescence at minimum concentration of EtBr were recorded to have over-expressed efflux pump activity, while those with fluorescence had no efflux pump activity.
2.9. Statistical Analysis
Descriptive statistic was used to describe the results of this research. Univariate analyses were performed to explore the association between social demographic factors, clinical manifestations, exposure sources, and the presence of DEC among children with diarrhea. The analyses were performed using the Chi-square test implemented in IBM SPSS version 20 (SPSS Inc. Chicago, IL, USA), and the level of significance was set at p < 0.05.
3. Results
3.1. Isolation of Presumptive DEC
A total of 228 diarrheal stool samples from children under 5 years attending or admitted in both private and public health facilities in Ogun State, Nigeria, were screened for DEC on selective agar, in which 1240 presumptive DEC isolates were obtained. In addition, 924 presumptive DEC isolates were obtained from 362 food sources (leftover household food = 18, fresh produce from markets = 252, and household drinking water = 92) and 183 wastewater samples from households and markets in proximity to the patients’ households.
3.2. Prevalence of Hetero-Pathogenic and DEC Pathotypes among the Diarrheic Children
A total of 139 isolates were confirmed to be DEC by multiplex PCR (Table 2, Figure S1a–e, Figure S2). The most prevalent pathotype was ETEC, with the isolates having the following virulent gene distribution: estA-p (69.1%), estA-h (9.4%), and estA-p/estA-h (8.6%), as shown in Table 2 The eae gene, which signifies EPEC, was found in 5% of the DEC isolates. Various combinations of virulent genes from two or more pathotypes, known as hetero-pathogenic DEC, were found in some of the DEC isolates. The most prevalent of these combinations were estA-p/eae (4.3%), indicative of the ETEC-EPEC heteropathotype, while four virulent genes combination of vtx1/estA-h/estA-p, vtx2/estA-h, vtx1/vtx2/estA-h, and vtx1/vtx2/estA-p representing the ETEC-VTEC hetero-pathotype. The EIEC-ETEC hetero-pathotype with the virulent gene combination of ipaH/estA-h/estA-p was also detected. However, the eltA gene was not detected in any of the isolates. The most prevalent DEC pathotype among the index cases was ETEC (21.05%), while the ETEC-EPEC (2.13%) was the most prevalent DEC hetero-pathotype. Overall, the prevalence of hetero-pathogenic and DEC pathotypes was 25.9, as presented in Table 3 (Figure S3).

Table 2.
Distribution of virulence genes among DEC isolated (n = 139) in diarrheic children under 5 years in Ogun State, Nigeria.

Table 3.
Prevalence of DEC pathotypes among cases (n = 228) of diarrheic children under 5 years in Ogun State, Nigeria.
3.3. Distribution of Virulence Genes and DEC Pathotypes in Various Food and Environmental Sources
In the food samples, the most prevalent pathotype was ETEC, with the isolates having the following virulent genes: estA-h (11.1%), estA-p (6.4%), and estA-p/estA-h (4.7%) (Table 4). The least prevalent single pathotypes were EPEC and EIEC, with both having a prevalence of 0.6% (Table 4). Furthermore, ETEC-VTEC (2.2%) was the most prevalent heteropathotype, while ETEC-EPEC-VTEC (0.3%) was the least prevalent (Table 4). Investigation into the specific food sources showed that fresh produce had the highest prevalence and distribution of hetero-pathogenic and DEC pathotypes, followed by drinking water and leftover food, as presented in Table 5. Conversely, in the wastewater samples, ETEC (2.2%) was the only DEC pathotype detected (Table 5). The distribution of DEC in the fresh farm produce is presented in Table 6, with the highest prevalence and the largest diversity of DEC pathotypes observed in cabbage.

Table 4.
Distribution of virulence genes among DEC pathotypes isolated from food and water sources in Ogun State, Nigeria.

Table 5.
Distribution of hetero-pathogenic and DEC pathotypes among various food and environmental sources in Ogun State, Nigeria.

Table 6.
Distribution of hetero-pathogenic and DEC isolates in fresh farm produce in Ogun State, Nigeria.
3.4. Socio-Demographic and Risk Factors of DEC Pathotypes
The results of univariate risk factor analysis for the occurrence of DEC infection among diarrheic children under the age of five are presented in Table 7. DEC-infected children were more likely to experience loss of appetite (OR = 2.3, p = 0.012) and abdominal pain (OR = 2.4, p = 0.013) compared to diarrheic children with other etiologies. No statistically significant association (OR = 1.5, p = 0.37) was observed between DEC infection in males compared to females. Children with DEC infection were more likely to be exposed to street-vended foods (OR = 2.1, p = 0.04) than children with diarrhea from other causes.

Table 7.
Socio-demographic and risk factors predictor of DEC pathotypes among cases of diarrheic children under 5 years in Ogun State, Nigeria.
3.5. Antimicrobial Susceptibility and Multidrug Resistance Profiles of Selected DEC Isolates
The antimicrobial susceptibility profiles of the selected DEC isolates are summarized in Figure 1. Notably, very high resistance to commonly used antibiotics such as Augmentin (94%), Ceftazidime (86%), Cefuroxime (86%), and Cefixime (84%) was observed, while a relatively lower percentage was recorded for Nitrofurantoin (26%) (Figure 1). The majority of DEC pathotypes tested were MDR, as shown in Table 8. A total of 50 DEC isolates were analysed for antimicrobial resistance; 40 (80%) of the DEC can be categorised as multidrug-resistant (MDR) strains being resistant to more than three unrelated antimicrobial agents. However, the most predominant resistance phenotype (n = 19) was CAZ, CRX, GEN, CXM, OFL, AUG, and CPR. Furthermore, overexpression of efflux pump activity known to potentiate MDR phenotype was found in ETEC and EPEC isolates, as presented in Table 8.
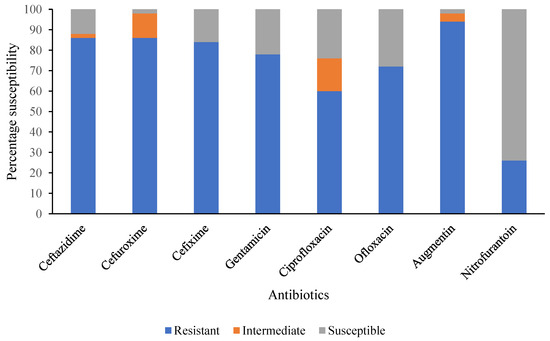
Figure 1.
Summary of antimicrobial susceptibility pattern of selected DEC isolates from diarrheic children under 5 years in Ogun State, Nigeria.

Table 8.
Antimicrobial and multidrug resistance (MDR) profile of selected DEC isolated from diarrheic children under 5 years in Ogun State, Nigeria.
4. Discussion
Diarrhea of bacterial etiology is often self-limiting, requiring no prior identification for patient treatment. However, in cases of prolonged or severe diarrhea (indicative of invasive infection), the identification of etiological agents becomes necessary for effective treatment [28]. The availability of surveillance and other epidemiological data is vital in the empirical management of and policy-making on preventing diarrheal diseases, including those of DEC etiology [29,30]. Regrettably, there is a paucity of such data in resource-limited countries like Nigeria due to the lack of sufficient functional laboratories and the limitations of currently used diagnostic techniques in routine laboratories. In Nigeria, data on the molecular epidemiology of DEC pathotypes in children under five years and their exposure environment is limited.
The current study revealed the relative prevalence and risk factors of hetero-pathogenic and diarrheagenic E. coli isolated from children under 5 years presented with diarrhea in hospitals situated in Ogun state. Outcomes from this study showed that DEC pathotypes were detectable in approximately 25.9% of the total diarrhea cases. This prevalence is within the prevalence range of DEC pathotypes earlier reported in Nigeria (18.4–73.8%) [7,17,31,32,33,34] and in Africa (7.4–86.5%) [35,36,37,38,39]. The observed variations among studies in DEC prevalence may be associated with differences in demography, geography, study population, and reservoir of DEC pathotypes. Still, the occurrence of DEC pathotypes is of clinical importance and underscores its significance as a major cause of infectious diarrhea in children in Ogun State, Nigeria.
Most significant and of great concern in the current study is the high prevalence of ETEC pathotype (21.05%), which was higher than previously reported cases (2.4–18.0%) in other regions in Nigeria [7,17,31,32,33,34]. Enterotoxigenic E. coli is known to harbor the heat-stable enterotoxin A (estA), which is further classified into two subtypes, known as estA-p and estA-h, because they were initially isolated from strains of pigs and human origin, respectively [40]. Heat-stable toxins producing ETEC isolated in this study are of public health significance and are commonly associated with severe diarrhea among children in low-income countries [41,42,43]. Interestingly, the high prevalence of estA-p and estA-h in the studied population and in fresh produce suggests environmental contamination with the possibility of a vibrant non-human reservoir of infection, which constitutes an exposure source for children in these communities.
The presence of the eae (E. coli attachment effacement) gene within the studied population is indicative of EPEC [44]. The eae gene encodes an outer membrane protein, intimin, which mediates adherence of these pathotypes to enterocytes, thereby starting the effacement of gastric epithelial–cell microvilli [44,45,46]. EPEC has been implicated as a leading cause of infantile diarrhea in developing countries [6,47]. The occurrence of EPEC pathotype in Nigeria is not unusual; however, the prevalence in this study (0.44%) was lower than that previously reported (1.8–15.0%) in other parts of the country [7,17,31,32,33,34]. Age category, study location, and study period may contribute to this observed discrepancy. Humans and animals are reservoirs for EPEC [6]. So, the absence of potential risk factors (such as the practice of open defecation) that could mediate transmission from the human reservoir may also be a possible reason for a lower prevalence.
Hetero- or hybrid pathotypes are terms used to describe new combinations of virulence factors among DEC [13]. In 2015, an EPEC expressing the ETEC heat-labile toxin was observed in India [15]. The same combinations in the present study were reported in a study done in South Africa [48]. Approximately 55% (6/11) of the ETEC/EPEC hybrid strains in the present study were detected in diarrheic children. In addition, the relative prevalence of EPEC/ETEC hetero-pathogenic pathotype was similar to that reported previously in the northern part of Nigeria [7,15,33]. The vtx1 and vtx2 genes occurred as VTEC/ETEC hetero-pathotypes with a prevalence of 1.3%, which was lower than a single VTEC pathotype earlier reported in other studies [17,31,34,49]. The presence of estA/vtx1/vtx2 producing hetero-pathogenic VTEC/ETEC pathotype could increase the progression of clinical manifestations such as hemolytic uremic syndrome (HUS), thrombotic thrombocytopenic purpura, and hemorrhagic colitis [50,51,52] that are commonly associated with single pathotype infection. Likewise, the detection of the ipaH/estA gene indicative of the EIEC/ETEC hetero-pathotype with a prevalence of 0.44% was reported in this study. Previous studies have implicated a single EIEC pathotype as the etiology of different outbreaks of bacillary dysentery across the globe [45,53,54]; however, there is a paucity of scientific data on the prevalence or detection of EIEC/ETEC hetero-pathotype from human cases. The low prevalence of hetero-pathotype suggests a possible low but emerging epidemiological pattern in Nigeria. The genomic plasticity of E. coli allows for the emergence of new hybrid strains, which could result in severe outbreaks, heightened levels of pathogenesis, and immune evasion [7,13,55,56,57,58,59,60,61]. Heteropathotype infection could also increase the transmission of resistant genes, which may complicate the available treatment options for DEC infections.
In the food samples, the wide distribution of various hetero-pathogenic and DEC pathotypes in the fresh produce samples suggests these food types are an important source of DEC infection in the studied population. This is anticipated because fresh produce is often served raw to children as a form of nutritional supplement and undergoes minimal processing. Moreover, fresh produce is often grown with organic manure laden with a high number of microorganisms [62,63,64,65,66]. The presence of DEC pathotypes in drinking water and leftover food samples further implicates these sources as being probable sources of infection. Conversely, only ETEC was found in the wastewater samples, indicating this as a likely reservoir.
Generally, episodes of diarrhea are often accompanied by clinical manifestations such as watery diarrhea, vomiting, fever, loss of appetite as well as abdominal pain [67]. In this study, diarrheic children with associated DEC pathotypes showed these clinical manifestations. These symptoms were not related to specific DEC pathotypes, which is consistent with other studies describing that infections with any DEC pathotypes are indistinguishable based on clinical manifestations [30,68]. The age distribution analysis of the DEC pathotype in the current research agrees with previous findings that the incidence of DEC reduces with an increase in age [7,33]. The UNICEF/WHO encourages exclusive breastfeeding for the first 6 months of a child’s life [69] as a possible means of reducing the incidence of diarrhea. Despite this advocacy, DEC was still detectable among children who were exclusively breastfed. In addition, the incidence of DEC among the other feeding type sub-groups is indicative of a hygiene-related problem. Analysis of the associated risk factors of DEC infection revealed that diarrheic children exposed to street-vended foods were more likely to be infected with DEC infection than children fed homemade foods. This agrees with the findings of Estrada-Garcia et al. [70], Islam et al. [71], and Ugboko et al. [72] that DEC is more often associated with street-vended foods in developing countries. A possible explanation for this might be the prolonged exposure of these foods to open air and the likelihood of inadequate quality assurance measures in the production process. Such findings call for extra caution on the part of parents and caregivers to selectively buy ready-to-eat foods from vendors with good hygiene. The occupational status of caregivers does not correlate with the frequency of DEC infections. Hence, consumption of street-vended foods and contaminated fresh produce could be the drivers of DEC infection in children in Ogun State, Nigeria. These results, notwithstanding, underscore the significance of non-human reservoirs as sources of infection, as other studies had previously reported the presence of DEC in water and other non-human reservoirs within the state [73,74,75,76]. Provision of a potable water supply is still recommended, and in cases when such is not available, the boiling of drinking water is not negotiable in the prevention of diarrhea due to DEC.
Accessibility, availability, and timeliness of interventions such as supportive therapy, anti-secretory drug therapy, and specific antimicrobial chemotherapy are critical to reducing diarrhea-associated morbidity and mortality, especially in children under 5 years found in rural and resource-limited populations [74]. Administration of ORS and zinc is considered the first line of treatment in the management of diarrhea infection in children under 5 years [3]. In the current study, ORS was only administered in 50.9% of all the DEC-associated diarrhea cases. This implies that a good number of the caregivers knew what actions to perform during episodes of diarrhea. However, there is a need to create more awareness of the use of ORS in the management of diarrhea, especially in rural communities.
DEC infections are often self-limiting, requiring no interventions [6,7]. Conversely, infections with ETEC, EPEC, and EIEC pathotypes can result in persistent, severe, and invasive diseases that may require the use of antibiotics for effective treatment [6]. Antibiotics such as Cephalosporin, Fluoroquinolones, Penicillin (Augmentin), and sulfonamides are among the commonly used antibiotics for the management of bacterial diarrhea in Nigeria. In recent years, the alarming rising incidence of MDR DEC, especially extended-spectrum β-lactamase (ESBL) and carbapenemase-producing strains is of global public health significance [6]. The inappropriate usage and lack of antibiotic stewardship in the management of diarrheal diseases in children is a problem in Nigeria [77]. In the present study, representatives of the isolated DEC pathotypes subjected to antimicrobial susceptibility assay showed different levels of susceptibility and resistance to all the tested antibiotics, with Cephalosporin and Augmentin having the highest observed resistance. Resistance to the tested antibiotics, as well as the observed multidrug resistance to the Cephalosporin–Aminoglycoside–Penicillin class of antibiotics in this study, is suggestive of extended-spectrum β-lactamase (ESBL) activity, which is an emerging problem in the treatment of DEC infection in children under 5 years in Nigeria [78]. Resistance to some of these commonly used antibiotics was also found in Ethiopia [6], South Africa [79], and Gabon [80]. Investigation into the probable cause of observed multidrug resistance revealed over-expression of efflux pump activity among some of the ETEC and EPEC pathotypes. Efflux pump activity has been shown to potentiate the evolution of antibiotic resistance among bacteria isolates [81].
5. Conclusions
Enterotoxigenic E. coli (ETEC) was found to be the predominant pathotype implicated in cases of childhood diarrhea in the studied population. The detection of DEC in fresh farm produce is a clear indication of food safety risk, especially among children. Consumers of farm products should wash them thoroughly with potable water before consumption to reduce the risk of infection with DEC. Continuous surveillance of DEC pathotypes as part of the routine surveillance program is highly recommended. Further studies to increase our understanding of the implication of DEC hetero-pathotypes on disease progression and severity are recommended. Information from studies of this nature will further enrich empirical treatment, especially in locations where such diagnosis cannot be routinely performed. Antibiotic stewardship should be given priority in LMIC to stem down cases of multidrug resistance. Additionally, the persistence of the DEC pathotype in this study calls for the requirement of re-appraisal of current diarrhea prevention programs available in the country. Continuous education on diarrhea prevention and care for every stakeholder involved in pediatric care is, thereby, recommended.
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens12111358/s1: Figure S1a–e: Agarose gel electrophoresis image of multiplex PCR amplicon of DEC isolates; Figure S2: Growth of presumptive DEC colonies on Sorbitol MacConkey Agar (A-Sorbitol fermenter and B—Non Sorbitol fermenter); Figure S3: Over expression of efflux pump assay for multidrug resistant DEC isolates.
Author Contributions
T.S.O., O.E.F., G.B.A., C.I.A. and T.H. conceived, designed, and developed the methodology of the study. T.S.O., O.E.F. and G.B.A. analyzed and interpreted the data, and T.S.O. drafted the manuscript. O.E.F., G.B.A., C.I.A. and T.H. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of the “FOCAL (Foodborne disease epidemiology, surveillance, and control in African LMIC)” Project, a multi-partner, multi-study research grant co-funded by the Bill & Melinda Gates Foundation and the Foreign, Commonwealth and Development Office (FCDO) of the United Kingdom Government (Grant Agreement Investment ID OPP1195617).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Federal Medical Centre Research Ethics Committee (FMCREC), Idi–Aba, Abeokuta, Ogun State (FMCA/243/HREC/03/2016/14), and Sacred Heart Hospital Ethical Committee (SHH/EC/EA/03/07/20). The aims and objectives of the study were thoroughly explained to the parents/caregivers of the diarrheal child. Each parent decided whether or not to consent to their child’s participation in the study. The parent(s) were also enlightened about their freedom to withdraw from the study at any time without any consequences.
Informed Consent Statement
The aims and objectives of the study were thoroughly explained to the parents/caregivers of the diarrheal child. Each parent decided whether or not to consent to their child’s participation in the study. The parent(s) were also enlightened about their freedom to withdraw from the study at any time without any consequences. Informed consent was obtained from the parents of all diarrheal children. Also, written informed consent has been obtained from the child’s parent to publish this paper.
Data Availability Statement
Data are available on request.
Acknowledgments
We appreciate the funders for providing the required funding for this study. Special thanks to the medical staff in all the health facilities who participated in this study and all the parents/caretakers of children who enrolled in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mokomane, M.; Kasvosve, I.; Melo, E.D.; Pernica, J.M.; Goldfarb, D.M. The global problem of childhood diarrheal diseases: Emerging strategies in prevention and management. Ther. Adv. Infect. Dis. 2018, 5, 29–43. [Google Scholar] [PubMed]
- Olufunke, A.; Peter, A.K.; Akinniyi, A.P. Resistant Plasmid Profile Analysis of Shigella spp Isolated from Stool Samples of School Children from Selected Communities in Odeda Local Government, Ogun State. Int. J. Microbiol. Biotechnol. 2019, 4, 49. [Google Scholar] [CrossRef]
- Peter, A.K.; Umar, U. Combating diarrhea in Nigeria: The way forward. J. Microbiol. Exp. 2018, 6, 191–197. [Google Scholar]
- Kotloff, K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. N. Am. 2017, 64, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Baye, A.; Adane, M.; Sisay, T.; Hailemeskel, H.S. Priorities for intervention to prevent diarrhea among children aged 0–23 months in northeastern Ethiopia: A matched case-control study. BMC Pediatr. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Zelelie, T.Z.; Eguale, T.; Yitayew, B.; Abeje, D.; Alemu, A.; Seman, A.; Jass, J.; Mihret, A.; Abebe, T. Molecular epidemiology and antimicrobial susceptibility of diarrheagenic Escherichia coli isolated from children under age five with and without diarrhea in Central Ethiopia. PLoS ONE 2023, 18, e0288517. [Google Scholar] [CrossRef] [PubMed]
- Saka, H.K.; Dabo, N.T.; Muhammad, B.; García-Soto, S.; Ugarte-Ruiz, M.; Alvarez, J. Diarrheagenic Escherichia coli pathotypes from children younger than 5 years in Kano State, Nigeria. Front. Public Health 2019, 7, 348. [Google Scholar] [CrossRef]
- Pawłowska, B.; Sobieszczańska, B.M. Intestinal epithelial barrier: The target for pathogenic Escherichia coli. Adv. Clin. Exp. Med. 2017, 26, 1437–1445. [Google Scholar] [CrossRef]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Cabrera-Sosa, L.; Ochoa, T.J. Escherichia coli diarrhea. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 481–485. [Google Scholar]
- García, A.; Fox, J.G. A one health perspective for defining and deciphering Escherichia coli pathogenic potential in multiple hosts. Comp. Med. 2021, 71, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.d.M.; Santos, F.F.; Silva, R.M.; Gomes, T.A.T. Diversity of hybrid- and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.; Harvey, E.; Bidol, S.A.; Henderson, T.; Njord, R.; DeSalvo, T.; Haupt, T.; Mba-Jonas, A.; Bailey, C.; Bopp, C.; et al. Outbreak of Escherichia coli O104: H4 infections associated with sprout consumption—Europe and North America, May–July 2011. Morb. Mortal. Wkly. Rep. 2013, 62, 1029. [Google Scholar]
- Dutta, S.; Pazhani, G.P.; Nataro, J.P.; Ramamurthy, T. Heterogenic virulence in a diarrheagenic Escherichia coli: Evidence for an EPEC expressing heat-labile toxin of ETEC. Int. J. Med. Microbiol. 2015, 305, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Santos, A.R.R.; Rocha, L.B.; Caetano, B.A.; Mitsunari, T.; Santos, L.I.; Polatto, J.M.; Horton, D.S.P.Q.; Guth, B.E.C.; dos Santos, L.F.; et al. Development and validation of Shiga toxin-producing Escherichia coli immunodiagnostic assay. Microorganisms 2019, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Onanuga, A.; Igbeneghu, O.; Lamikanra, A. A study of the prevalence of diarrheagenic Escherichia coli in children from Gwagwalada, Federal Capital Territory, Nigeria. Pan Afr. Med. J. 2014, 17, 146. [Google Scholar] [CrossRef]
- Canizalez-Roman, A.; Flores-Villaseñor, H.M.; Gonzalez-Nuñez, E.; Velazquez-Roman, J.; Vidal, J.E.; Muro-Amador, S.; Alapizco-Castro, G.; Díaz-Quiñonez, J.A.; León-Sicairos, N. Surveillance of diarrheagenic Escherichia coli strains isolated from diarrhea cases from children, adults and elderly at Northwest of Mexico. Front. Microbiol. 2016, 7, 1924. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Hou, H.; Lu, Y.; Yu, J.; Mao, L.; Mao, L.; Sun, Z. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: A hospital based study. BMC Infect. Dis. 2018, 18, 63. [Google Scholar] [CrossRef]
- Omolajaiye, S.A.; Afolabi, K.O.; Iweriebor, B.C. Pathotyping and antibiotic resistance profiling of Escherichia coli isolates from children with acute diarrhea in amatole district municipality of Eastern Cape, South Africa. BioMed Res. Int. 2020, 2020, 4250165. [Google Scholar] [CrossRef]
- Hazen, T.H.; Michalski, J.; Luo, Q.; Shetty, A.C.; Daugherty, S.C.; Fleckenstein, J.M.; Rasko, D.A. Comparative genomics and transcriptomics of Escherichia coli isolates carrying virulence factors of both enteropathogenic and enterotoxigenic E. coli. Sci. Rep. 2017, 7, 3513. [Google Scholar] [CrossRef]
- Choo, E.; Jang, S.S.; Kim, K.; Lee, K.-G.; Heu, S.; Ryu, S. Prevalence and genetic diversity of Bacillus cereus in dried red pepper in Korea. J. Food Prot. 2007, 70, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Olsen, K.E.; Scheutz, F.; Krogfelt, K.A.; Gerner-Smidt, P. A method for fast and simple detection of major diarrheagenic Escherichia coli in the routine diagnostic laboratory. Clin. Microbiol. Infect. 2007, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, CLSI M100, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; McCusker, M.P.; Viveiros, M.; Couto, I.; Fanning, S.; Pagès, J.-M.; Amaral, L. A simple method for assessment of MDR bacteria for over-expressed efflux pumps. Open Microbiol. J. 2013, 7, 72–82. [Google Scholar] [CrossRef]
- Humphries, R.M.; Linscottb, A.J. Practical Guidance for Clinical Microbiology Laboratories: Diagnosis of Bacterial Gastroenteritis. Clin. Microbiol. Rev. 2015, 28, 3–31. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Operario, D.J.; Houpt, E.R. Molecular Diagnosis of Diarrhea: Current Status and Future Potential. Curr. Infect. Dis. Rep. 2011, 14, 41–46. [Google Scholar] [CrossRef]
- Miliwebsky, E.; Schelotto, F.; Varela, G.; Luz, D.; Chinen, I.; Piazza, R.M.F. Human diarrheal infections: Diagnosis of diarrheagenic Escherichia coli pathotypes. In Escherichia coli in the Americas; Torres, A.G., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 343–369. [Google Scholar] [CrossRef]
- Okeke, I.N.; Lamikanra, A.; Steinrück, H.; Kaper, J.B. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 2000, 38, 7–12. [Google Scholar] [CrossRef]
- Nweze, E.I. Aetiology of diarrhea and virulence properties of diarrheagenic Escherichia coli among patients and healthy subjects in southeast Nigeria. J. Health Popul. Nutr. 2010, 28, 245. [Google Scholar] [CrossRef][Green Version]
- Ifeanyi, C.I.C.; Ikeneche, N.F.; Bassey, B.E.; Al-Gallas, N.; Ben Aissa, R.; Boudabous, A. Diarrheagenic Escherichia coli pathotypes isolated from children with diarrhea in the Federal Capital Territory Abuja, Nigeria. J. Infect. Dev. Ctries. 2015, 9, 165–174. [Google Scholar] [CrossRef][Green Version]
- Odetoyin, B.W.; Hofmann, J.; Aboderin, A.O.; Okeke, I.N. Diarrheagenic Escherichia coli in mother-child pairs in ile-ife, Southwestern Nigeria. BMC Infect. Dis. 2015, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Moyo, S.J.; Maselle, S.Y.; I Matee, M.; Langeland, N.; Mylvaganam, H. Identification of diarrheagenic Escherichia coli isolated from infants and children in Dar es Salaam, Tanzania. BMC Infect. Dis. 2007, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Oundo, J.O.; Hibino, T.; Saidi, S.M.; Hinenoya, A.; Osawa, K.; Shirakawa, T.; Osawa, R.; Yamasaki, S. High prevalence of diarrheagenic Escherichia coli among children with diarrhea in Kenya. Jpn. J. Infect. Dis. 2017, 70, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Kathiiko, C.; Wada, A.; Odoyo, E.; Bundi, M.; Miringu, G.; Guyo, S.; Karama, M.; Ichinose, Y. Prevalence, seasonal variation, and antibiotic resistance pattern of enteric bacterial pathogens among hospitalized diarrheic children in suburban regions of central Kenya. Trop. Med. Health 2016, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Konaté, A.; Dembélé, R.; Kagambèga, A.; Soulama, I.; Kaboré, W.A.D.; Sampo, E.; Cissé, H.; Sanou, A.; Serme, S.; Zongo, S.; et al. Molecular characterization of diarrheagenic Escherichia coli in children less than 5 years of age with diarrhea in Ouagadougou, Burkina Faso. Eur. J. Microbiol. Immunol. 2017, 7, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.M.; Foster, M.A.; Iqbal, J.; Fonnesbeck, C.; Payne, D.C.; Zhang, C.; Chappell, J.D.; Halasa, N.; Gómez-Duarte, O.G. Diarrheagenic Escherichia coli and acute gastroenteritis in children in Davidson County, Tennessee, United States: A Case-control Study. Pediatr. Infect. Dis. J. 2018, 37, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Hartadi, E.B.; Effendi, M.H.; Plumeriastuti, H.; Sofiana, E.D.; Wibisono, F.M.; Hidayatullah, A.R. A review of enterotoxigenic Escherichia coli infection in piglets: Public health importance. Syst. Rev. Pharm. 2020, 11, 687–698. [Google Scholar]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Turunen, K.; Antikainen, J.; Lääveri, T.; Kirveskari, J.; Svennerholm, A.-M.; Kantele, A. Clinical aspects of heat-labile and heat-stable toxin-producing enterotoxigenic Escherichia coli: A prospective study among Finnish travellers. Travel Med. Infect. Dis. 2020, 38, 101855. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Luo, Q.; Davis, S.M.; Westra, C.; Vickers, T.J.; Fleckenstein, J.M. Molecular determinants of enterotoxigenic Escherichia coli heat-stable toxin secretion and delivery. Infect. Immun. 2018, 86, e00526-18. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Fröhlicher, E.; Krause, G.; Zweifel, C.; Beutin, L.; Stephan, R. Characterization of attaching and effacing Escherichia coli (AEEC) isolated from pigs and sheep. BMC Microbiol. 2008, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.A.; Yamamoto, D.; Vieira, M.A.; Hernandes, R.T. Atypical enteropathogenic Escherichia coli. In Escherichia coli in the Americas; Springer: Cham, Switzerland, 2016; pp. 77–96. [Google Scholar]
- Trabulsi, L.R.; Keller, R.; Gomes, T.A.T. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 2002, 8, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Bolukaoto, J.Y.; Singh, A.; Alfinete, N.; Barnard, T.G. Occurrence of Hybrid Diarrhoeagenic Escherichia coli Associated with Multidrug Resistance in Environmental Water, Johannesburg, South Africa. Microorganisms 2021, 9, 2163. [Google Scholar] [CrossRef]
- Enyi-Idoh, K.H.; Akwa, O.A.; Bassey, I.U.; Idim, V.D.; Egeonu, S.U. Prevalence and Antibiotic Susceptibility Patterns of Escherichia coli O157: H7 in Children 0–24 Months in Calabar South LGA of Cross River State, Nigeria. Prevalence 2017, 22, 1–10. [Google Scholar] [CrossRef]
- Orth, D.; Würzner, R. What Makes an Enterohemorrhagic Escherichia coli? Clin. Infect. Dis. 2006, 43, 1168–1169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, Y.; Sperandio, V. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front. Cell. Infect. Microbiol. 2012, 2, 90. [Google Scholar] [CrossRef]
- Spickler, A.R. Enterohemorrhagic Escherichia coli Infections. 2016. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php (accessed on 26 September 2022).
- Michelacci, V.; Prosseda, G.; Maugliani, A.; Tozzoli, R.; Sanchez, S.; Herrera-León, S.; Dallman, T.; Jenkins, C.; Caprioli, A.; Morabito, S. Characterization of an emergent clone of enteroinvasive Escherichia coli circulating in Europe. Clin. Microbiol. Infect. 2015, 22, 278.e11–287.e19. [Google Scholar] [CrossRef]
- Newitt, S.; MacGregor, V.; Robbins, V.; Bayliss, L.; Chattaway, M.A.; Dallman, T.; Ready, D.; Aird, H.; Puleston, R.; Hawker, J. Two linked enteroinvasive Escherichia coli outbreaks, Nottingham, UK, June 2014. Emerg. Infect. Dis. 2016, 22, 1178. [Google Scholar] [CrossRef]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef]
- Bratoeva, M.P.; Wolf, M.K.; Marks, J.K.; Cantey, J.R. A case of diarrhea, bacteremia, and fever caused by a novel strain of Escherichia coli. J. Clin. Microbiol. 1994, 32, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Garcia, F. Escherichia coli O104: H4 pathogenesis: An enteroaggregative E. coli/Shiga toxin-producing E. coli explosive cocktail of high virulence. In Enterohemorrhagic Escherichia coli and Other Shiga Toxin-Producing E. coli; American Society for Microbiology: Washington, DC, USA, 2015; pp. 503–529. [Google Scholar]
- Kessler, R.; Nisa, S.; Hazen, T.H.; Horneman, A.; Amoroso, A.; Rasko, D.A.; Donnenberg, M.S. Diarrhea, bacteremia and multiorgan dysfunction due to an extraintestinal pathogenic Escherichia coli strain with enteropathogenic E. coli genes. Pathog. Dis. 2015, 73, ftv076. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.W.; Bouts, A.H.; Rossen, J.W.; Van der Kuip, M.; Van Heerde, M.; Bökenkamp, A. Diarrhea, urosepsis and hemolytic uremic syndrome caused by the same heteropathogenic Escherichia coli strain. Pediatr. Infect. Dis. J. 2016, 35, 1045–1047. [Google Scholar] [CrossRef]
- Soysal, N.; Mariani-Kurkdjian, P.; Smail, Y.; Liguori, S.; Gouali, M.; Loukiadis, E.; Fach, P.; Bruyand, M.; Blanco, J.; Bidet, P.; et al. Enterohemorrhagic Escherichia coli hybrid pathotype O80:H2 as a new therapeutic challenge. Emerg. Infect. Dis. 2016, 22, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, K.L.; Schijvens, A.M.; Rossen, J.W.A.; Kooistra-Smid, A.M.D.; Schreuder, M.F.; van de Kar, N.C.A.J. Unusual severe case of hemolytic uremic syndrome due to Shiga toxin 2d-producing E. coli O80:H2. Pediatr. Nephrol. 2017, 32, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Reuben, C.R.; Makut, M.D. Occurrence of Escherichia coli O157: H7 in vegetables grown and sold in Lafia metropolis, Nigeria. World J. Microbiol. 2014, 1, 017–021. [Google Scholar]
- Moses, A.E.; James, R.A.; Ekanem, U.S. Prevalence of Escherichia coli O157 in fruits, vegetables and animal feacal waste used as manure in farms of some communities of Akwa Ibom State-Nigeria. Central Afr. J. Public Health 2016, 1, 22–27. [Google Scholar]
- Maikai, B.; Akubo, D. Coliform count and isolation of Escherichia coli in fresh fruits and vegetables sold at retail outlets in Samaru, Kaduna State, Nigeria. Niger. Veter-J. 2019, 39, 327. [Google Scholar] [CrossRef]
- Ehim, N.H.; Muk, M.F.; Salisu, N. Prevalence of bacterial loads on some fruits and vegetables sold in kaduna central market, Northwestern Nigeria. J. Appl. Sci. 2018, 19, 20–24. [Google Scholar] [CrossRef]
- Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in today’s world. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [CrossRef]
- A Shetty, V.; Kumar, S.H.; Shetty, A.K.; Karunasagar, I.; Karunasagar, I. Prevalence and characterization of diarrheagenic Escherichia coli isolated from adults and children in Mangalore, India. J. Lab. Physicians 2012, 4, 024–029. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, S.; Reyes, D.; Paniagua, M.; Bucardo, F.; Möllby, R.; Weintraub, A. Prevalence of diarrheagenic Escherichia coli in children from Leon, Nicaragua. J. Med. Microbiol. 2009, 58, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Garcia, T.; Cerna, J.F.; Thompson, M.R.; Lopez-Saucedo, C. Faecal contamination and enterotoxigenic Escherichia coli in street-vended chili sauces in Mexico and its public health relevance. Epidemiology Infect. 2002, 129, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Nasrin, N.; Rizwan, F.; Nahar, L.; Bhowmik, A.; Esha, S.A.; Talukder, K.A.; Akter, M.; Roy, A.; Ahmed, M. Microbial contamination of street vended foods from a university campus in Bangladesh. Southeast Asian J. Trop. Med. Public Health 2015, 46, 480–485. [Google Scholar] [PubMed]
- Ugboko, H.U.; Fatoki, T.H.; Nwinyi, O.C.; Ibraheem, O.; Omonhinmin, C.A.; Fatoki, J.M.; Adetuyi, O.Y. Computational Study of 16S rRNA of Microbe Cluster Implicated in Diarrheal: Phylogeny, Docking, and Dynamics. Res. Sq. 2021, 1–19. [Google Scholar] [CrossRef]
- Adenodi, S.; Oyejide, N.; Fayemi, S.; Ayoade, F. Prevalence of Antibiotic-Resistant Strains of Escherichia coli in Drinking Water Samples from Mowe Metropolis, Ogun State, Nigeria. Afr. J. Clin. Exp. Microbiol. 2014, 15, 69. [Google Scholar] [CrossRef]
- Ivbade, A.; Ojo, O.E.; Dipeolu, M.A. Shiga toxin-producing Escherichia coli O157:H7 in milk and milk products in Ogun State, Nigeria. Vet. Ital. 2014, 50, 185–191. [Google Scholar] [CrossRef]
- Odumosu, B.; Akintimehin, A. Occurrence of extended-spectrum beta-lactamase producing Enterobacteriaceae isolates in communal water sources in Ogun State, Nigeria. Afr. J. Clin. Exp. Microbiol. 2014, 16, 28. [Google Scholar] [CrossRef]
- Akpan, S.N.; Odeniyi, O.A.; Adebowale, O.O.; Alarape, S.A.; Adeyemo, O.K. Antibiotic resistance profile of Gram-negative bacteria isolated from Lafenwa abattoir effluent and its receiving water (Ogun River) in Abeokuta, Ogun state, Nigeria. Onderstepoort J. Veter-Res. 2020, 87, 8. [Google Scholar] [CrossRef]
- Efunshile, A.M.; Ezeanosike, O.; Nwangwu, C.C.; König, B.; Jokelainen, P.; Robertson, L.J. Apparent overuse of antibiotics in the management of watery diarrhea in children in Abakaliki, Nigeria. BMC Infect. Dis. 2019, 19, 275. [Google Scholar] [CrossRef]
- Ogunsola, F.T.; Jewoola, O.O.; Bode-Sojobi, I.O.; Okonji, P. High carriage rates of extended-spectrum beta-lactamase-producing enterobacteriaceae in children at admission into paediatric wards of a university teaching hospital in Lagos, Nigeria. Niger. Postgrad. Med. J. 2020, 27, 136–142. [Google Scholar] [CrossRef]
- Msolo, L.; Iweriebor, B.C.; I Okoh, A. Antimicrobial Resistance Profiles of Diarrheagenic E. coli (DEC) and Salmonella Species Recovered from Diarrheal Patients in Selected Rural Communities of the Amathole District Municipality, Eastern Cape Province, South Africa. Infect. Drug Resist. 2020, 13, 4615–4626. [Google Scholar] [CrossRef]
- Mabika, R.M.; Liabagui, S.L.O.; Moundounga, H.K.; Mounioko, F.; Souza, A.; Yala, J.F. Molecular Prevalence and Epidemiological Characteristics of Diarrheagenic E. coli in Children under 5 Years Old in the City of Koula-Moutou, East-Central Gabon. Open J. Med. Microbiol. 2021, 11, 157–175. [Google Scholar] [CrossRef]
- Papkou, A.; Hedge, J.; Kapel, N.; Young, B.; MacLean, R.C. Efflux pump activity potentiates the evolution of antibiotic resistance across S. aureus isolates. Nat. Commun. 2020, 11, 3970. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).