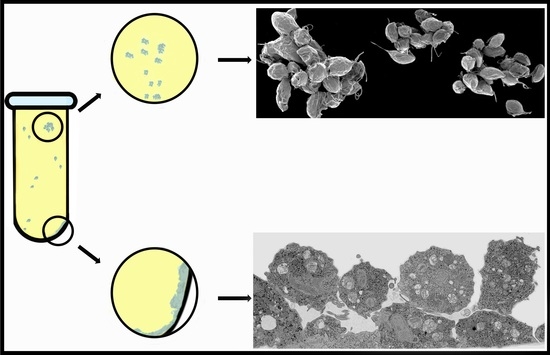
Trichomonas vaginalis: Monolayer and Cluster Formation—Ultrastructural Aspects Using High-Resolution Scanning Electron Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Culture
2.2. Fraction of T. vaginalis Cytoskeleton
2.3. Cluster Formation
2.4. Ruthenium Red
2.5. Thiéry Technique [19]
2.6. Immunolabeling
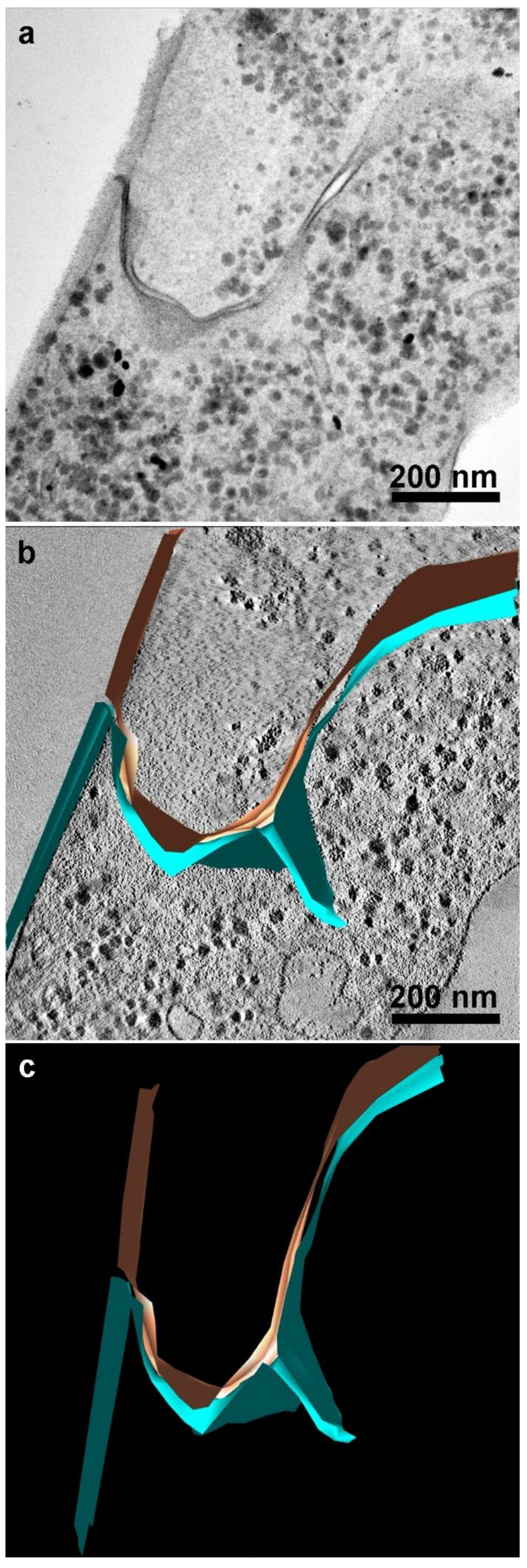
2.7. Electron Microscopy Tomography
2.8. Dye Injection
3. Results
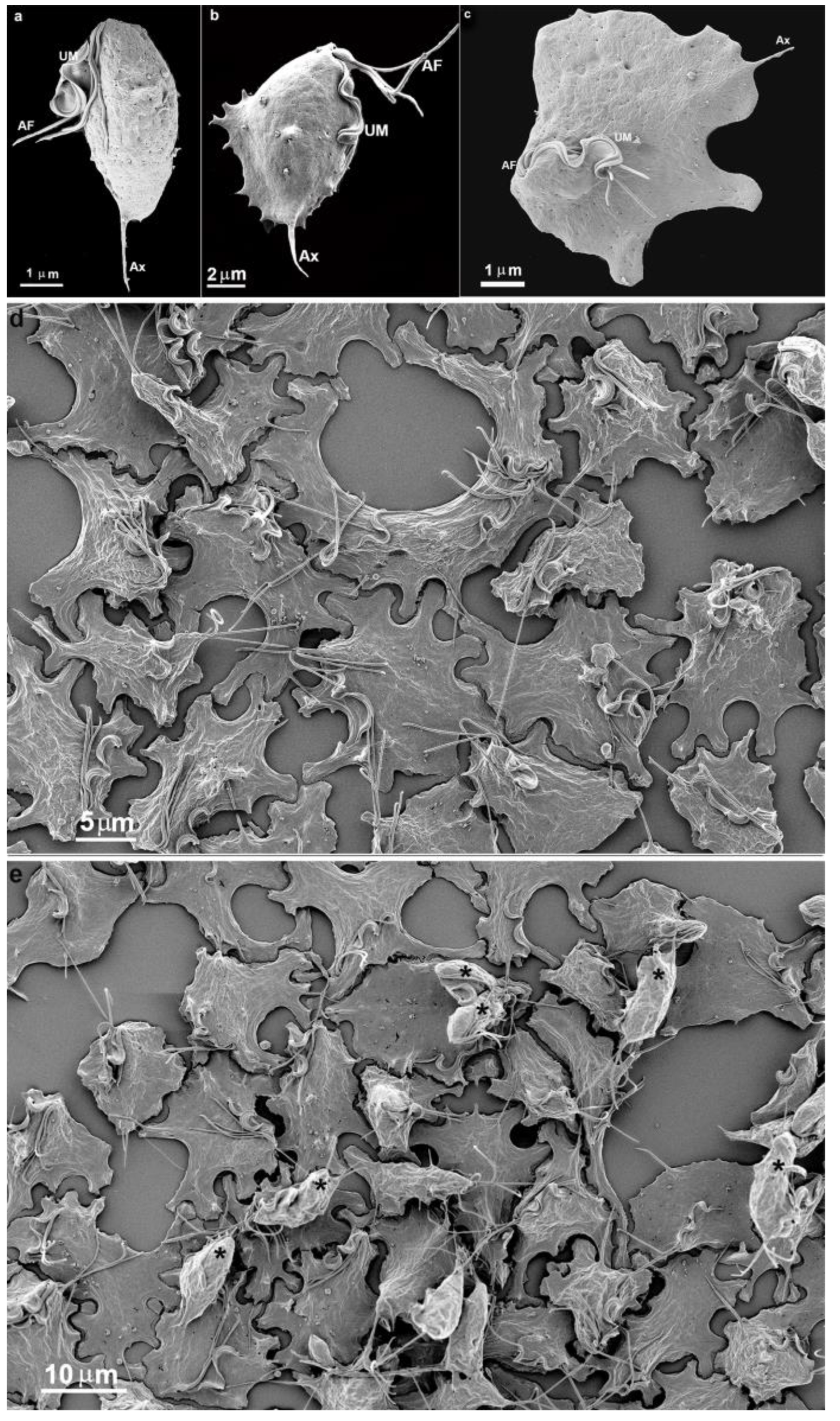
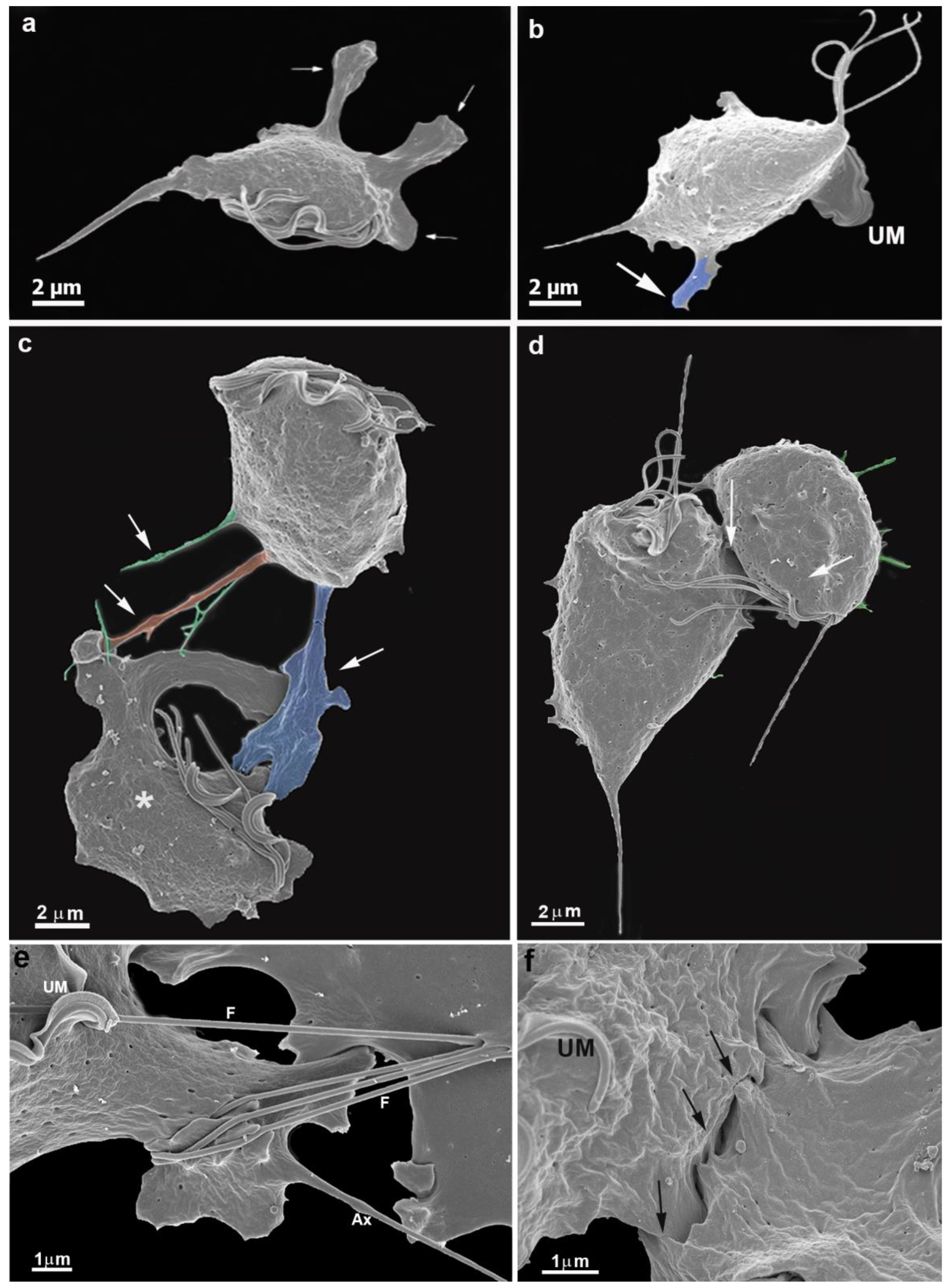
3.1. The First Cell Contacts
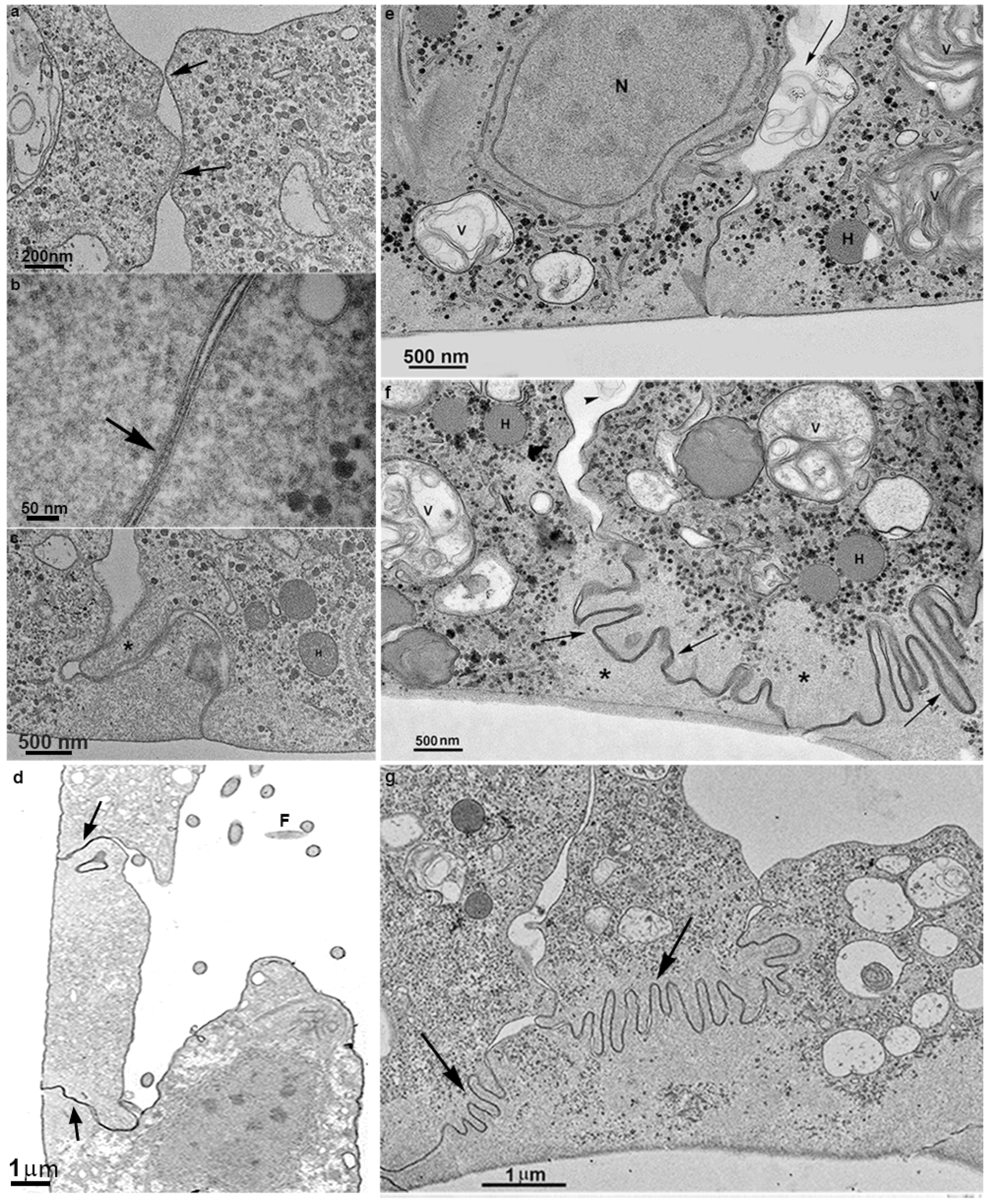
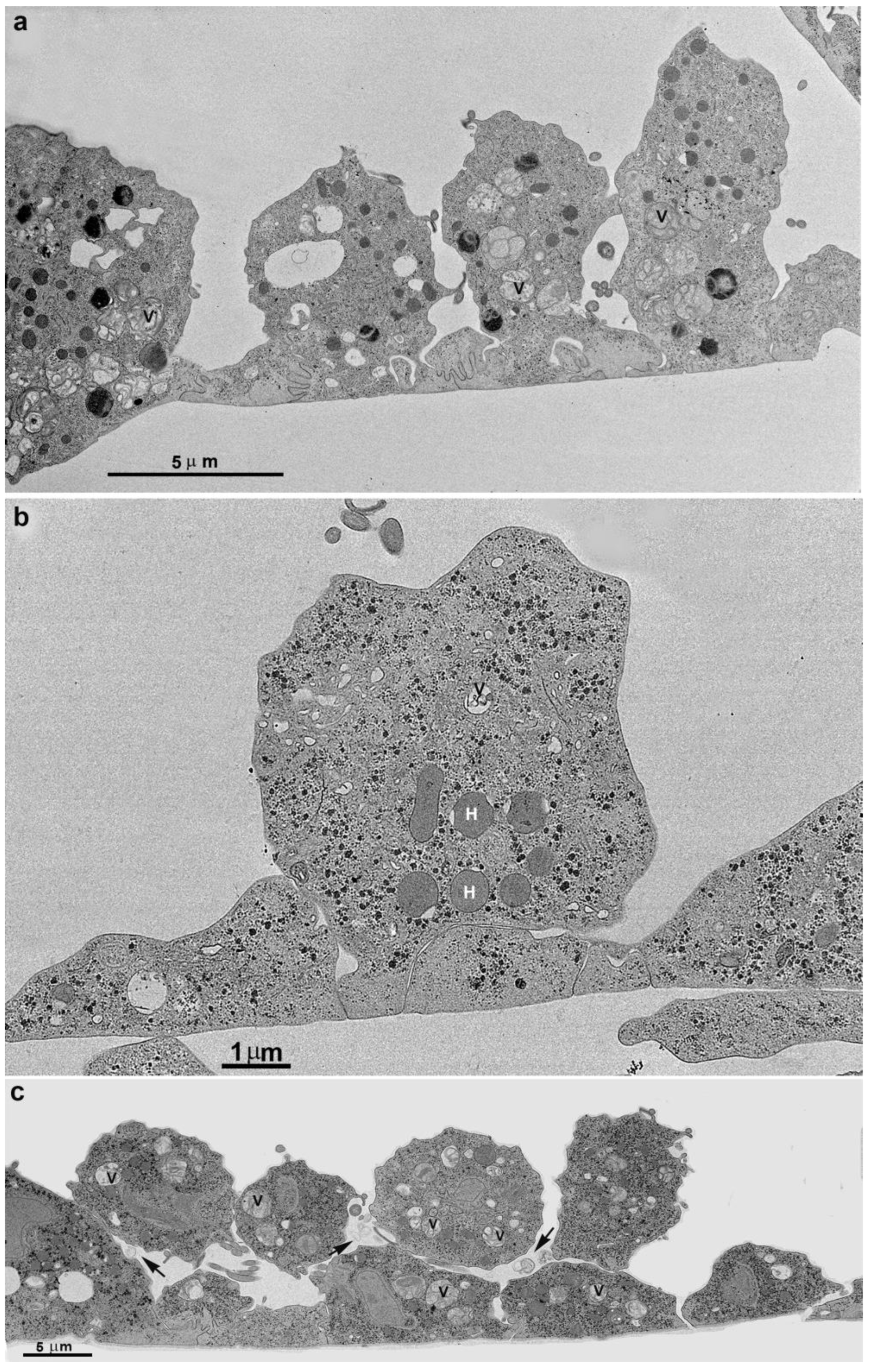
3.2. Monolayer Formation and Cell–Cell Attachment
3.3. Extrusion of T. vaginalis Extracellular Vesicles (TvEVs)
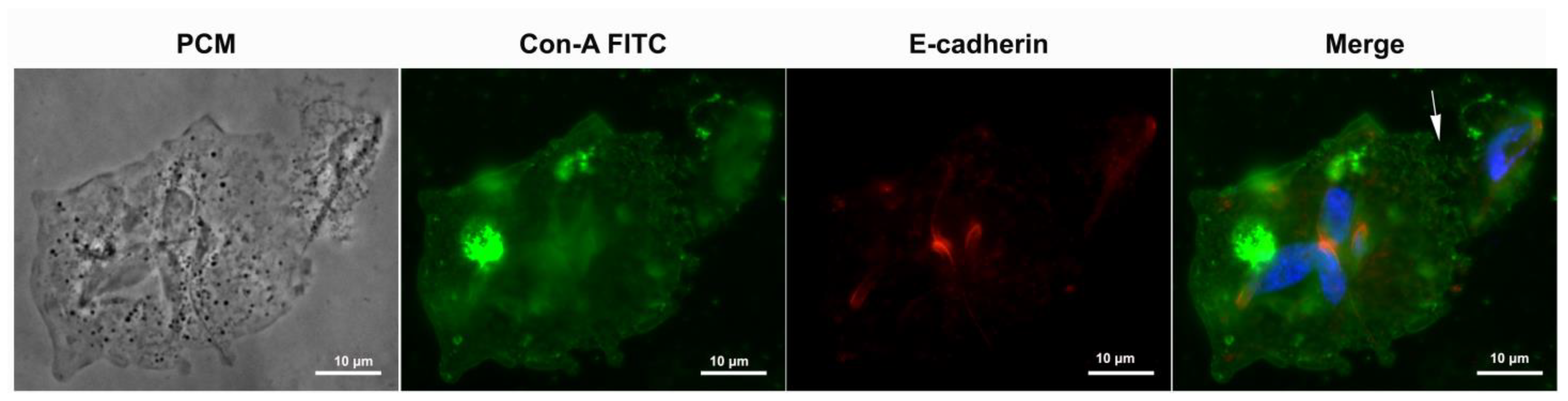
3.4. Proteins of Junctional Areas
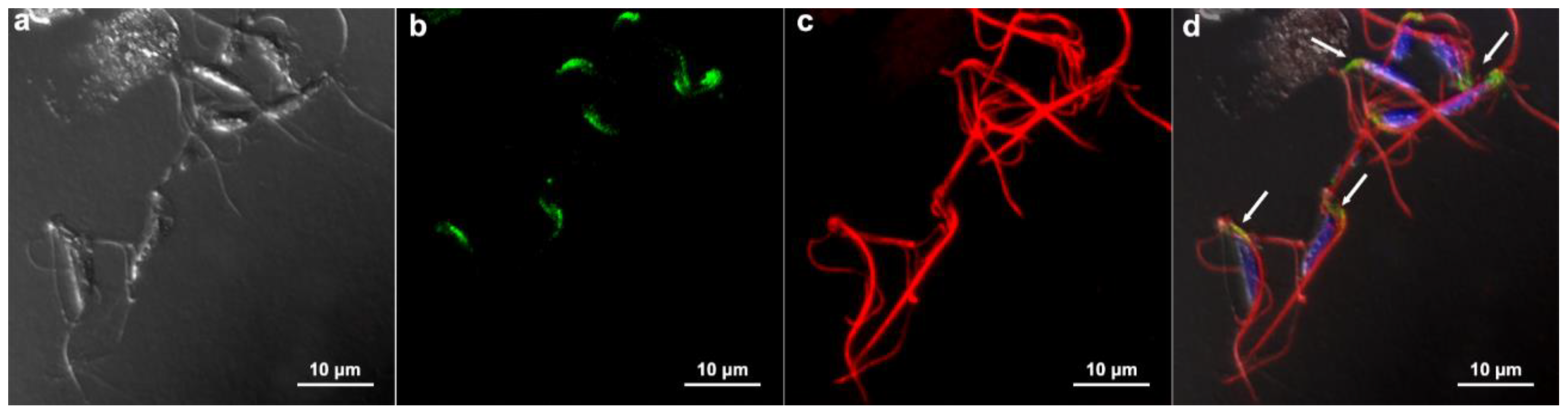
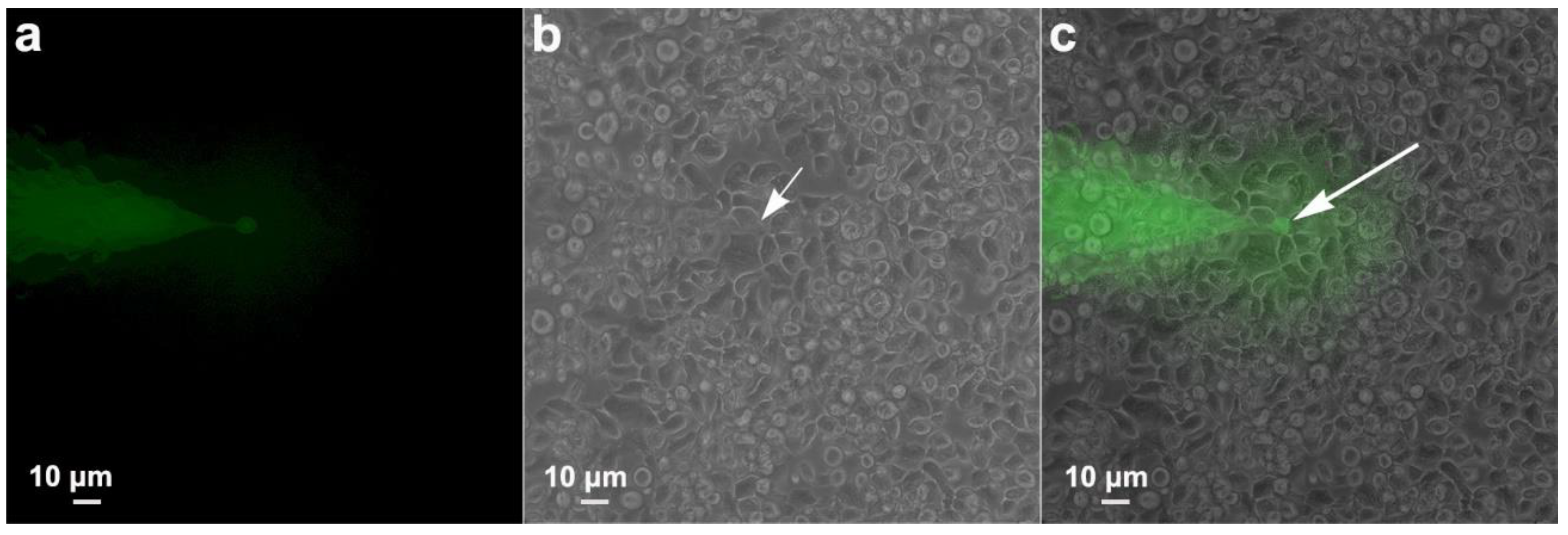
3.5. Communication between Parasites: Injection Tests
4. Cluster Formation
5. Discussion
5.1. Monolayer Formation by T. vaginalis
5.2. Clumping
5.3. E-Cadherin
5.4. Extracellular Vesicles
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, S.; Li, H.; Song, X.; Zhang, H.; Duan, Y.; Luo, C.; Wang, B.; Ji, S.; Xie, Q.; et al. Development of a convenient detection method for Trichomonas vaginalis based on loop-mediated isothermal amplification targeting adhesion protein 65. BMC Infect. Dis. 2020, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Lu, H.; Li, D.; Zhang, R.; Xie, X.; Guo, L.; Hao, L.; Tian, X.; Yang, Z.; et al. A systematic review of the correlation between Trichomonas vaginalis infection and infertility. Acta Trop. 2022, 236, 106693. [Google Scholar] [CrossRef] [PubMed]
- Langston, M.E.; Bhalla, A.; Alderete, J.F.; Nevin, R.L.; Pakpahan, R.; Hansen, J.; Elliott, D.; De Marzo, A.M.; Gaydos, C.A.; Isaacs, W.B.; et al. Trichomonas vaginalis infection and prostate-specific antigen concentration: Insights into prostate involvement and prostate disease risk. Prostate 2019, 79, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Li, Y.; Zhang, R.; Xie, X.; Yao, Y.; Zhao, L.; Tian, X.; Yang, Z.; Wang, S.; et al. The correlation between Trichomonas vaginalis infection and reproductive system cancer: A systematic review and meta-analysis. Infect. Agent Cancer 2023, 18, 15. [Google Scholar] [CrossRef]
- Maritz, J.M.; Land, K.M.; Carlton, J.M.; Hirt, R.P. What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 2014, 30, 333–341. [Google Scholar] [CrossRef] [PubMed]
- McLaren, L.C.; Davis, L.E.; Healy, G.R.; James, C.G. Isolation of Trichomonas vaginalis from the respiratory tract of infants with respiratory disease. Pediatrics 1983, 71, 888–890. [Google Scholar] [CrossRef]
- Masha, S.C.; Cools, P.; Sanders, E.J.; Vaneechoutte, M.; Crucitti, T. Trichomonas vaginalis and HIV infection acquisition: A systematic review and meta-analysis. Sex Transm. Infect. 2019, 95, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, X.; Zhang, Z.; Li, H.; Duan, Y.; Zhang, H.; Lu, H.; Luo, C.; Wang, M. The molecular characterization and immune protection of adhesion protein 65 (AP65) of Trichomonas vaginalis. Microb. Pathog. 2021, 152, 104750. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Deng, Y.; Li, Y.; Li, F.; Sheng, W.; Tian, X.; Yang, Z.; Mei, X.; Wang, S. Trichomonas vaginalis adhesion protein 65 (TvAP65) modulates parasite pathogenicity by interacting with host cell proteins. Acta Tropica 2023, 246, 106996. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Sheng, W.; Song, X.; Li, Y.; Li, F.; Pan, Y.; Tian, X.; Yang, Z.; Wang, S.; et al. The interaction between adhesion protein 33 (TvAP33) and BNIP3 mediates the adhesion and pathogenicity of Trichomonas vaginalis to host cells. Parasit Vectors 2023, 16, 210. [Google Scholar] [CrossRef]
- Lustig, G.; Ryan, C.M.; Secor, W.E.; Johnson, P.J. Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect Immun 2013, 81, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Coceres, V.M.; Iriarte, L.S.; Miranda-Magalhães, A.; de Andrade, T.A.S.; de Miguel, N.; Pereira-Neves, A. Ultrastructural and Functional Analysis of a Novel Extra-Axonemal Structure in Parasitic Trichomonads. Front. Cell. Infect. Microbiol. 2021, 11, 757185. [Google Scholar] [CrossRef] [PubMed]
- Molgora, B.M.; Rai, A.K.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Johnson, P.J. A Novel Trichomonas vaginalis Surface Protein Modulates Parasite Attachment via Protein:Host Cell Proteoglycan Interaction. mBio 2021, 12, e03374-20. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.S. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 1957, 43, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Midlej, V.; Benchimol, M. Trichomonas vaginalis kills and eats--evidence for phagocytic activity as a cytopathic effect. Parasitology 2010, 137, 65–76. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, A.; Lázaro-Haller, A.; Espinosa-Cantellano, M.; Anaya-Velázquez, F.; Martínez-Palomo, A. Trichomonas vaginalis: Ultrastructural bases of the cytopathic effect. J. Eukaryot. Microbiol. 1995, 42, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Pachano, T.; Nievas, Y.R.; Lizarraga, A.; Johnson, P.J.; Strobl-Mazzulla, P.H.; de Miguel, N. Epigenetics regulates transcription and pathogenesis in the parasite Trichomonas vaginalis. Cell. Microbiol. 2017, 19, e12716. [Google Scholar] [CrossRef] [PubMed]
- Coceres, V.M.; Alonso, A.M.; Nievas, Y.R.; Midlej, V.; Frontera, L.; Benchimol, M.; Johnson, P.J.; de Miguel, N. The C-terminal tail of tetraspanin proteins regulates their intracellular distribution in the parasite Trichomonas vaginalis. Cell. Microbiol. 2015, 17, 1217–1229. [Google Scholar] [CrossRef]
- Thiery, J.P. Mise en evidence des polysaccharides sur coupes fines en microscopie electronique. J. Microsc. 1967, 6, 987–1018. [Google Scholar]
- Srinivas, M.; Costa, M.; Gao, Y.; Fort, A.; Fishman, G.I.; Spray, D.C. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J. Physiol. 1999, 517 Pt 3, 673–689. [Google Scholar] [CrossRef]
- Chen, Y.P.; Riestra, A.M.; Rai, A.K.; Johnson, P.J. A Novel Cadherin-like Protein Mediates Adherence to and Killing of Host Cells by the Parasite Trichomonas vaginalis. mBio 2019, 10, e00720-19. [Google Scholar] [CrossRef]
- Ryan, C.M.; de Miguel, N.; Johnson, P.J. Trichomonas vaginalis: Current understanding of host-parasite interactions. Essays Biochem. 2011, 51, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Nievas, Y.R.; Lizarraga, A.; Salas, N.; Cóceres, V.M.; de Miguel, N. Extracellular vesicles released by anaerobic protozoan parasites: Current situation. Cell. Microbiol. 2020, 22, e13257. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.; Ofek, I. Adhesion of Trichomonas vaginalis to plastic surfaces: Requirement for energy and serum constituents. Parasitology 1992, 105 Pt 1, 55–62. [Google Scholar] [CrossRef]
- Silva Filho, F.C.; Elias, C.A.; De Souza, W. Role of divalent cations, pH, cytoskeleton components and surface charge on the adhesion of Trichomonas vaginalis to a polystyrene substrate. Mem. Inst. Oswaldo Cruz. 1987, 82, 379–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brugerolle, G.; Bricheux, G.; Coffe, G. Actin cytoskeleton demonstration in Trichomonas vaginalis and in other trichomonads. Biol. Cell 1996, 88, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Corcuera, F.D.; Okumura, C.Y.; Colocoussi, A.; Johnson, P.J. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell 2005, 4, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.M.; Yang, Y.Y.; Huang, Y.H.; Chu, C.H.; Tu, T.J.; Wu, Y.T.; Chiang, C.J.; Yang, S.B.; Hsu, D.K.; Liu, F.T.; et al. Distinct features of the host-parasite interactions between nonadherent and adherent Trichomonas vaginalis isolates. PLoS Negl. Trop. Dis. 2023, 17, e0011016. [Google Scholar] [CrossRef]
- Dos Santos, O.; Rigo, G.V.; Macedo, A.J.; Tasca, T. Trichomonas vaginalis clinical isolates: Cytoadherence and adherence to polystyrene, intrauterine device, and vaginal ring. Parasitol. Res. 2017, 116, 3275–3284. [Google Scholar] [CrossRef]
- Weiner, A.; Dahan-Pasternak, N.; Shimoni, E.; Shinder, V.; von Huth, P.; Elbaum, M.; Dzikowski, R. 3D nuclear architecture reveals coupled cell cycle dynamics of chromatin and nuclear pores in the malaria parasite Plasmodium falciparum. Cell. Microbiol. 2011, 13, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Santos, T.C.; de Souza, W.; Attias, M. Dynamics and 3D organization of secretory organelles of Toxoplasma gondii. J. Struct. Biol. 2012, 177, 420–430. [Google Scholar] [CrossRef]
- Medeiros, L.C.; De Souza, W.; Jiao, C.; Barrabin, H.; Miranda, K. Visualizing the 3D architecture of multiple erythrocytes infected with Plasmodium at nanoscale by focused ion beam-scanning electron microscopy. PLoS ONE 2012, 7, e33445. [Google Scholar] [CrossRef]
- Lee, K.E.; Kim, J.H.; Jung, M.K.; Arii, T.; Ryu, J.S.; Han, S.S. Three-dimensional structure of the cytoskeleton in Trichomonas vaginalis revealed new features. J. Electron. Microsc. 2009, 58, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Salas, N.; Pedreros, M.B.; Melo, T.d.S.; Maguire, V.G.; Sha, J.; A Wohlschlegel, J.; Pereira-Neves, A.; de Miguel, N. Role of cytoneme structures and extracellular vesicles in Trichomonas vaginalis parasite-parasite communication. eLife 2023, 12, e86067. [Google Scholar] [CrossRef]
- Nievas, Y.R.; Vashisht, A.A.; Corvi, M.M.; Metz, S.; Johnson, P.J.; Wohlschlegel, J.A.; de Miguel, N. Protein Palmitoylation Plays an Important Role in Trichomonas vaginalis Adherence. Mol. Cell Proteomics 2018, 17, 2229–2241. [Google Scholar] [CrossRef]
- Aquino, M.F.K.; Hinderfeld, A.S.; Simoes-Barbosa, A. Trichomonas vaginalis. Trends Parasitol. 2020, 36, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Kusdian, G.; Woehle, C.; Martin, W.F.; Gould, S.B. The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell. Microbiol. 2013, 15, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Okumura, C.Y.; Baum, L.G.; Johnson, P.J. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol 2008, 10, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V.; Baines, A.J. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001, 81, 1353–1392. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.R.; Mohler, P.J. Ankyrin protein networks in membrane formation and stabilization. J. Cell Mol. Med. 2009, 13, 4364–4376. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.H.; Otto, E.; Bennett, V. Specific 33-residue repeat(s) of erythrocyte ankyrin associate with the anion exchanger. J. Biol. Chem. 1991, 266, 11163–11169. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.D.; Hirakawa, M.P.; House, S.A.; Schwartz, C.L.; Pham, J.K.; Cipriano, M.J.; De La Torre, M.J.; Sek, A.C.; Du, G.; Forsythe, B.M.; et al. Novel structural components of the ventral disc and lateral crest in Giardia intestinalis. PLoS Negl. Trop. Dis. 2011, 5, e1442. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. BioRxiv 2022. [Google Scholar] [CrossRef]
- Eisenhaber, B.; Bork, p.; Eisenhaber, F. Sequence properties of GPI-anchored proteins near the omega-site: Constraints for the polypeptide binding site of the putative transamidase. Protein Eng. 1998, 11, 1155–1161. [Google Scholar] [CrossRef]
- Artuyants, A.; Campos, T.L.; Rai, A.K.; Johnson, P.J.; Dauros-Singorenko, P.; Phillips, A.; Simoes-Barbosa, A. Extracellular vesicles produced by the protozoan parasite Trichomonas vaginalis contain a preferential cargo of tRNA-derived small RNAs. Int J. Parasitol. 2020, 50, 1145–1155. [Google Scholar] [CrossRef]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host: Parasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef]
- Olmos-Ortiz, L.M.; Barajas-Mendiola, M.A.; Barrios-Rodiles, M.; Castellano, L.E.; Arias-Negrete, S.; Avila, E.E.; Cuéllar-Mata, P. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. 2017, 39, e12426. [Google Scholar] [CrossRef]
- Rai, A.K.; Johnson, P.J. Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc. Natl. Acad. Sci. USA 2019, 116, 21354–21360. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Molgora, B.M.; Mukherjee, S.K.; Baumel-Alterzon, S.; Santiago, F.M.; Muratore, K.A.; Sisk, A.E., Jr.; Mercer, F.; Johnson, P.J. Trichomonas vaginalis adherence phenotypes and extracellular vesicles impact parasite survival in a novel in vivo model of pathogenesis. PLoS Negl. Trop. Dis. 2023, 17, e0011693. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, S.F.d.N.; Verdan, R.; Fortes, F.d.S.d.A.; Benchimol, M. Trichomonas vaginalis: Monolayer and Cluster Formation—Ultrastructural Aspects Using High-Resolution Scanning Electron Microscopy. Pathogens 2023, 12, 1381. https://doi.org/10.3390/pathogens12121381
Ortiz SFdN, Verdan R, Fortes FdSdA, Benchimol M. Trichomonas vaginalis: Monolayer and Cluster Formation—Ultrastructural Aspects Using High-Resolution Scanning Electron Microscopy. Pathogens. 2023; 12(12):1381. https://doi.org/10.3390/pathogens12121381
Chicago/Turabian StyleOrtiz, Sharmila Fiama das Neves, Raphael Verdan, Fabio da Silva de Azevedo Fortes, and Marlene Benchimol. 2023. "Trichomonas vaginalis: Monolayer and Cluster Formation—Ultrastructural Aspects Using High-Resolution Scanning Electron Microscopy" Pathogens 12, no. 12: 1381. https://doi.org/10.3390/pathogens12121381
APA StyleOrtiz, S. F. d. N., Verdan, R., Fortes, F. d. S. d. A., & Benchimol, M. (2023). Trichomonas vaginalis: Monolayer and Cluster Formation—Ultrastructural Aspects Using High-Resolution Scanning Electron Microscopy. Pathogens, 12(12), 1381. https://doi.org/10.3390/pathogens12121381