Current Understanding of Bacillus Calmette-Guérin-Mediated Trained Immunity and Its Perspectives for Controlling Intracellular Infections
Abstract
:1. Introduction
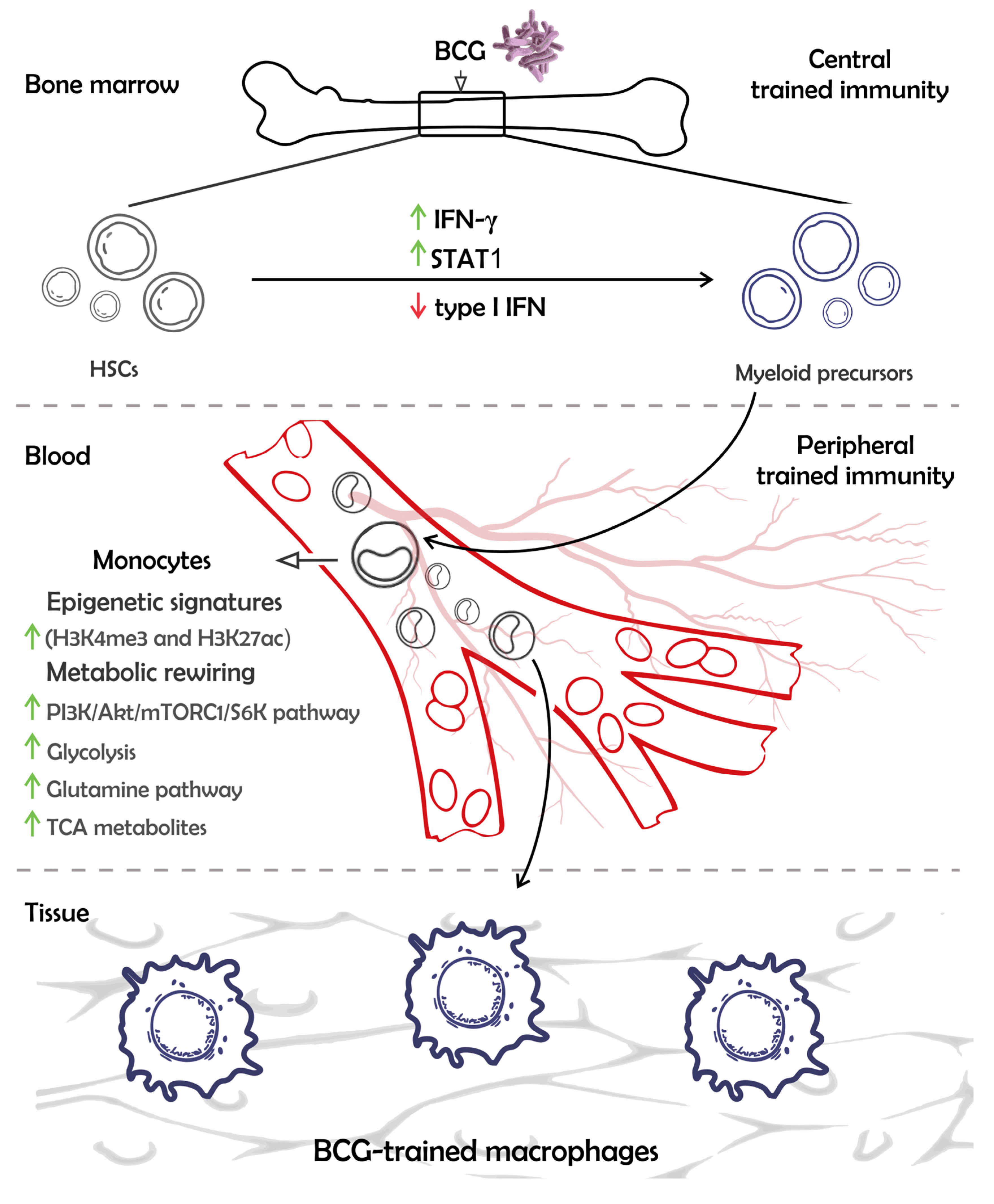
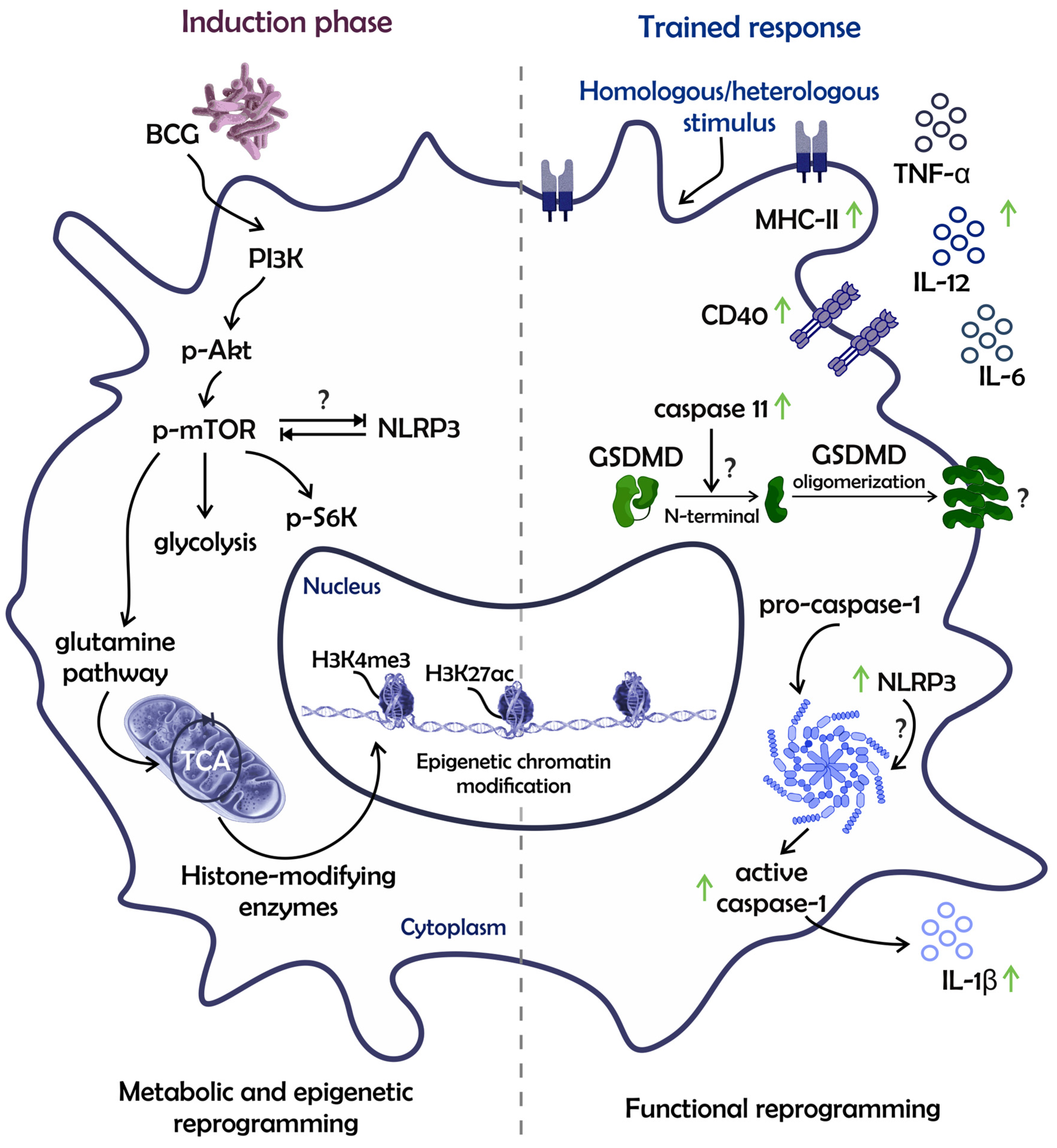
2. The Mechanisms of BCG-Mediated Trained Immunity in Multipotent Progenitors and Monocytic Lineage
3. Trained Immunity Mechanisms in Neutrophils and Dendritic Cells (DCs)
4. Crosstalk between Trained Innate Immune Cells and Lymphocytes
5. The BCG NSEs against Intracellular Pathogens Comprise TI and Heterologous T cell Responses
6. Trained-Immunity-Based Vaccines (TIbVs) and What It Adds to the Field of Vaccinology
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Lobo, N.; Brooks, N.A.; Zlotta, A.R.; Cirillo, J.D.; Boorjian, S.; Black, P.C.; Meeks, J.J.; Bivalacqua, T.J.; Gontero, P.; Steinberg, G.D.; et al. 100 years of Bacillus Calmette-Guerin immunotherapy: From cattle to COVID-19. Nat. Rev. Urol. 2021, 18, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Netea, M.G.; Bishai, W.R. BCG turns 100: Its nontraditional uses against viruses, cancer, and immunologic diseases. J. Clin. Investig. 2021, 131, e148291. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; p. 43. [Google Scholar]
- Butkeviciute, E.; Jones, C.E.; Smith, S.G. Heterologous effects of infant BCG vaccination: Potential mechanisms of immunity. Future Microbiol. 2018, 13, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- van Puffelen, J.H.; Novakovic, B.; van Emst, L.; Kooper, D.; Zuiverloon, T.C.M.; Oldenhof, U.T.H.; Witjes, J.A.; Galesloot, T.E.; Vrieling, A.; Aben, K.K.H.; et al. Intravesical BCG in patients with non-muscle invasive bladder cancer induces trained immunity and decreases respiratory infections. J. Immunother. Cancer 2023, 11, e005518. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Nofal, A.; Hosny, D. Treatment of common and plane warts in children with topical viable Bacillus Calmette-Guerin. Pediatr. Dermatol. 2013, 30, 60–63. [Google Scholar] [CrossRef]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Dominguez-Andres, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.E.; Damoraki, G.; et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 2020, 183, 315–323.e9. [Google Scholar] [CrossRef]
- Marchant, A.; Goetghebuer, T.; Ota, M.O.; Wolfe, I.; Ceesay, S.J.; De Groote, D.; Corrah, T.; Bennett, S.; Wheeler, J.; Huygen, K.; et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 1999, 163, 2249–2255. [Google Scholar] [CrossRef]
- Messina, N.L.; Zimmermann, P.; Curtis, N. The impact of vaccines on heterologous adaptive immunity. Clin. Microbiol. Infect. 2019, 25, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, A.; de Queiroz, N.; Marinho, F.V.; Oliveira, S.C. Bacillus Calmette-Guerin-Trained Macrophages Elicit a Protective Inflammatory Response against the Pathogenic Bacteria Brucella abortus. J. Immunol. 2023, 211, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Goncalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334.e5. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Downey, J.; Sanz, J.; Kaufmann, E.; Blankenhaus, B.; Pacis, A.; Pernet, E.; Ahmed, E.; Cardoso, S.; Nijnik, A.; et al. M. tuberculosis Reprograms Hematopoietic Stem Cells to Limit Myelopoiesis and Impair Trained Immunity. Cell 2020, 183, 752–770.e22. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Khan, N.; Novakovic, B.; Kaufmann, E.; Jansen, T.; van Crevel, R.; Divangahi, M.; Netea, M.G. beta-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1. Cell Rep. 2020, 31, 107634. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Kaufmann, E.; Khan, N.; Tran, K.A.; Ulndreaj, A.; Pernet, E.; Fontes, G.; Lupien, A.; Desmeules, P.; McIntosh, F.; Abow, A.; et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022, 38, 110502. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Barroso de Figueiredo, A.M.; Teodoro Silva, M.V.; Cirovic, B.; de Bree, L.C.J.; Damen, M.; Moorlag, S.; Gomes, R.S.; Helsen, M.M.; Oosting, M.; et al. beta-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: A Crucial Role for IL-32. Cell Rep. 2019, 28, 2659–2672.e6. [Google Scholar] [CrossRef]
- Lachmandas, E.; Beigier-Bompadre, M.; Cheng, S.C.; Kumar, V.; van Laarhoven, A.; Wang, X.; Ammerdorffer, A.; Boutens, L.; de Jong, D.; Kanneganti, T.D.; et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 2016, 46, 2574–2586. [Google Scholar] [CrossRef]
- Lin, L.R.; Gao, Z.X.; Lin, Y.; Zhu, X.Z.; Liu, W.; Liu, D.; Gao, K.; Tong, M.L.; Zhang, H.L.; Liu, L.L.; et al. Akt, mTOR and NF-kappaB pathway activation in Treponema pallidum stimulates M1 macrophages. Int. Immunopharmacol. 2018, 59, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Ma, Y.; Zheng, M.M.; Chen, N.; Hu, M.N.; Wu, L.Y.; Zheng, Y.; Lou, Y.L.; Xie, D.L. NLRP3 and mTOR Reciprocally Regulate Macrophage Phagolysosome Formation and Acidification Against Vibrio vulnificus Infection. Front. Cell Dev. Biol. 2020, 8, 587961. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Q.; Romagnani, C. About Training and Memory: NK-Cell Adaptation to Viral Infections. Adv. Immunol. 2017, 133, 171–207. [Google Scholar] [CrossRef]
- Stary, V.; Stary, G. NK Cell-Mediated Recall Responses: Memory-Like, Adaptive, or Antigen-Specific? Front. Cell. Infect. Microbiol. 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Terren, I.; Orrantia, A.; Astarloa-Pando, G.; Amarilla-Irusta, A.; Zenarruzabeitia, O.; Borrego, F. Cytokine-Induced Memory-Like NK Cells: From the Basics to Clinical Applications. Front. Immunol. 2022, 13, 884648. [Google Scholar] [CrossRef]
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 2019, 10, 2955. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.J.; Xu, J.; Bermik, J.; Potchen, N.; den Dekker, A.; Neal, L.M.; Zhao, G.; Malachowski, A.; Schaller, M.; Kunkel, S.; et al. Epigenetic stabilization of DC and DC precursor classical activation by TNFalpha contributes to protective T cell polarization. Sci. Adv. 2019, 5, eaaw9051. [Google Scholar] [CrossRef]
- Yao, Y.; Jeyanathan, M.; Haddadi, S.; Barra, N.G.; Vaseghi-Shanjani, M.; Damjanovic, D.; Lai, R.; Afkhami, S.; Chen, Y.; Dvorkin-Gheva, A.; et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 2018, 175, 1634–1650.e17. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Findlay, E.G. Orchestration of Adaptive T Cell Responses by Neutrophil Granule Contents. Mediat. Inflamm. 2019, 2019, 8968943. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Chen, F.; Wu, W.; Millman, A.; Craft, J.F.; Chen, E.; Patel, N.; Boucher, J.L.; Urban, J.F., Jr.; Kim, C.C.; Gause, W.C. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 2014, 15, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Larsen, N.; Biering-Sorensen, S.; Andersen, A.; Eriksen, H.B.; Monteiro, I.; Hougaard, D.; Aaby, P.; Netea, M.G.; Flanagan, K.L.; et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: A randomized-controlled trial. J. Infect. Dis. 2015, 211, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Spencer, J.C.; Ganguly, R.; Waldman, R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guerin. J. Infect. Dis. 1977, 136, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, T.; Okuyama, T.; Yoshida, I.; Azuma, M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: Participation of interferon in enhanced resistance. Infect. Immun. 1978, 20, 312–314. [Google Scholar] [CrossRef]
- Sakuma, T.; Suenaga, T.; Yoshida, I.; Azuma, M. Mechanisms of enhanced resistance of Mycobacterium bovis BCG-treated mice to ectromelia virus infection. Infect. Immun. 1983, 42, 567–573. [Google Scholar] [CrossRef]
- Lodmell, D.L.; Ewalt, L.C. Enhanced resistance against encephalomyocarditis virus infection in mice, induced by a nonviable Mycobacterium tuberculosis oil-droplet vaccine. Infect. Immun. 1978, 19, 225–230. [Google Scholar] [CrossRef]
- Lodmell, D.L.; Ewalt, L.C. Induction of enhanced resistance against encephalomyocarditis virus infection of mice by nonviable Mycobacterium tuberculosis: Mechanisms of protection. Infect. Immun. 1978, 22, 740–745. [Google Scholar] [CrossRef]
- Mathurin, K.S.; Martens, G.W.; Kornfeld, H.; Welsh, R.M. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J. Virol. 2009, 83, 3528–3539. [Google Scholar] [CrossRef]
- Charoenlap, S.; Piromsopa, K.; Charoenlap, C. Potential role of Bacillus Calmette-Guerin (BCG) vaccination in COVID-19 pandemic mortality: Epidemiological and Immunological aspects. Asian Pac. J. Allergy Immunol. 2020, 38, 150–161. [Google Scholar] [CrossRef]
- Koneru, G.; Batiha, G.E.; Algammal, A.M.; Mabrok, M.; Magdy, S.; Sayed, S.; AbuElmagd, M.E.; Elnemr, R.; Saad, M.M.; Abd Ellah, N.H.; et al. BCG Vaccine-Induced Trained Immunity and COVID-19: Protective or Bystander? Infect. Drug Resist. 2021, 14, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Hilligan, K.L.; Namasivayam, S.; Sher, A. BCG mediated protection of the lung against experimental SARS-CoV-2 infection. Front. Immunol. 2023, 14, 1232764. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Shuai, H.; Gong, H.R.; Hu, J.C.; Yan, B.; Yuen, T.T.; Hu, Y.F.; Yoon, C.; Wang, X.L.; Hou, Y.; et al. Bacillus Calmette-Guerin-induced trained immunity protects against SARS-CoV-2 challenge in K18-hACE2 mice. JCI Insight 2022, 7, e157393. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Wang, R.; Lombardo, K.A.; Praharaj, M.; Bullen, C.K.; Um, P.; Davis, S.; Komm, O.; Illei, P.B.; Ordonez, A.A.; et al. Dynamic single-cell RNA sequencing reveals BCG vaccination curtails SARS-CoV-2 induced disease severity and lung inflammation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hilligan, K.L.; Namasivayam, S.; Clancy, C.S.; O’Mard, D.; Oland, S.D.; Robertson, S.J.; Baker, P.J.; Castro, E.; Garza, N.L.; Lafont, B.A.P.; et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022, 219, e20211862. [Google Scholar] [CrossRef]
- White, A.D.; Sibley, L.; Sarfas, C.; Morrison, A.L.; Bewley, K.; Churchward, C.; Fotheringham, S.; Gkolfinos, K.; Gooch, K.; Handley, A.; et al. Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques. Front. Immunol. 2021, 12, 801799. [Google Scholar] [CrossRef]
- Netea, M.G.; Ziogas, A.; Benn, C.S.; Giamarellos-Bourboulis, E.J.; Joosten, L.A.B.; Arditi, M.; Chumakov, K.; van Crevel, R.; Gallo, R.; Aaby, P.; et al. The role of trained immunity in COVID-19: Lessons for the next pandemic. Cell Host Microbe 2023, 31, 890–901. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef]
- Silva, M.V.T.; Dos Santos, J.C.; Figueiredo, A.M.B.; Teufel, L.U.; Pereira, J.X.; Matos, G.G.; Pinto, S.A.; Netea, M.G.; Gomes, R.S.; Joosten, L.A.B.; et al. The role of IL-32 in Bacillus Calmette-Guerin (BCG)-induced trained immunity in infections caused by different Leishmania spp. Microb. Pathog. 2021, 158, 105088. [Google Scholar] [CrossRef]
- Parra, M.; Liu, X.; Derrick, S.C.; Yang, A.; Tian, J.; Kolibab, K.; Kumar, S.; Morris, S.L. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PLoS ONE 2013, 8, e66115. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Ye, G.; Singh, R.; Afkhami, S.; Bavananthasivam, J.; Luo, X.; Vaseghi-Shanjani, M.; Aleithan, F.; Zganiacz, A.; Jeyanathan, M.; et al. Subcutaneous BCG vaccination protects against streptococcal pneumonia via regulating innate immune responses in the lung. EMBO Mol. Med. 2023, 15, e17084. [Google Scholar] [CrossRef] [PubMed]
- Mata, E.; Tarancon, R.; Guerrero, C.; Moreo, E.; Moreau, F.; Uranga, S.; Gomez, A.B.; Marinova, D.; Domenech, M.; Gonzalez-Camacho, F.; et al. Pulmonary BCG induces lung-resident macrophage activation and confers long-term protection against tuberculosis. Sci. Immunol. 2021, 6, eabc2934. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Lee, A.; Wimmers, F.; Pulendran, B. Epigenetic adjuvants: Durable reprogramming of the innate immune system with adjuvants. Curr. Opin. Immunol. 2022, 77, 102189. [Google Scholar] [CrossRef]
- Bayram, Z.; Musharrafieh, U.; Bizri, A.R. Revisiting the potential role of BCG and MMR vaccines in COVID-19. Sci. Prog. 2022, 105, 368504221105172. [Google Scholar] [CrossRef]
- Barton, E.S.; White, D.W.; Cathelyn, J.S.; Brett-McClellan, K.A.; Engle, M.; Diamond, M.S.; Miller, V.L.; Virgin, H.W.T. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007, 447, 326–329. [Google Scholar] [CrossRef]
- Ifrim, D.C.; Quintin, J.; Joosten, L.A.; Jacobs, C.; Jansen, T.; Jacobs, L.; Gow, N.A.; Williams, D.L.; van der Meer, J.W.; Netea, M.G. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vaccine Immunol. 2014, 21, 534–545. [Google Scholar] [CrossRef]
- Guevara-Hoyer, K.; Saz-Leal, P.; Diez-Rivero, C.M.; Ochoa-Grullon, J.; Fernandez-Arquero, M.; Perez de Diego, R.; Sanchez-Ramon, S. Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study. Biomedicines 2020, 8, 203. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.Y.; Jang, Y.H.; Seo, S.U.; Chang, J.; Seong, B.L. Non-specific Effect of Vaccines: Immediate Protection against Respiratory Syncytial Virus Infection by a Live Attenuated Influenza Vaccine. Front. Microbiol. 2018, 9, 83. [Google Scholar] [CrossRef]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Donath, S.; Perrett, K.P.; Messina, N.L.; Ritz, N.; Netea, M.G.; Flanagan, K.L.; van der Klis, F.R.M.; Curtis, N.; MIS BAIR Group. The influence of neonatal Bacille Calmette-Guerin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine 2019, 37, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Zhang, L.; Woda, M.; Acosta, L.P.; Obcena, A.; Brion, J.D.; Capeding, R.Z. Neonatal BCG vaccination is associated with enhanced T-helper 1 immune responses to heterologous infant vaccines. Trials Vaccinol. 2014, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Johansen, M.D.; Stella, A.O.; Nguyen, D.H.; Ferguson, A.L.; Aggarwal, A.; Bhattacharyya, N.D.; Grey, A.; Hutchings, O.; Patel, K.; et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines 2021, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Mouhoub, E.; Domenech, P.; Ndao, M.; Reed, M.B. The Diverse Applications of Recombinant BCG-Based Vaccines to Target Infectious Diseases Other Than Tuberculosis: An Overview. Front. Microbiol. 2021, 12, 757858. [Google Scholar] [CrossRef]
- Bastos, R.G.; Borsuk, S.; Seixas, F.K.; Dellagostin, O.A. Recombinant Mycobacterium bovis BCG. Vaccine 2009, 27, 6495–6503. [Google Scholar] [CrossRef]
- Bastos, R.G.; Dellagostin, O.A.; Barletta, R.G.; Doster, A.R.; Nelson, E.; Osorio, F.A. Construction and immunogenicity of recombinant Mycobacterium bovis BCG expressing GP5 and M protein of porcine reproductive respiratory syndrome virus. Vaccine 2002, 21, 21–29. [Google Scholar] [CrossRef]
- Kanno, A.I.; Goulart, C.; Rofatto, H.K.; Oliveira, S.C.; Leite, L.C.C.; McFadden, J. New Recombinant Mycobacterium bovis BCG Expression Vectors: Improving Genetic Control over Mycobacterial Promoters. Appl. Environ. Microbiol. 2016, 82, 2240–2246. [Google Scholar] [CrossRef]
- Desel, C.; Dorhoi, A.; Bandermann, S.; Grode, L.; Eisele, B.; Kaufmann, S.H. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect. Dis. 2011, 204, 1573–1584. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Dias, W.O.; Mazzantini, R.P.; Miyaji, E.N.; Gamberini, M.; Quintilio, W.; Gebara, V.C.; Cardoso, D.F.; Ho, P.L.; Raw, I.; et al. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect. Immun. 2000, 68, 4877–4883. [Google Scholar] [CrossRef] [PubMed]
- Ohara, N.; Matsuoka, M.; Nomaguchi, H.; Naito, M.; Yamada, T. Protective responses against experimental Mycobacterium leprae infection in mice induced by recombinant Bacillus Calmette-Guerin over-producing three putative protective antigen candidates. Vaccine 2001, 19, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Galvez, N.M.S.; Rivera, C.A.; Palavecino, C.E.; Cespedes, P.F.; Rey-Jurado, E.; Bueno, S.M.; Kalergis, A.M. Recombinant BCG Vaccines Reduce Pneumovirus-Caused Airway Pathology by Inducing Protective Humoral Immunity. Front. Immunol. 2018, 9, 2875. [Google Scholar] [CrossRef]
- Bontempi, I.; Leal, K.; Prochetto, E.; Diaz, G.; Cabrera, G.; Bortolotti, A.; Morbidoni, H.R.; Borsuk, S.; Dellagostin, O.; Marcipar, I. Recombinant Mycobacterium bovis BCG is a promising platform to develop vaccines against Trypansoma cruzi infection. Clin. Exp. Immunol. 2020, 201, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Mambelli, F.; Marinho, F.V.; Andrade, J.M.; de Araujo, A.; Abuna, R.P.F.; Fabri, V.M.R.; Santos, B.P.O.; da Silva, J.S.; de Magalhaes, M.T.Q.; Homan, E.J.; et al. Recombinant Bacillus Calmette-Guerin Expressing SARS-CoV-2 Chimeric Protein Protects K18-hACE2 Mice against Viral Challenge. J. Immunol. 2023, 210, 1925–1937. [Google Scholar] [CrossRef]
- de Queiroz, N.; Marinho, F.V.; de Araujo, A.; Fahel, J.S.; Oliveira, S.C. MyD88-dependent BCG immunotherapy reduces tumor and regulates tumor microenvironment in bladder cancer murine model. Sci. Rep. 2021, 11, 15648. [Google Scholar] [CrossRef]
- Gu, H.; Zeng, X.; Peng, L.; Xiang, C.; Zhou, Y.; Zhang, X.; Zhang, J.; Wang, N.; Guo, G.; Li, Y.; et al. Vaccination induces rapid protection against bacterial pneumonia via training alveolar macrophage in mice. eLife 2021, 10, e69951. [Google Scholar] [CrossRef]
- Chan, L.C.; Chaili, S.; Filler, S.G.; Miller, L.S.; Solis, N.V.; Wang, H.; Johnson, C.W.; Lee, H.K.; Diaz, L.F.; Yeaman, M.R. Innate Immune Memory Contributes to Host Defense against Recurrent Skin and Skin Structure Infections Caused by Methicillin-Resistant Staphylococcus aureus. Infect. Immun. 2017, 85, e00876-16. [Google Scholar] [CrossRef]
- Suen, T.K.; Moorlag, S.; Li, W.; de Bree, C.L.J.; Koeken, V.; Mourits, V.P.; Dijkstra, H.; Lemmers, H.; Bhat, J.; Xu, C.J.; et al. BCG vaccination induces innate immune memory in gamma delta T cells in humans. J. Leukoc. Biol. 2023, qiad103. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araujo, A.C.V.S.C.; Mambelli, F.; Sanches, R.O.; Marinho, F.V.; Oliveira, S.C. Current Understanding of Bacillus Calmette-Guérin-Mediated Trained Immunity and Its Perspectives for Controlling Intracellular Infections. Pathogens 2023, 12, 1386. https://doi.org/10.3390/pathogens12121386
de Araujo ACVSC, Mambelli F, Sanches RO, Marinho FV, Oliveira SC. Current Understanding of Bacillus Calmette-Guérin-Mediated Trained Immunity and Its Perspectives for Controlling Intracellular Infections. Pathogens. 2023; 12(12):1386. https://doi.org/10.3390/pathogens12121386
Chicago/Turabian Stylede Araujo, Ana Carolina V. S. C., Fábio Mambelli, Rodrigo O. Sanches, Fábio V. Marinho, and Sergio C. Oliveira. 2023. "Current Understanding of Bacillus Calmette-Guérin-Mediated Trained Immunity and Its Perspectives for Controlling Intracellular Infections" Pathogens 12, no. 12: 1386. https://doi.org/10.3390/pathogens12121386
APA Stylede Araujo, A. C. V. S. C., Mambelli, F., Sanches, R. O., Marinho, F. V., & Oliveira, S. C. (2023). Current Understanding of Bacillus Calmette-Guérin-Mediated Trained Immunity and Its Perspectives for Controlling Intracellular Infections. Pathogens, 12(12), 1386. https://doi.org/10.3390/pathogens12121386







