Impact of Sclerotinia sclerotiorum Infection on Lettuce (Lactuca sativa L.) Survival and Phenolics Content—A Case Study in a Horticulture Farm in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lettuce Cultivars, Planting, and Cultivation
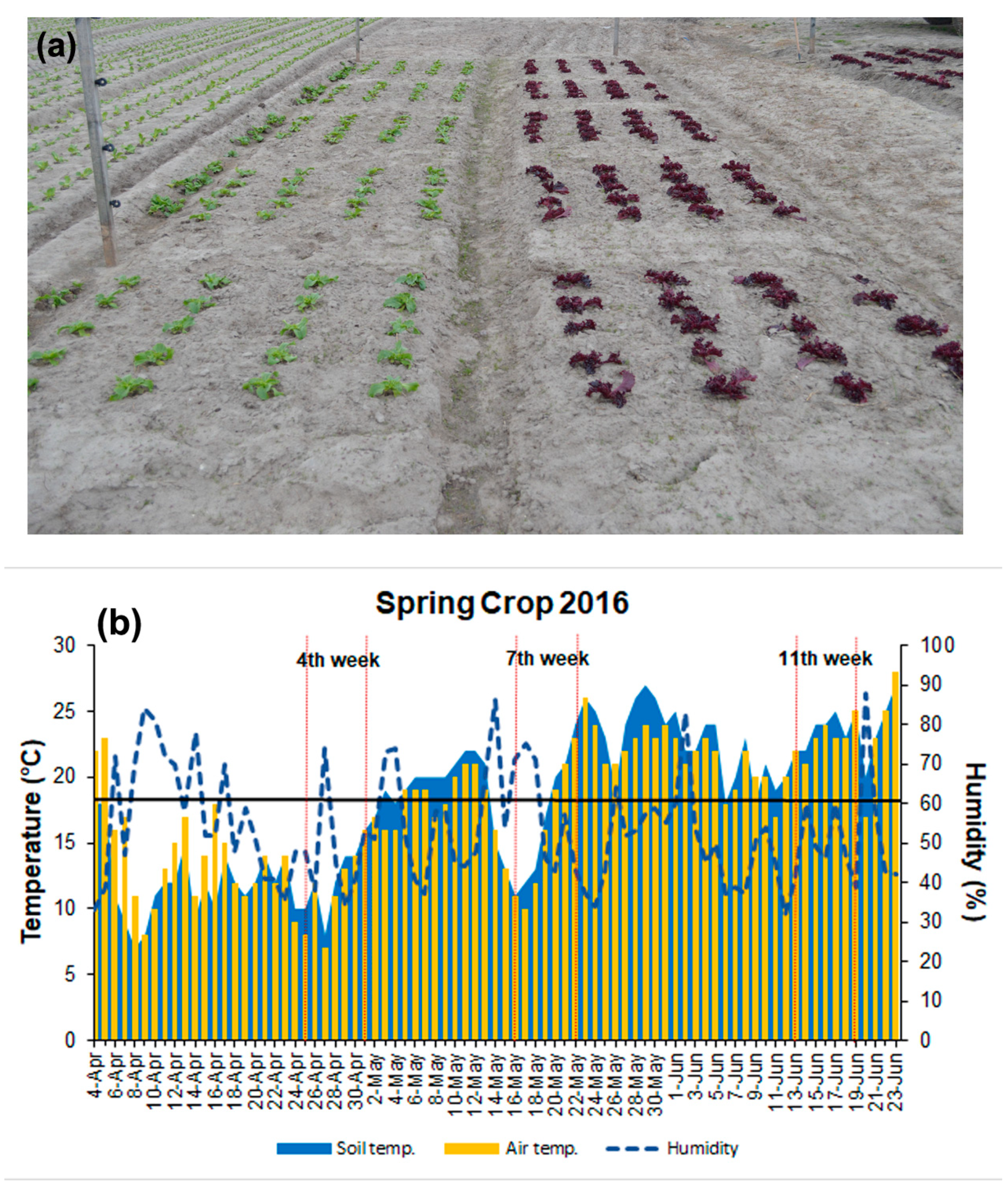
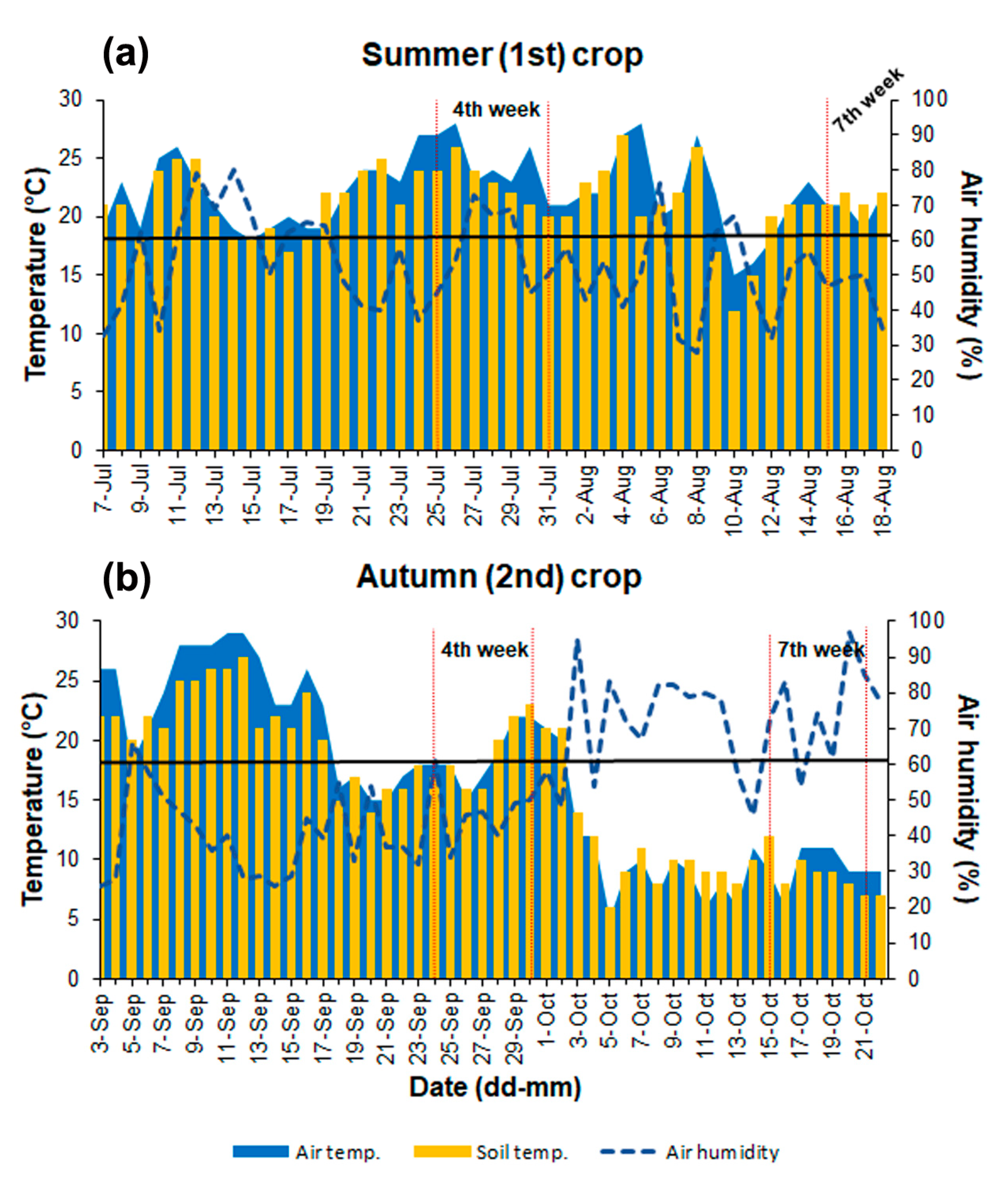
2.1.1. The Summer (1st) Crop, 2016
2.1.2. The Autumn (2nd) Crop, 2016
2.2. Air and Soil Parameters
2.3. S. sclerotiorum Soil Contamination and Re-Isolation of the Fungus
2.4. Evaluation of Lettuce Infection
2.5. Analyses of Phenolics Contents
2.5.1. Sample Collection and Preparation of Plant Extracts
2.5.2. Total Phenolic Compounds (TPCs)
2.5.3. Flavonoids
2.5.4. Anthocyanins
2.6. Statistical Analysis
3. Results
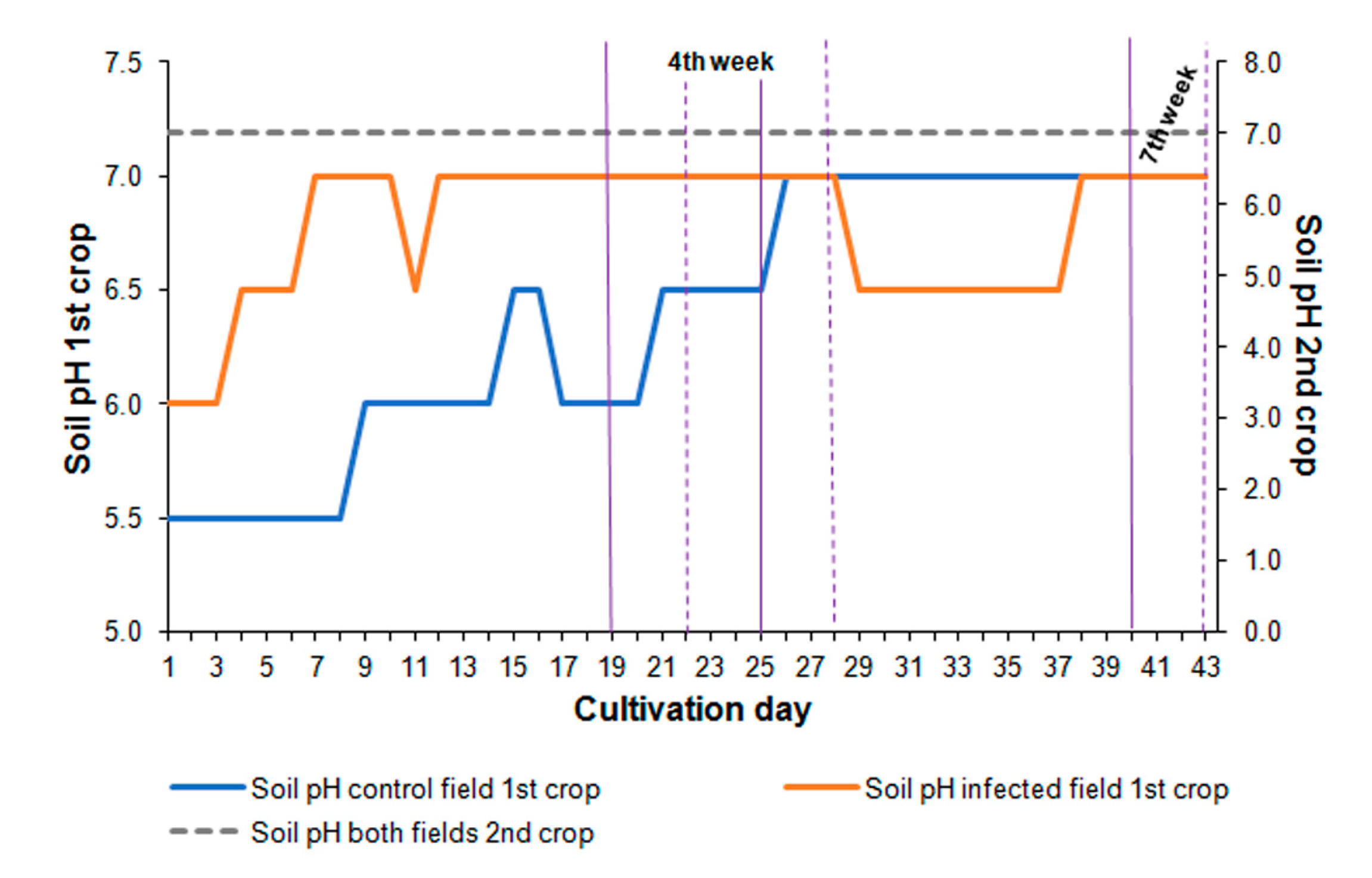
3.1. Changes in the Air and Soil Parameters
3.2. Re-Isolation of the Pathogen
3.3. Evaluation of Disease Progression and Lettuce Survival
3.4. Analysis of Phenolics Content
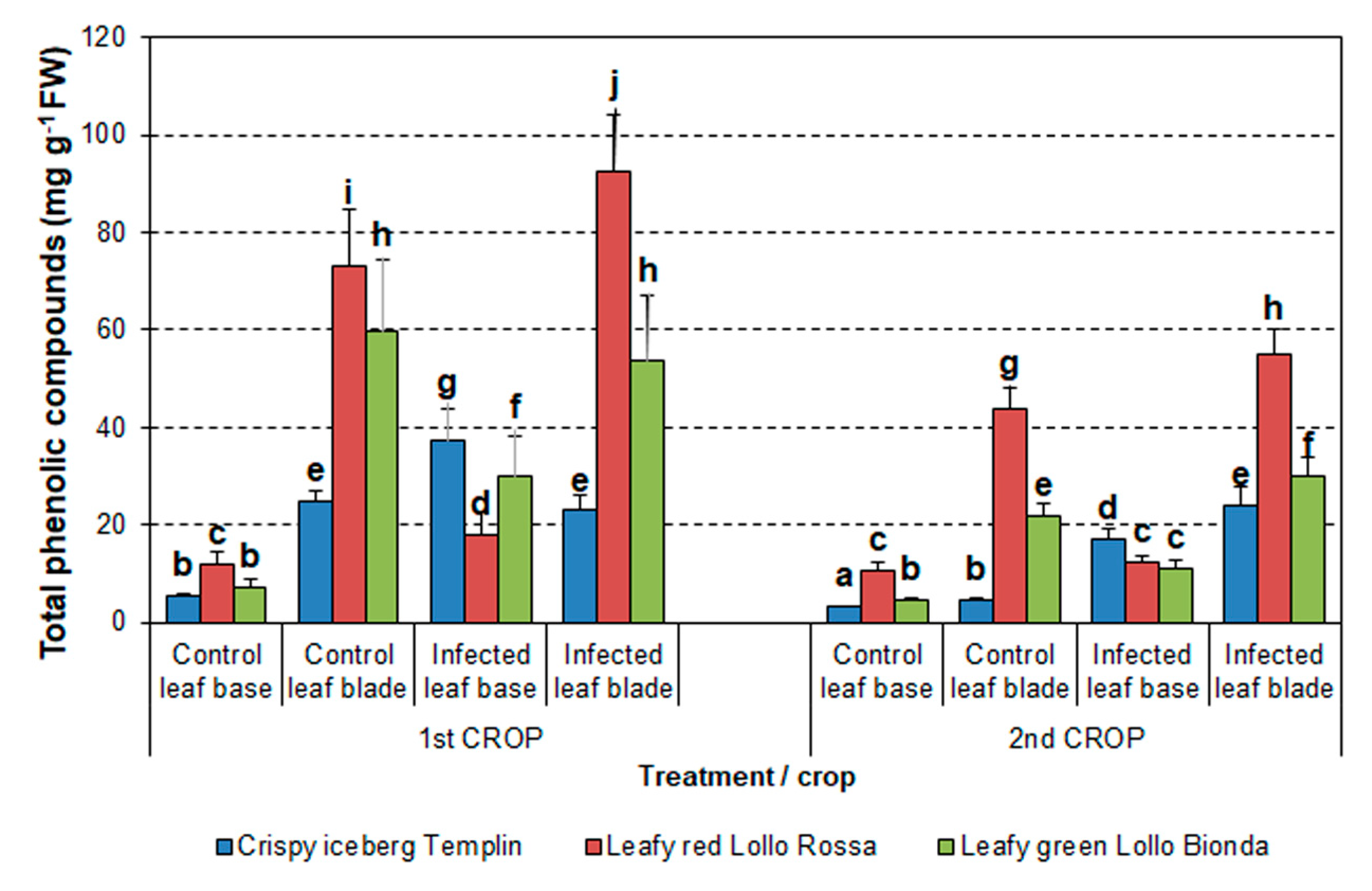
3.4.1. Total Content of Phenolic Compounds (TPCs)
3.4.2. Flavonoid Content
3.4.3. Anthocyanin Content
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Křístková, E.; Doležalová, I.; Lebeda, A.; Vinter, V.; Novotná, A. Description of morphological characters of lettuce (Lactuca sativa L.) genetic resources. Hortic. Sci. 2008, 35, 113–129. [Google Scholar] [CrossRef]
- Han, R.; Truco, M.J.; Lavelle, D.O.; Michelmore, R.W. A Composite Analysis of Flowering Time Regulation in Lettuce. Front. Plant Sci. 2021, 12, 632708. [Google Scholar] [CrossRef] [PubMed]
- Mou, B. Lettuce. Vegetables I—Asteraceae, Brassicaceae, Chenopodiaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 75–116. [Google Scholar]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S. Lactuca sativa L. (Asteraceae/Compositae). In Handbook of 200 Medicinal Plants; Akbar, S., Ed.; Springer: Cham, Switzerland, 2020; pp. 1067–1075. [Google Scholar] [CrossRef]
- Ryder, E.J. Lettuce. In Leafy Salad Vegetables; Ryder, E.J., Ed.; Springer: Dordrecht, The Netherlands, 1979; pp. 13–94. [Google Scholar]
- Damerum, A.; Chapman, M.A.; Taylor, G. Innovative breeding technologies in lettuce for improved post-harvest quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A.L.; Nair, M.G. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010, 118, 300–306. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Kik, G.-S.; Lee, W.S.; Kim, Y.-H.; Kang, N.J.; Jin, J.S.; Lee, G.M.; Kim, S.T.; El-Aty, A.M.; Shim, J.H.; et al. The effects of different night-time temperatures and cultivation durations on the polyphenolic contents of lettuce: Application of principal component analysis. J. Adv. Res. 2015, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Rasiukevičiūtė, N.; Viršilė, A.; Miliauskienė, J.; Laužikė, K.; Valiuškaitė, A.; Dėnė, L.; Chrapačienė, S.; et al. Phenolic Compounds Content Evaluation of Lettuce Grown under Short-Term Preharvest Daytime or Nighttime Supplemental LEDs. Plants 2022, 11, 1123. [Google Scholar] [CrossRef]
- de Souza, A.S.N.; de Oliveira Schmidt, H.; Pagno, C.; Rodrigues, E.; Silva da Silva, M.A.; Hickmann Flôres, S.; de Oliveira Rios, A. Influence of cultivar and season on carotenoids and phenolic compounds from red lettuce influence of cultivar and season on lettuce. Food Res. Int. 2022, 155, 111110. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Frank Quartacci, M.; Muñoz-Rued, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT-Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Llorach, R.; Martınez-Sanchez, A.; Tomas-Barberan, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Sinkovič, L.; Sinkovič, D.K.; Ugrinović, K. Yield and nutritional quality of soil-cultivated crisphead lettuce (Lactuca sativa L. var. capitata) and corn salad (Valerianella spp.) harvested at different growing periods. Food Sci. Nutr. 2023, 11, 1755–1769. [Google Scholar] [CrossRef] [PubMed]
- Turini, T.A.; Joseph, S.V.; Baldwin, R.A.; Koike, S.T.; Natwick, E.T.; Ploeg, A.T.; Smith, R.F.; Dara, S.K.; Fennimore, S.A.; LeStrange, M.; et al. UC IPM Pest Management Guidelines: Lettuce. UC ANR Publication 3450. Davis, CA, USA. Available online: https://ipm.ucanr.edu/agriculture/lettuce/ (accessed on 30 April 2023).
- Fall, M.L.; Van der Heyden, H.; Carisse, O. Bremia lactucae infection efficiency in lettuce is modulated by temperature and leaf wetness duration under Quebec field conditions. Plant Dis. 2015, 99, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.K.; Kim, M.J.; Kim, Y.K.; Jee, H.J. Evaluation of lettuce germplasm resistance to gray mold disease for organic cultivations. Plant Pathol. J. 2014, 30, 90–95. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Pink, H.; Talbot, A.; Graceson, A.; Graham, J.; Higgins, G.; Taylor, A.; Jackson, A.C.; Truco, M.; Michelmore, R.; Yao, C.; et al. Identification of genetic loci in lettuce mediating quantitative resistance to fungal pathogens. Theor. Appl. Genet. 2022, 135, 2481–2500. [Google Scholar] [CrossRef]
- Wu, B.M.; Subbarao, K.V. Analyses of Lettuce Drop Incidence and Population Structure of Sclerotinia sclerotiorum and S. minor. Phytopathology 2006, 96, 1322–1329. [Google Scholar] [CrossRef]
- Liang, X.; Rollins, J.A. Mechanisms of Broad Host Range Necrotrophic Pathogenesis in Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1128–1140. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Saharan, G.S.; Mehta, N. History and Host Range. In Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Saharan, G.S., Mehta, N., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 19–39. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Newman, T.E.; Khentry, Y.; Taiwo, A.O. The evolutionary and molecular features of the broad-host-range plant pathogen Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Durman, S.B.; Menendez, A.B.; Godeas, A.M. Variation in oxalic acid production and mycelial compatibility within field populations of Sclerotinia sclerotiorum. Soil Biol. Biochem. 2005, 37, 2180–2184. [Google Scholar] [CrossRef]
- Williams, B.; Kabbage, M.; Kim, H.-J.; Britt, R.; Dickman, M.B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011, 7, e1002107. [Google Scholar] [CrossRef] [PubMed]
- Erental, A.; Dickman, M.B.; Yarden, O. Sclerotial development in Sclerotinia sclerotiorum: Awakening molecular analysis of a ‘‘Dormant’’ structure. Fungal Biol. Rev. 2008, 22, 6–16. [Google Scholar] [CrossRef]
- Mamo, B.E.; Eriksen, R.L.; Adhikari, N.D.; Hayes, R.J.; Mou, B.; Simko, I. Epidemiological Characterization of Lettuce Drop (Sclerotinia spp.) and Biophysical Features of the Host Identify Soft Stem as a Susceptibility Factor. PhytoFrontiers 2021, 1, 182–204. [Google Scholar] [CrossRef]
- Mbengue, M.; Navaud, O.; Peyraud, R.; Barascud, M.; Badet, T.; Vincent, R.; Barbacci, A.; Raffaele, S. Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Front. Plant Sci. 2016, 7, 422. [Google Scholar] [CrossRef]
- Hayes, R.J.; Wu, B.M.; Pryor, B.M.; Chitrampalam, P.; Subbarao, K.V. Assessment of Resistance in Lettuce (Lactuca sativa L.) to Mycelial and Ascospore Infection by Sclerotinia minor Jagger and S. sclerotiorum (Lib.) de Bary. HortScience 2010, 45, 333–341. [Google Scholar] [CrossRef]
- Mamo, B.E.; Hayes, R.J.; Truco, M.J.; Puri, K.D.; Michelmore, R.W.; Subbarao, K.; Simko, I. The genetics of resistance to lettuce drop (Sclerotinia spp.) in lettuce in a recombinant inbred line population from Reine des Glaces × Eruption. Theor. Appl. Genet. 2019, 132, 2439–2460. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Wielanek, M.; Morkunas, I.; Ciereszko, I.; Kononowicz, A.K. Leaf position-dependent effect of Alternaria brassicicola development on host cell death, photosynthesis and secondary metabolites in Brassica juncea. Physiol. Plant. 2020, 168, 601–616. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Aziz, T.; Maqsood, M.A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application. Metabolites 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Mori, N.; Tomari, Y.; Mitsugi, N.; Deguchi, A.; Kashima, M.; Tezuka, A.; Nagano, A.J.; Usami, H.; Tanabata, T.; et al. Effect of differences in light source environment on transcriptome of leaf lettuce (Lactuca sativa L.) to optimize cultivation conditions. PLoS ONE 2022, 17, e0265994. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Engeseth, N.J. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT—Food Sci. Technol. 2021, 150, 111931. [Google Scholar] [CrossRef]
- Becker, C.; Urlić, B.; Špika, M.J.; Kläring, H.P.; Krumbein, A.; Baldermann, S.; Ban, S.G.; Perica, S.; Schwarz, D. Nitrogen limited red and green leaf lettuce accumulate flavonoid glycosides, caffeic acid derivatives, and sucrose while losing chlorophylls, β-carotene and xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef]
- Duchenne-Moutien, R.A.; Neetoo, H. Climate Change and Emerging Food Safety Issues: A Review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef]
- Raza, M.M.; Bebber, D.P. Climate change and plant pathogens. Curr. Opin. Microbiol. 2022, 70, 102233. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Chaloner, T.M.; Gurr, S.J.; Bebber, D.P. Plant pathogen infection risk tracks global crop yields under climate change. Nat. Clim. Chang. 2021, 11, 710–715. [Google Scholar] [CrossRef]
- Miranda-Apodaca, J.; Artetxe, U.; Aguado, I.; Martin-Souto, L.; Ramirez-Garcia, A.; Lacuesta, M.; Becerril, J.M.; Estonba, A.; Ortiz-Barredo, A.; Hernández, A.; et al. Stress Response to Climate Change and Postharvest Handling in Two Differently Pigmented Lettuce Genotypes: Impact on Alternaria alternata Invasion and Mycotoxin Production. Plants 2023, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Shahoveisi, F.; Manesh, M.R.; Del Río Mendoza, L.E. Modeling risk of Sclerotinia sclerotiorum-induced disease development on canola and dry bean using machine learning algorithms. Sci. Rep. 2022, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Shahoveisi, F.; Del Río Mendoza, L.E. Effect of Wetness Duration and Incubation Temperature on Development of Ascosporic Infections by Sclerotinia sclerotiorum. Plant Dis. 2020, 104, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.P.; Fawcett, L.; Anthony, S.D.; Young, C. A model for Sclerotinia sclerotiorum infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS ONE 2014, 9, e94049. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Jajor, E.; Wójtowicz, M.; Pasternak, M. Influence of temperature on mycelial growth and number of sclerotia of Sclerotinia sclerotiorum the cause of Sclerotinia stem rot. Prog. Plant Prot. 2016, 56, 241–244. [Google Scholar] [CrossRef]
- Grube, R.; Ryder, E.J. Identification of lettuce (Lactuca sativa L.) germplasm with genetic resistance to drop caused by Sclerotinia minor. J. Am. Soc. Hortic. Sci. 2004, 129, 70–76. [Google Scholar] [CrossRef]
- Tak, Y.; Kumar, M. Phenolics: A Key Defence Secondary Metabolite to Counter Biotic Stress. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 309–329. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef]
- Gan, Y.Z.; Azrina, A. Antioxidant properties of selected varieties of lettuce (Lactuca sativa L.) commercially available in Malaysia. Int. Food Res. J. 2016, 23, 2357–2362. [Google Scholar]
- Kang, H.-M.; Saltveit, M.-E. Antioxidant capacity of lettuce leaf tissue increases after wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, L.; Liang, X.; Dai, P.; Zhang, Y.; Li, B.; Lin, X.; Sun, C. Enhancement of polyphenolic metabolism as an adaptive response of lettuce (Lactuca sativa) roots to aluminum stress. Environ. Pollut. 2020, 261, 114230. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, A.; Singh, S.; Singh, H.B. Phenols enhancement effect of microbial consortium in pea plants restrains Sclerotinia sclerotiorum. Biol. Control 2015, 89, 23–32. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Gazula, A.; Kleinhenz, M.D.; Scheerens, J.C.; Ling, P.P. Anthocyanin Levels in Nine Lettuce (Lactuca sativa) Cultivars: Influence of Planting Date and Relations among Analytic, Instrumented, and Visual Assessments of Color. HortScience 2007, 42, 232–238. [Google Scholar] [CrossRef]
- Chon, S.-U.; Boo, H.-O.; Heo, B.-G.; Gorinstein, S. Anthocyanin content and the activities of polyphenol oxidase, peroxidase and phenylalanine ammonia-lyase in lettuce cultivars. Int. J. Food Sci. Nutr. 2012, 63, 45–48. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.D.; Streeter, J.G.; Miller, A.R. Temperature and cultivar effects on anthocyanin and chlorophyll b concentrations in three related Lollo Rosso lettuce cultivars. HortScience 2005, 40, 1731–1733. [Google Scholar] [CrossRef]
- Singh, M.; Avtar, R.; Lakra, N.; Pal, A.; Singh, V.K.; Punia, R.; Kumar, N.; Bishnoi, M.; Kumari, N.; Khedwal, R.S.; et al. Early oxidative burst and anthocyanin-mediated antioxidant defense mechanism impart resistance against Sclerotinia sclerotiorum in Indian mustard. Physiol. Mol. Plant Pathol. 2022, 120, 101847. [Google Scholar] [CrossRef]
- Shalaby, S.; Larkov, O.; Lamdan, N.L.; Goldshmidt-Tran, O.; Horwitz, B.A. Plant phenolic acids induce programmed cell death of a fungal pathogen: MAPK signaling and survival of Cochliobolus heterostrophus. Environ. Microbiol. 2016, 18, 4188–4199. [Google Scholar] [CrossRef]
- Świerczyńska, I.; Perek, A.; Pieczul, K.; Jajor, E. Sensitivity of Sclerotinia sclerotiorum to active substances of fungicides. Prog. Plant Prot. 2016, 56, 348–353. [Google Scholar] [CrossRef]
- Chen, X.; Pizzatti, C.; Bonaldi, M.; Saracchi, M.; Erlacher, A.; Kunova, A.; Berg, G.; Cortesi, P. Biological Control of Lettuce Drop and Host Plant Colonization by Rhizospheric and Endophytic Streptomycetes. Front. Microbiol. 2016, 7, 714. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, F.; Ziogas, I.; Gkizi, D.; Fragkogeorgi, G.A.; Tjamos, S.E. Novel biocontrol agents against Rhizoctonia solani and Sclerotinia sclerotiorum in lettuce. BioControl 2020, 65, 763–773. [Google Scholar] [CrossRef]
- Smolińska, U.; Kowalska, B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum—A review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.-A. Phenolic Compound Induction in Plant-Microbe and Plant-Insect Interactions: A Meta-Analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef] [PubMed]

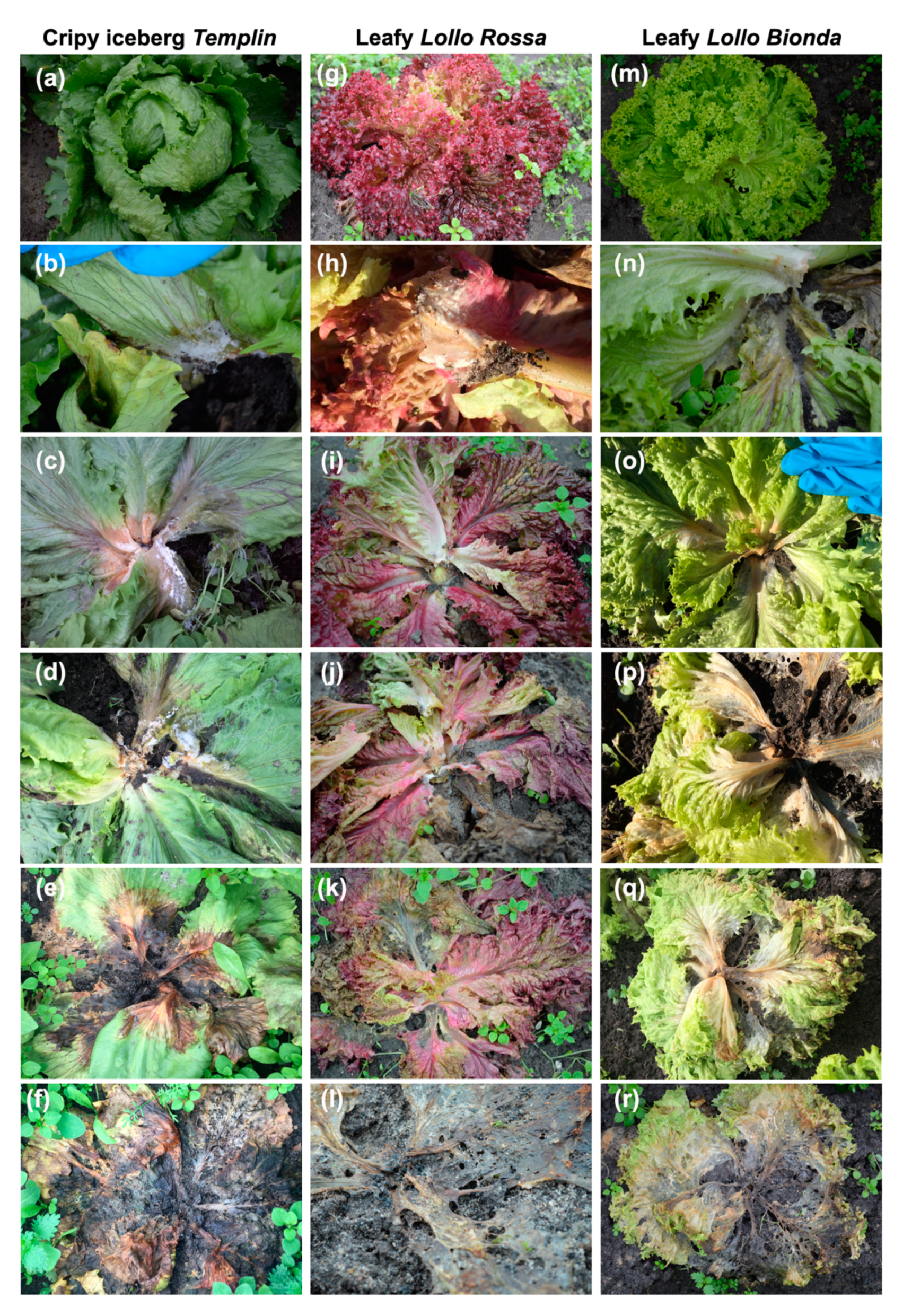


| Stage of Infection | Presence of the Fungus | Symptom on Plant |
|---|---|---|
| 0 | No fungus | No symptoms |
| 1 | Small white mycelium | Small necroses on the stem |
| 2 | White mycelium and black sclerotia on the stem | Wilted leaf base, necroses on leaves and stem |
| 3 | White mycelium and sclerotia on leaves and stems | Necroses on leaves and stems, marginal leaves wilted and brown |
| 4 | Mycelium spread and many black sclerotia | Wilted, rotten, and discolored leaves, rotten stem |
| 5 | Spreading fungus on the whole plant | Decoyed, dead plant |
| Cultivation Week | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|
| Cultivar | Plants in control field (%) | ||||||
| Crispy iceberg Templin | 98 | 92 1 | 90 1 | 82 1 | 80 1 | 80 1 | 80 1 |
| Leafy red Lollo Rossa | 100 | 100 | 99 | 99 | 99 | 99 | 99 |
| Leafy green Lollo Bionda | 99 | 99 | 93 | 92 | 92 | 92 | 92 |
| Plants in infected field (%) | |||||||
| Crispy iceberg Templin | 100 | 97 | 95 | 80 | 70 | 21 | 10 |
| Leafy red Lollo Rossa | 97 | 96 | 90 | 89 | 89 | 84 | 83 |
| Leafy green Lollo Bionda | 100 | 94 | 93 | 93 | 79 | 70 | 70 |
| Cultivation Week | I | II | III | IV | V | VI | VII |
|---|---|---|---|---|---|---|---|
| Cultivar | Plants in control field (%) | ||||||
| Crispy iceberg Templin | 100 | 100 | 100 | 98 | 96 | 96 | 92 |
| Leafy red Lollo Rossa | 100 | 100 | 100 | 100 | 99 | 99 | 97 |
| Leafy green Lollo Bionda | 100 | 100 | 100 | 99 | 99 | 99 | 97 |
| Plants in infected field (%) | |||||||
| Crispy iceberg Templin | 100 | 97 | 94 | 70 | 52 | 48 | 30 |
| Leafy red Lollo Rossa | 98 | 88 | 81 | 80 | 76 | 65 | 51 |
| Leafy green Lollo Bionda | 100 | 99 | 98 | 88 | 85 | 80 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macioszek, V.K.; Marciniak, P.; Kononowicz, A.K. Impact of Sclerotinia sclerotiorum Infection on Lettuce (Lactuca sativa L.) Survival and Phenolics Content—A Case Study in a Horticulture Farm in Poland. Pathogens 2023, 12, 1416. https://doi.org/10.3390/pathogens12121416
Macioszek VK, Marciniak P, Kononowicz AK. Impact of Sclerotinia sclerotiorum Infection on Lettuce (Lactuca sativa L.) Survival and Phenolics Content—A Case Study in a Horticulture Farm in Poland. Pathogens. 2023; 12(12):1416. https://doi.org/10.3390/pathogens12121416
Chicago/Turabian StyleMacioszek, Violetta Katarzyna, Paulina Marciniak, and Andrzej Kiejstut Kononowicz. 2023. "Impact of Sclerotinia sclerotiorum Infection on Lettuce (Lactuca sativa L.) Survival and Phenolics Content—A Case Study in a Horticulture Farm in Poland" Pathogens 12, no. 12: 1416. https://doi.org/10.3390/pathogens12121416







