Innovative Elastomers with Antimicrobial Activity May Decrease Infection Risks during Milking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Elastomer Characteristics
2.2. Bacterial Strains and Culture Conditions
2.3. Preparation of Silicone Sheets and Microplate Loading
2.4. Statistical Analysis
3. Results
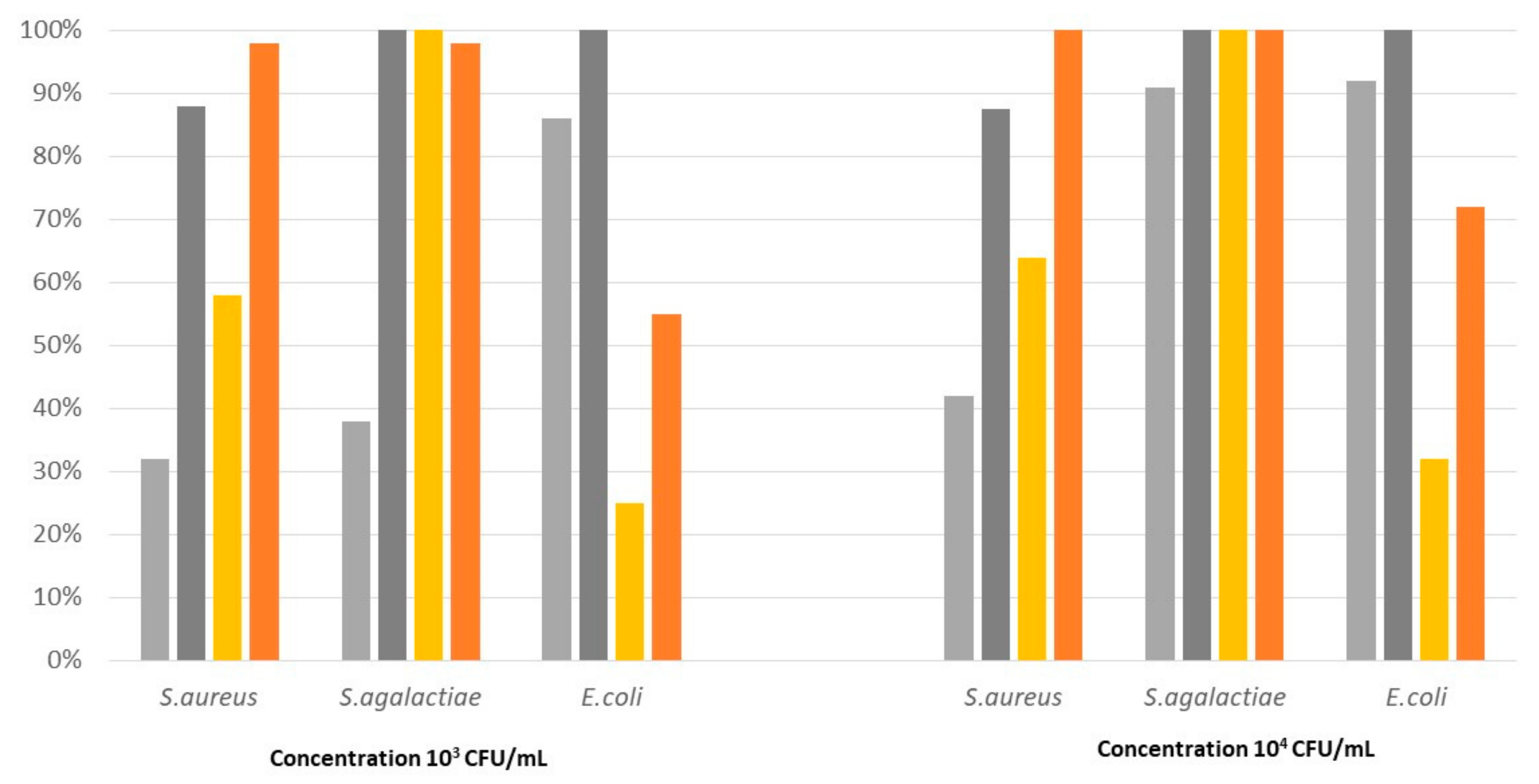
3.1. Rubber Elastomers
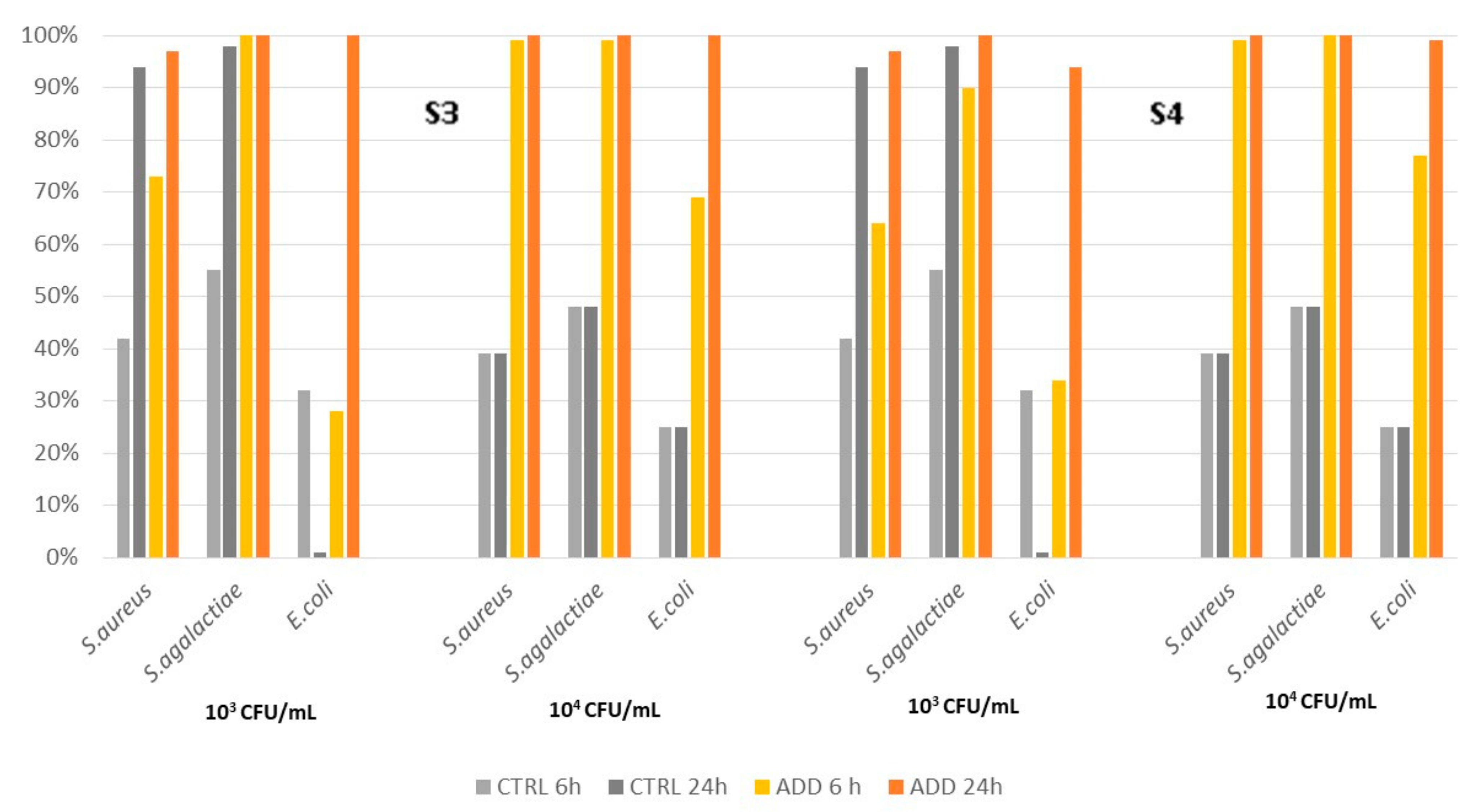
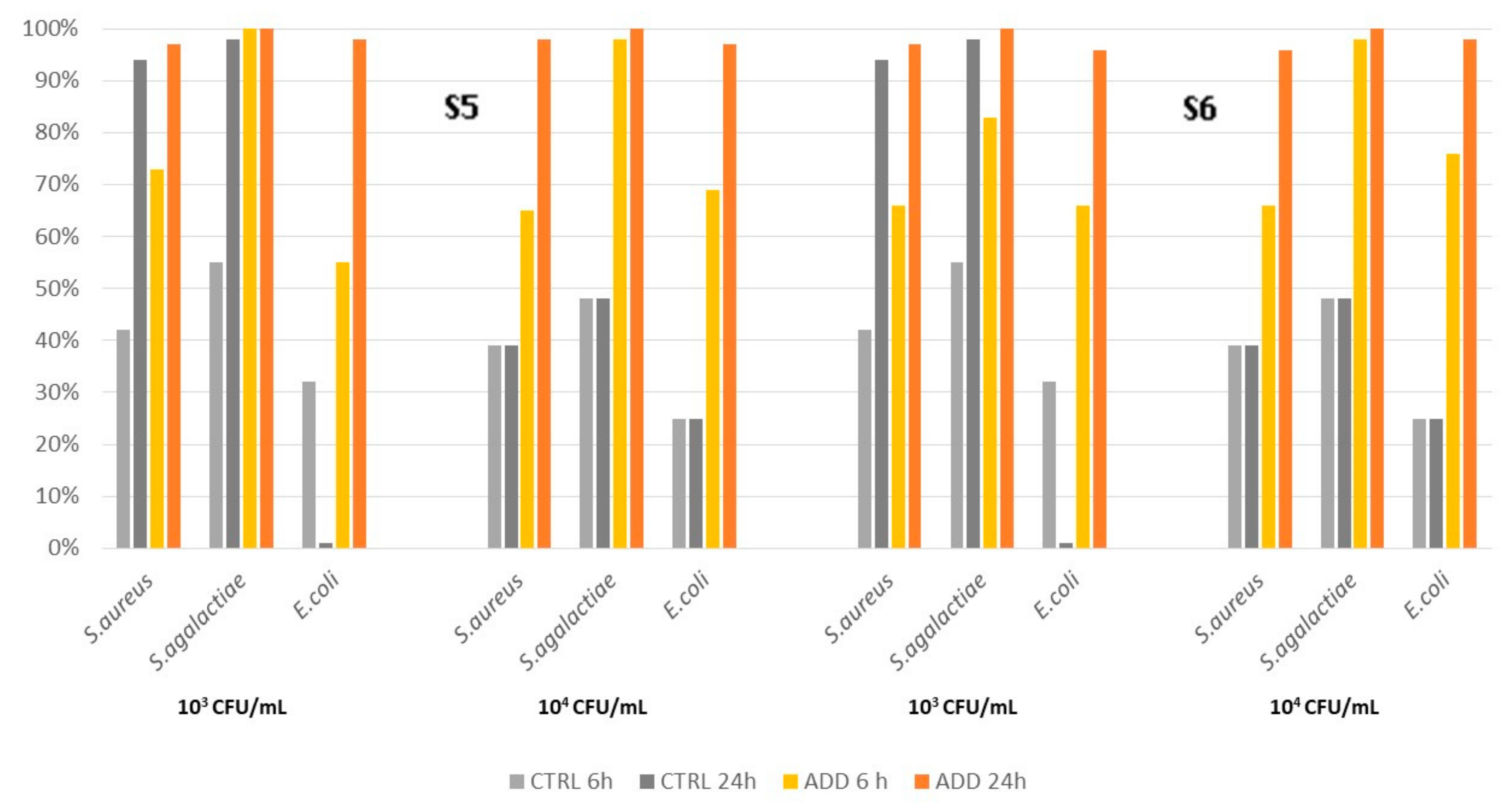
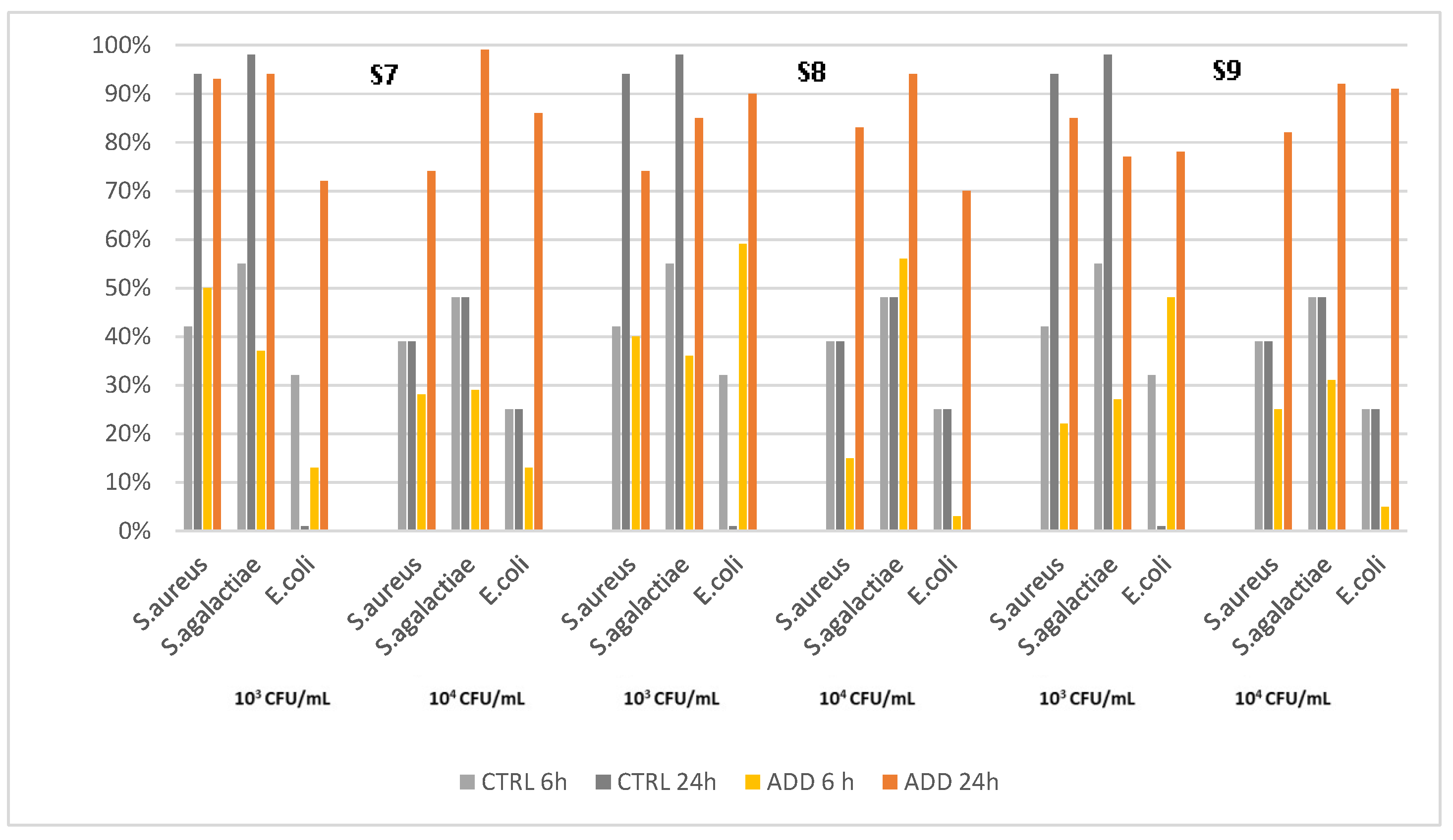
3.2. Silicone Elastomers
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Steeneveld, W.; Wolf, C.A. Production Diseases Reduce the Efficiency of Dairy Production: A Review of the Results, Methods, and Approaches Regarding the Economics of Mastitis. Annu. Rev. Resour. Econ. 2019, 11, 289–312. [Google Scholar] [CrossRef]
- Luo, T.; Steeneveld, W.; Nielen, M.; Zanini, L.; Zecconi, A. Linear Mixed-Effects Model to Quantify the Association between Somatic Cell Count and Milk Production in Italian Dairy Herds. Animals 2023, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Sora, V.M.; Martino, P.A.; Sbernini, A.; Laterza, G.; Zaghen, F.; Soggiu, A.; Zecconi, A. Epidemiology of Antimicrobial Resistance Genes in Streptococcus agalactiae Sequences from a Public Database in a One Health Perspective. Antibiotics 2022, 11, 1236. [Google Scholar] [CrossRef]
- Crestani, C.; Forde, T.L.; Lycett, S.J.; Holmes, M.A.; Fasth, C.; Persson-Waller, K.; Zadoks, R.N. The fall and rise of group B Streptococcus in dairy cattle: Reintroduction due to human-to-cattle host jumps? Microb. Genom. 2021, 7, 000648. [Google Scholar] [CrossRef]
- Zecconi, A. Contagious mastitis control. FIL-IDF Bull. 2007, 416, 34–40. [Google Scholar]
- Zaghen, F.; Sora, V.M.; Meroni, G.; Laterza, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Epidemiology of Antimicrobial Resistance Genes in Staphyloccocus aureus Isolates from a Public Database in a One Health Perspective—Sample Characteristics and Isolates’ Sources. Antibiotics 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Sora, V.M.; Panseri, S.; Nobile, M.; Di Cesare, F.; Meroni, G.; Chiesa, L.M.; Zecconi, A. Milk Quality and Safety in a One Health Perspective: Results of a Prevalence Study on Dairy Herds in Lombardy (Italy). Life 2022, 12, 786. [Google Scholar] [CrossRef] [PubMed]
- Pennone, V.; Prieto, M.; Alvarez-Ordonez, A.; Cobo-Diaz, J.F. Antimicrobial Resistance Genes Analysis of Publicly Available Staphylococcus aureus Genomes. Antibiotics 2022, 11, 1632. [Google Scholar] [CrossRef]
- Latorre, A.A.; Pacha, P.A.; Gonzalez-Rocha, G.; San Martin, I.; Quezada-Aguiluz, M.; Aguayo-Reyes, A.; Bello-Toledo, H.; Oliva, R.; Estay, A.; Pugin, J.; et al. On-Farm Surfaces in Contact with Milk: The Role of Staphylococcus aureus-Containing Biofilms for Udder Health and Milk Quality. Foodborne Pathog. Dis. 2020, 17, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mein, G.A. The Role of the Milking Machine in Mastitis Control. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Du, B.Y.; Meng, L.; Liu, H.M.; Zheng, N.; Zhang, Y.D.; Guo, X.D.; Zhao, S.G.; Li, F.D.; Wang, J.Q. Impacts of Milking and Housing Environment on Milk Microbiota. Animals 2020, 10, 2339. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Piccinini, R.; Fox, K.L. Epidemiologic study of intramammary infections with Staphylococcus aureus during a control program in nine commercial dairy herds. J. Am. Vet. Med. Assoc. 2003, 223, 684–688. [Google Scholar] [CrossRef]
- Scheib, S.; Leimbach, S.; Avramidis, G.; Bellmann, M.; Nitz, J.; Ochs, C.; Tellen, A.; Wente, N.; Zhang, Y.C.; Viöl, W.; et al. Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique. Pathogens 2023, 12, 560. [Google Scholar] [CrossRef] [PubMed]
- Tenhagen, B.A.; Heuwieser, W. Cluster disinfection to reduce new intramammary infections in lactating dairy cattle. Tierarztl. Umsch. 2007, 62, 364–368. [Google Scholar]
- Riekerink, R.; Ohnstad, I.; van Santen, B.; Barkema, H.W. Effect of an automated dipping and backflushing system on somatic cell counts. J. Dairy Sci. 2012, 95, 4931–4938. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Koop, G.; Hogeveen, H.; Fischer, E.A.J.; van den Borne, B.H.P.; van der Tol, R.; Lam, T. Transmission dynamics of Staphylococcus aureus and Streptococcus agalactiae in a Dutch dairy herd using an automatic milking system. Prev. Vet. Med. 2021, 192, 105384. [Google Scholar] [CrossRef]
- Bonestroo, J.; Fall, N.; Hogeveen, H.; Emanuelson, U.; Klaas, I.C.; van der Voort, M. The costs of chronic mastitis: A simulation study of an automatic milking system farm. Prev. Vet. Med. 2023, 210, 105799. [Google Scholar] [CrossRef]
- Skarbye, A.P.; Krogh, M.A.; Ostergaard, S.R. Retrospective cohort study of management procedures associated with dairy herd-level eradication of Streptococcus agalactiae in the Danish surveillance program. J. Dairy Sci. 2021, 104, 5988–5997. [Google Scholar] [CrossRef]
- Bohrz, D.D.S.; Webber, B.; Vancin, F.R.; Daroit, L.; Pilotto, F.; dos Santos, L.R.; Rodrigues, L.B. Staphylococcus aureus and Mesophilic Aerobic Bacteria Quantification in Hygienization Process of Milking Equipment. Acta Sci. Vet. 2019, 47, 6. [Google Scholar] [CrossRef]
- Santos, A.L.; Pires, A.C.S.; Behaine, J.J.S.; Araújo, E.A.; de Andrade, N.J.; de Carvalho, A.F. Effect of Cleaning Treatment on Adhesion of Streptococcus agalactiae to Milking Machine Surfaces. Food Bioprocess Technol. 2013, 6, 1868–1872. [Google Scholar] [CrossRef]
- Sukthavorn, K.; Nootsuwan, N.; Jongrungruangchok, S.; Veranitisagul, C.; Koonsaeng, N.; Laobuthee, A. Effect of nano-silver coated carbon black on curing, mechanical, antimicrobial, and electrical properties of natural rubber composite. J. Appl. Polym. Sci. 2022, 139, 51680. [Google Scholar] [CrossRef]
- Tomacheski, D.; Pittol, M.; Ribeiro, V.F.; Santana, R.M.C. Efficiency of silver-based antibacterial additives and its influence in thermoplastic elastomers. J. Appl. Polym. Sci. 2016, 133, 10. [Google Scholar] [CrossRef]
- Peddinti, B.S.T.; Scholle, F.; Ghiladi, R.A.; Spontak, R.J. Photodynamic Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat. ACS Appl. Mater. Interfaces 2018, 10, 25955–25959. [Google Scholar] [CrossRef]
- Boenigk, J.; Beisser, D.; Zimmermann, S.; Bock, C.; Jakobi, J.; Grabner, D.; Großmann, L.; Rahmann, S.; Barcikowski, S.; Sures, B. Effects of Silver Nitrate and Silver Nanoparticles on a Planktonic Community: General Trends after Short-Term Exposure. PLoS ONE 2014, 9, e95340. [Google Scholar] [CrossRef]
- Kaczor, P.; Bazan, P.; Kuciel, S. Bioactive Polyoxymethylene Composites: Mechanical and Antibacterial Characterization. Materials 2023, 16, 5718. [Google Scholar] [CrossRef] [PubMed]
- Przybylek, M.; Bakar, M.; Mendrycka, M.; Kosikowska, U.; Malm, A.; Worzakowska, M.; Szymborski, T.; Kedra-Krlik, K. Rubber elastomeric nanocomposites with antimicrobial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 269–277. [Google Scholar] [CrossRef]
- Zecconi, A.; Dell’Orco, F.; Rizzi, N.; Vairani, D.; Cipolla, M.; Pozzi, P.; Zanini, L. Cross-sectional study on the prevalence of contagious pathogens in bulk tank milk and their effects on somatic cell counts and milk yield. Ital. J. Anim. Sci. 2019, 19, 66–74. [Google Scholar] [CrossRef]
- Holmoy, I.H.; Toftaker, I.; Kirkeby, C.; Osteras, O.; Jorgensen, H.J.; Nodtvedt, A. A cohort study of the effect of Streptococcus agalactiae on milk yield and somatic cell count in Norwegian dairy cows. J. Dairy Sci. 2019, 102, 8385–8399. [Google Scholar] [CrossRef]
- Cuny, C.; Friedrich, A.; Kozytska, S.; Layer, F.; Nübel, U.; Ohlsen, K.; Strommenger, B.; Walther, B.; Wieler, L.; Witte, W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med. Microbiol. 2010, 300, 109–117. [Google Scholar] [CrossRef]
- Svennesen, L.; Nielsen, S.S.; Mahmmod, Y.S.; Kromker, V.; Pedersen, K.; Klaas, I.C. Association between teat skin colonization and intramammary infection with Staphylococcus aureus and Streptococcus agalactiae in herds with automatic milking systems. J. Dairy Sci. 2019, 102, 629–639. [Google Scholar] [CrossRef]
- Skarbye, A.P.; Krogh, M.A.; Denwood, M.; Bjerring, M.; Ostergaard, S. Effect of enhanced hygiene on transmission of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in dairy herds with automatic milking systems. J. Dairy Sci. 2021, 104, 7195–7209. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Piccinini, R.; Casirani, G.; Binda, E.; Migliorati, L. Effects of automatic milking system on teat tissues, intramammary infections and somatic cell counts. Ital. J. Anim. Sci. 2003, 2, 275–282. [Google Scholar] [CrossRef]
- Bazan, P.; Mazur, K.E.; Rybicka, K.; Kuciel, S. The influence of organic and inorganic antibacterial additives on the strength and biocidal properties of thermoplastic elastomers (TPO). Ind. Crops Prod. 2023, 198, 116682. [Google Scholar] [CrossRef]
- Tomacheski, D.; Pittol, M.; Simoes, D.N.; Ribeiro, V.F.; Santana, R.M.C. Influence of natural ageing on mechanical, thermal and antimicrobial properties of thermoplastic elastomers containing silver nanoparticles and titanium dioxide. Polym. Bull. 2018, 75, 3917–3934. [Google Scholar] [CrossRef]

| Main Components | Patented Antimicrobial Additive (1%) | Acronym (in Tables and Figures) |
|---|---|---|
| NBR Acrylonitrile Copolymer (CAS 9003-18-3) 50–60% Carbon black (CAS: 1333-86-4) 3–7% Plasticizer (CAS 103-23-1) 6–10% Calcined kaolin (CAS 92704-41-1) 20–24% Precipitated silica (112926-00-8) 4–6% Stearic acid (CAS: 57-11-4) 0.1–1% TMTM (CAS 97-74-5) 0.1–1% ZDBC (CAS 136-23-2) 0.1–1% Sulfur (CAS 7704-14-9) 0.1–2% | Zinc oxide (CAS 1314-13-2) Magnesium oxide (CAS 1309-48-4) Silver chloride (CAS 7783-90-6) Silver phosphate glass (CAS- 308069-39-8) Zinc pyrithione (CAS 13463-41-7) | R1 |
| Butilic rubber (CAS 9010-85-5) 54–57% Carbon black (CAS: 1333-86-4) 26–29% Calcium carbonate (CAS 1317-65-3) 9.5–11% Stearic acid (CAS: 57-11-4) 0.1–1% ZOEC (CAS 14324-55-1) 0.25–1% TMDM (CAS 137-26-8) 0.4–1.8% Sulfur (CAS 7704-14-9) 0.1–2% | Zinc oxide (CAS 1314-13-2) Magnesium oxide (CAS 1309-48-4) Silver chloride (CAS 7783-90-6) Silver phosphate glass (CAS 308069-39-8) Zinc pyrithione (CAS 13463-41-7) | R2 |
| Main Components | Patented Antimicrobial Additive | Acronym (in Table and Figures) |
|---|---|---|
| Silicone rubber (CAS: 63394-02-5) 95–99% 50% Dicumyl peroxide (CAS 80-43-3)– Organic peroxides, H242 50%2.5-Dimethyl-2,5-di(tert-butylperox) hexane (CAS 78-63-7)–Organic peroxides, H24 | Silver chloride (CAS 7783-90-6) Silver phosphate glass (CAS 308069-39-8) Zinc pyrithione (CAS 13463-41-7) | S3 |
| Zinc oxide (CAS 1314-13-2) Magnesium oxide (CAS 1309-48-4) Silver chloride (CAS 7783-90-6) Silver phosphate glass (CAS 308069-39-8) Zinc pyrithione (CAS 13463-41-7) | S4 | |
| Magnesium oxide (CAS 1309-48-4) Silver chloride (CAS 7783-90-6) Silver phosphate glass (CAS 308069-39-8) Zinc pyrithione (CAS 13463-41-7) | S5 | |
| Zinc oxide (CAS 1314-13-2) Magnesium oxide (CAS 1309-48-4) Silver chloride (CAS 7783-90-6) Silver phosphate glass (CAS 308069-39-8) | S6 | |
| 2% Silver phosphate glass (CAS 308069-39-8) Zinc pyrithione (CAS 13463-41-7) | S7 | |
| Same as above but at 3% concentration | S8 | |
| Same as above but at 4% concentration | S9 |
| Pathogen | Median Survival Rate (%) | Control vs. R1 (PAA-Added) p = | |||||
|---|---|---|---|---|---|---|---|
| Control | R1 | ||||||
| S. aureus | T1 a | T6 | T24 | T1 | T6 | T24 | |
| 103 CFU/mL | 82 b | 81 | 0 | 70 | 50 | 0 | <0.0001 |
| 104 CFU/mL | 95 | 75 | 14 | 78 | 50 | 0 | <0.0001 |
| S. agalactiae | |||||||
| 103 CFU/mL | 96 | 61 | 0 | 70 | 50 | 0 | <0.0001 |
| 104 CFU/mL | 97 | 86 | 2 | 67 | 50 | 0 | <0.0001 |
| E. coli | |||||||
| 103 CFU/mL | 79 | 53 | 7 | 67 | 50 | 0 | <0.0001 |
| 104 CFU/mL | 80 | 56 | 3 | 67 | 50 | 0 | <0.0001 |
| Pathogen | Median Survival Rate (%) | Control vs. R2 (PAA-Added) p = | |||||
|---|---|---|---|---|---|---|---|
| Control | R2 | ||||||
| S. aureus | T1 a | T6 | T24 | T1 | T6 | T24 | |
| 103 CFU/mL | 100 b | 84 | 12 | 95 | 71 | 2 | <0.0001 |
| 104 CFU/mL | 92 | 78 | 12 | 86 | 65 | 0 | <0.0001 |
| S. agalactiae | |||||||
| 103 CFU/mL | 79 | 63 | 0 | 75 | 0 | 0 | <0.0001 |
| 104 CFU/mL | 85 | 55 | 0 | 73 | 0 | 0 | <0.0001 |
| E. coli | |||||||
| 103 CFU/mL | 88 | 57 | 0 | 90 | 85 | 44 | <0.0001 |
| 104 CFU/mL | 88 | 54 | 0 | 94 | 82 | 28 | <0.0001 |
| Pathogen | Median Survival Rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | S3 | S4 | |||||||
| S. aureus | T1 a | T6 | T24 | T1 | T6 | T24 | T1 | T6 | T24 |
| 103 CFU/mL | 98 b | 79 | 6 | 87 | 62 | 3 | 95 | 68 | 3 |
| 104 CFU/mL | 86 | 77 | 8 | 80 | 40 | 0 | 78 | 51 | 0 |
| S. agalactiae | |||||||||
| 103 CFU/mL | 79 | 71 | 2 | 90 | 45 | 0 | 85 | 0 | 0 |
| 104 CFU/mL | 81 | 79 | 50 | 93 | 50 | 0 | 100 | 0 | 0 |
| E. coli | |||||||||
| 103 CFU/mL | 88 | 84 | 84 | 98 | 81 | 0 | 91 | 76 | 3 |
| 104 CFU/mL | 92 | 93 | 99 | 87 | 64 | 0 | 89 | 61 | 1 |
| Pathogen | Median Survival Rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | S5 | S6 | |||||||
| S. aureus | T1a | T6 | T24 | T1 | T6 | T24 | T1 | T6 | T24 |
| 103 CFU/mL | 98 b | 79 | 6 | 90 | 57 | 1 | 96 | 67 | 2 |
| 104 CFU/mL | 86 | 77 | 8 | 93 | 68 | 1 | 95 | 67 | 4 |
| S. agalactiae | |||||||||
| 103 CFU/mL | 79 | 71 | 2 | 100 | 0 | 0 | 100 | 51 | 0 |
| 104 CFU/mL | 81 | 79 | 50 | 100 | 51 | 0 | 100 | 58 | 0 |
| E. coli | |||||||||
| 103 CFU/mL | 88 | 84 | 84 | 85 | 74 | 2 | 86 | 68 | 4 |
| 104 CFU/mL | 92 | 93 | 99 | 76 | 50 | 1 | 80 | 61 | 1 |
| Pathogen | Median Survival Rate (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | S7 | S8 | S9 | |||||||||
| S. aureus | T1 a | T6 | T24 | T1 | T6 | T24 | T1 | T6 | ||||
| 103 CFU/mL | 98 b | 79 | 6 | 99 | 75 | 7 | 95 | 76 | 26 | 98 | 77 | 5 |
| 104 CFU/mL | 86 | 77 | 8 | 95 | 86 | 26 | 96 | 62 | 17 | 93 | 87 | 18 |
| S. agalactiae | ||||||||||||
| 103 CFU/mL | 79 | 71 | 2 | 91 | 81 | 6 | 98 | 81 | 14 | 94 | 86 | 22 |
| 104 CFU/mL | 81 | 79 | 50 | 99 | 84 | 0.4 | 99 | 72 | 6 | 97 | 84 | 8 |
| E. coli | ||||||||||||
| 103 CFU/mL | 88 | 84 | 84 | 97 | 93 | 27 | 99 | 71 | 7 | 94 | 78 | 22 |
| 104 CFU/mL | 92 | 93 | 99 | 96 | 93 | 15 | 100 | 98 | 31 | 99 | 95 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meroni, G.; Sora, V.; Zaghen, F.; Laterza, G.; Martino, P.A.; Zecconi, A. Innovative Elastomers with Antimicrobial Activity May Decrease Infection Risks during Milking. Pathogens 2023, 12, 1431. https://doi.org/10.3390/pathogens12121431
Meroni G, Sora V, Zaghen F, Laterza G, Martino PA, Zecconi A. Innovative Elastomers with Antimicrobial Activity May Decrease Infection Risks during Milking. Pathogens. 2023; 12(12):1431. https://doi.org/10.3390/pathogens12121431
Chicago/Turabian StyleMeroni, Gabriele, Valerio Sora, Francesca Zaghen, Giulia Laterza, Piera Anna Martino, and Alfonso Zecconi. 2023. "Innovative Elastomers with Antimicrobial Activity May Decrease Infection Risks during Milking" Pathogens 12, no. 12: 1431. https://doi.org/10.3390/pathogens12121431






