Antibiotic Resistance Genes, Virulence Factors, and Biofilm Formation in Coagulase-Negative Staphylococcus spp. Isolates from European Hakes (Merluccius merluccius, L.) Caught in the Northeast Atlantic Ocean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Collection
2.2. Sample Collection, Bacterial Isolation and Antimicrobial Activity Assays
2.3. Taxonomic Identification of Bacterial Isolates
2.4. Genetic Diversity Analysis by Enterobacterial Repetitive Intergenic Consensus—PCR (ERIC-PCR)
2.5. Biofilm Formation and Quantification Assays
2.6. Antibiotic Susceptibility Testing
2.7. Antibiotic Resistance and Virulence Factor Genes
2.8. Statistical Analyses
3. Results
3.1. Identification of the CoNS Isolated from Fecal and Intestinal Samples from European Hakes
3.2. Genetic Diversity Analysis by ERIC-PCR
3.3. Biofilm Formation
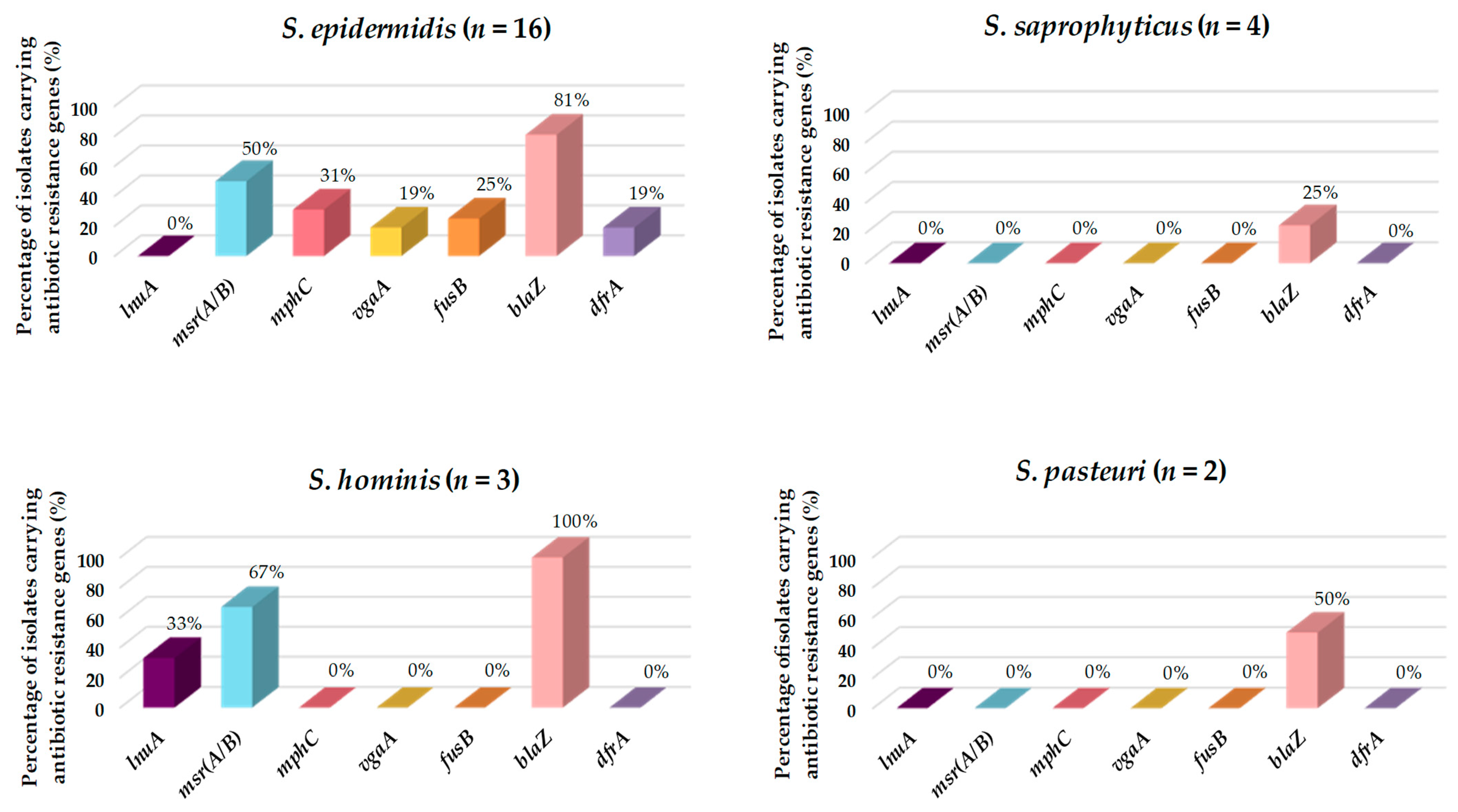
3.4. Antibiotic Resistance and Virulence Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The FAO Action Plan on Antimicrobial Resistance 2016–2020. 2016. Available online: https://www.fao.org/3/i5996s/i5996s.pdf (accessed on 1 September 2023).
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2022. 2022. Available online: https://www.fao.org/documents/card/en/c/cc0461es (accessed on 1 September 2023).
- Ministerio de Agricultura, Alimentación y Medio Ambiente (MAPA). El Mercado de la Merluza en España. El mercado de la Merluza en España. 2016. Available online: https://www.mapa.gob.es/es/pesca/temas/mercados-economia-pesquera/informemerluzaabril2016_tcm30-291641.pdf (accessed on 15 September 2023).
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Estadísticas Pesqueras: Pesca Marítima. 2022. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-pesqueras/pesca-maritima/ (accessed on 15 September 2023).
- World Health Organization (WHO). Antimicrobial Resistance. 2020. Available online: https://www.who.int/es/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 17 September 2023).
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rheman, S.; Debnath, N.; Delamare-Deboutteville, J.; Akhtar, Z.; Ghosh, S.; Parveen, S.; Islam, K.; Islam, A.; Rashid, M.; et al. Antibiotics usage practices in aquaculture in Bangladesh and their associate factors. One Health 2022, 15, 100445. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Reviews of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Muñoz-Atienza, E.; Gómez-Sala, B.; Araújo, C.; Campanero, C.; del Campo, R.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 2013, 13, 15–36. [Google Scholar] [CrossRef]
- Anadón, A. The EU ban of antibiotics as feed additives: Alternatives and consumer safety. J. Vet. Pharmacol. Ther. 2006, 29, 41–44. [Google Scholar] [CrossRef]
- Commission Regulation (UE). No. 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification as Regards Maximum Residue Limits in Foodstuffs of Animal Origin. DOUE. No 15, of 20 January 2010. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2010-80044 (accessed on 20 September 2023).
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaclture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents. 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Gravningen, K.; Sorum, H.; Horsberg, T.E. The future of therapeutic agents in aquaculture. Rev. Sci. Tech. 2009, 38, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Understanding Antimicrobial Resistances in Aquaculture. Asian Fisheries Society. 2020. Available online: http://www.fao.org/publications/card/en/c/CB2601EN/ (accessed on 20 September 2023).
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. mBio 2012, 3, 305–311. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations. Rev. Antimicrob. Resist. 2016, 1–84. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjElreVvIyDAxVskVYBHUb6C4oQFnoECAoQAQ&url=https%3A%2F%2Famr-review.org%2Fsites%2Fdefault%2Ffiles%2F160518_Final%2520paper_with%2520cover.pdf&usg=AOvVaw0kDaiLbLr8dtJAJDnpiBEF&opi=89978449 (accessed on 1 September 2023).
- Food and Agriculture Organization-World Organization for Animal Health-World Health Organization (FAO-OIE-WHO). The Tripartite’s Commitment Providing Multi-Sectoral, Collaborative Leadership in Addressing Health Challenges; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Chessa, D.; Ganau, G.; Mazzarello, V. An overview of Staphylococcus epidermidis and Staphylococcus aureus with a focus on developing countries. J. Infect. Dev. Ctries. 2015, 9, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Almeida, L.; Gaio, V.; Cerca, N.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Biofilm formation of multidrug-resistant MRSA strains isolated from different types of human infections. Pathogens 2021, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Coagulase-negative staphylococci (CoNS) isolated from ready-to-eat food of animal origin—Phenotypic and genotypic antibiotic resistance. Food Microbiol. 2015, 46, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, A.; Stahnke, L. Growth and aroma production by Staphylococcus xylosus, S. carnosus and S. equorum—A comparative study in model systems. Int. J. Food Microbiol. 2002, 75, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Piette, A.; Verschraegen, G. Role of coagulase-negative staphylococci in human disease. Vet. Microbiol. 2009, 134, 45–54. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [CrossRef]
- Gómez-Sanz, E.; Ceballos, S.; Ruiz-Ripa, L.; Zarazaga, M.; Torres, C. Clonally diverse methicillin and multidrug resistant coagulase negative staphylococci are ubiquitous and pose transfer ability between pets and their owners. Front. Microbiol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Vaiyapuri, M.; Joseph, T.C.; Rao, B.M.; Lalitha, K.V.; Prasad, M.M. Methicillin-Resistant Staphylococcus aureus in seafood: Prevalence, laboratory detection, clonal nature, and control in seafood chain. J. Food Sci. 2019, 84, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, W.; Korzeniewska, E.; Harnisz, M.; Drzymała, J.; Felis, E.; Bajkacz, S. Wastewater treatment plants as a reservoir of integrase and antibiotic resistance genes—An epidemiological threat to workers and environment. Environ. Int. 2021, 156, 106641. [Google Scholar] [CrossRef]
- Kukułowicz, A.; Steinka, I.; Siwek, A. Presence of antibiotic-resistant Staphylococcus aureus in fish and seafood originating from points of sale in the Tri-City Area (Poland). J. Food Prot. 2021, 84, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.A.; Smith, J.T.; Mydosh, J.L.; Ball, J.; Needle, D.B.; Gibson, R.; Andam, C.P. Shared antibiotic resistance and virulence genes in Staphylococcus aureus from diverse animal hosts. Sci. Rep. 2022, 12, 4413. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Fri, J.; Njom, H.A.; Ateba, C.N.; Ndip, R.N. Antibiotic resistance and virulence gene characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) isolated from healthy edible marine fish. Int. J. Microbiol. 2020, 9803903. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, M.; Dabrowska, A.; Murray, G.G.R.; Kett, S.M.; Vick, A.J.A.; Banister, S.C.; Munoz, L.P.; Cunningham, P.; Welch, J.J.; Holmes, M.A.; et al. Absence of Staphylococcus aureus in wild populations of fish supports a spillover hypothesis. Microbiol. Spectr. 2023, 11, e0485822. [Google Scholar] [CrossRef] [PubMed]
- Uniacke-Lowe, S.; Collins, F.W.J.; Hill, C.; Ross, R.P. Bioactivity Screening and Genomic Analysis Reveals Deep-Sea Fish Microbiome Isolates as Sources of Novel Antimicrobials. Mar. Drugs 2023, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commercial Designations. Fishing Zones. Available online: https://fish-commercial-names.ec.europa.eu/fish-names/fishing-areas_es (accessed on 15 October 2023).
- Cintas, L.M.; Rodríguez, J.M.; Fernández, M.F.; Sletten, K.; Nes, I.F.; Hernández, P.E.; Holo, H. Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl. Environ. Microbiol. 1995, 61, 2643–2648. [Google Scholar] [CrossRef]
- Jaffrès, E.; Sohier, D.; Leroi, F.; Pilet, M.F.; Prévost, H.; Joffraud, J.J.; Dousset, X. Study of the bacterial ecosystem in tropical cooked and peeled shrimps using a polyphasic approach. Int. J. Food Microbiol. 2009, 131, 20–29. [Google Scholar] [CrossRef]
- Araújo, C.; Muñoz-Atienza, E.; Ramírez, M.; Poeta, P.; Igrejas, G.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Safety assessment, genetic relatedness and bacteriocin activity of potential probiotic Lactococcus lactis strains from rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. Eur. Food Res. Technol. 2015, 241, 647–662. [Google Scholar] [CrossRef]
- Oniciuc, E.-A.; Cerca, N.; Nicolau, A.I. Compositional Analysis of Biofilms Formed by Staphylococcus aureus isolated from food sources. Front. Microbiol. 2016, 7, 390. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 13.1. 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf (accessed on 20 June 2023).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard—Second Edition; document M31-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002; ISBN 1-56238-461-9. [Google Scholar]
- Silva, V.; Almeida, F.; Silva, A.; Correia, S.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Caniça, M.; Igrejas, G.; et al. First report of linezolid-resistant cfr-positive methicillin-resistant Staphylococcus aureus in humans in Portugal. J. Glob. Antimicrob. Resist. 2019, 17, 323–325. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of panton-valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; van Kessel, K.P.M.; van Strijp, J.A.G. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Y.; Lv, J.; Qi, X.; Lu, C.; Ding, Y.; Li, D.; Liu, H.; Wang, L. Antimicrobial susceptibility, virulence determinant carriage and molecular characteristics of Staphylococcus aureus isolates associated with skin and soft tissue infections. Braz. J. Infect. Dis. 2015, 19, 614–622. [Google Scholar] [CrossRef]
- Silva, V.; Vieira-Pinto, M.; Saraiva, C.; Manageiro, V.; Reis, L.; Ferreira, E.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Prevalence and characteristics of multidrug-resistant livestock-associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) CC398 isolated from quails (Coturnix coturnix japonica) slaughtered for human consumption. Animals 2021, 11, 2038. [Google Scholar] [CrossRef] [PubMed]
- Proia, L.; von Schiller, D.; Sànchez-Melsió, A.; Sabater, S.; Borrego, C.M.; Rodríguez-Mozaz, S.; Balcázar, J.L. Occurrence and persistence of antibiotic resistance genes in river biofilms after wastewater inputs in small rivers. Environ. Pollut. 2016, 210, 121–128. [Google Scholar] [CrossRef]
- Silva, V.; Almeida, F.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Tejedor-Junco, M.T.; Caniça, M.; Igrejas, G.; Poeta, P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G.; Popa, M.; Gheorghe-Barbu, I.; Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.-F.; Kemper, M.A.; Spießberger, B.; Wengenroth, L.; et al. Wastewater treatment plants, an “escape gate” for ESCAPE pathogens. Front. Microbiol. 2023, 14, 1193907. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Lozano, C.; Benito, D.; Estepa, V.; Tenorio, C.; Zarazaga, M.; Torres, C. Characterization of staphylococci in urban wastewater treatment plants in Spain, with detection of methicillin resistant Staphylococcus aureus ST398. Environ. Pollut. 2016, 212, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Martineau, F.; Picard, F.J.; Ke, D.; Paradis, S.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol. 2001, 39, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Regecová, I.; Pipová, M.; Jevinová, P.; Marušková, K.; Kmeť, V.; Popelka, P. Species identification and antimicrobial resistance of coagulase-negative staphylococci isolated from the meat of sea fish. J. Food Sci. 2014, 79, M898–M902. [Google Scholar] [CrossRef] [PubMed]
- Canak, O.; Timur, G. An initial survey on the occurrence of staphylococcal infections in Turkish marine aquaculture (2013–1014). J. Appl. Ichthyol. 2020, 36, 932–941. [Google Scholar] [CrossRef]
- Hsu, B.M.; Chen, J.S.; Lin, I.C.; Hsu, G.J.; Koner, S.; Hussain, B.; Huang, S.W.; Tsai, H.C. Molecular and anti-microbial Resistance (AMR) profiling of Methicillin-Resistant Staphylococcus aureus (MRSA) from hospital and long-term care facilities (LTCF) environment. Antibiotics 2021, 10, 748. [Google Scholar] [CrossRef]
- Abdul-Aziz, A. ERIC-PCR fingerprint profiling and genetic diversity of coagulase negative Staphylococcus in Malaysia. Malays. J. Microbiol. 2020, 16, 7–16. [Google Scholar] [CrossRef]
- Feito, J.; Araújo, C.; Gómez-Sala, B.; Contente, D.; Campanero, C.; Arbulu, S.; Saralegui, C.; Peña, N.; Muñoz-Atienza, E.; Borrero, J.; et al. Antimicrobial activity, molecular typing and in vitro safety assessment of Lactococcus garvieae isolates from healthy cultured rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. LWT—Food Sci. Technol. 2022, 162, 113496. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Correia, T.M.A.; Oliveira, A.P.D.; Miyasato, I.F.; Santos, T.M.B.; Dias, F.S. Characterization of Staphylococcus aureus isolated from tilapia and utensils used in the commercialization of tilapia in the street markets of a semi-arid Brazilian municipality. Acta Sci. Pol. Technol. Aliment. 2019, 18, 413–425. [Google Scholar] [CrossRef]
- Hamza, M.; Sivaraman, G.K.; Mothadaka, M.P. Multidrug-resistant phenotypes among biofilm-producing staphylococcal isolates from farm-raised fish: A molecular scrutiny. J. Appl. Microbiol. 2023, 134, lxad136. [Google Scholar] [CrossRef] [PubMed]
- Feazel, L.M.; Baumgartner, L.K.; Peterson, K.L.; Frank, D.N.; Harris, J.K.; Pace, N.R. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. USA 2009, 106, 16393–16399. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.; Witt, N.; Flemming, H.-C.; Wingender, J. Faecal indicator bacteria in river biofilms. Water Sci. Technol. 2010, 61, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Flemming, H.-C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-negative staphylococci pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef]
- International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): Guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 2009, 53, 4961–4967. [Google Scholar] [CrossRef]
- Raz, R.; Colodner, R.; Kunin, C.M. Who are you: Staphylococcus saprophyticus? Clin. Infect. Dis. 2005, 40, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Hayami, H.; Takahashi, S.; Ishikawa, K.; Yasuda, M.; Yamamoto, S.; Wada, K.; Kobayashi, K.; Hamasuna, R.; Minamitani, S.; Matsumoto, T.; et al. Second nationwide surveillance of bacterial pathogens in patients with acute uncomplicated cystitis conducted by Japanese Surveillance Committee from 2015 to 2016: Antimicrobial susceptibility of Escherichia coli, Klebsiella pneumoniae, and Staphylococcus saprophyticus. J. Infect. Chemother. 2019, 25, 413–422. [Google Scholar] [CrossRef]
- Lawal, O.U.; Barata, M.; Fraqueza, M.J.; Worning, P.; Bartels, M.D.; Goncalves, L.; Paixão, P.; Goncalves, E.; Toscano, C.; Empel, J.; et al. Staphylococcus saprophyticus from clinical and environmental origins have distinct biofilm composition. Front. Microbiol. 2021, 12, 663768. [Google Scholar] [CrossRef]
- Otto, M. Virulence factors of the coagulase-negative staphylococci. Front. Biosci. 2004, 9, 841–863. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Yamashita, A.; Hirakawa, H.; Kumano, M.; Morikawa, K.; Higashide, M.; Maruyama, A.; Inose, Y.; Matoba, K.; Toh, H.; et al. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc. Natl. Acad. Sci. USA 2005, 102, 13272–13277. [Google Scholar] [CrossRef] [PubMed]
- Tevell, S.; Hellmark, B.; Nilsdotter-Augustinsson, Å.; Söderquist, B. Staphylococcus capitis isolated from prosthetic joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Laurent, F.; Butin, M. Staphylococcus capitis and NRCS-A clone: The story of an unrecognized pathogen in neonatal intensive care units. Clin. Microbiol. Infect. 2019, 25, 1081–1085. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.R.; Jiang, J.-H.; Hassan, K.A.; Elbourne, L.D.H.; Tuck, K.L.; Paulsen, I.T.; Peleg, A.Y. Insights on virulence from Staphylococcus capitis. Front. Microbiol. 2015, 6, 980. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.E.; Bengtsson, R.J.; Horsburgh, M.J. Comparative genomics of Staphylococcus capitis reveals species determinants. Front. Microbiol. 2022, 13, 1005949. [Google Scholar] [CrossRef]
- D’Azevedo, P.A.; Trancesi, R.; Sales, T.; Monteiro, J.; Gales, A.C.; Pignatari, A.C. Outbreak of Staphylococcus hominis subsp. novobiosepticus bloodstream infections in São Paulo city, Brazil. J. Med. Microbiol. 2008, 57, 256–257. [Google Scholar] [CrossRef]
- Sorlozano, A.; Gutierrez, J.; Martinez, T.; Yuste, M.E.; Perez-Lopez, J.A.; Vindel, A.; Guillen, J.; Boquete, T. Detection of new mutations conferring resistance to linezolid in glycopeptide-intermediate susceptibility Staphylococcus hominis subspecies hominis circulating in an intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 73–80. [Google Scholar] [CrossRef]
- Ruiz de Gopegui, E.; Iuliana Marinescu, C.; Diaz, P.; Socias, A.; Garau, M. Nosocomial spread of linezolid-resistant Staphylococcus hominis in two hospitals in Majorca. Enferm. Infecc. Microbiol. Clin. 2011, 29, 339–344. [Google Scholar] [CrossRef]
- Szczuka, E.; Krzymińska, S.; Bogucka, N.; Kaznowski, A. Multifactorial mechanisms of the pathogenesis of methicillin-resistant Staphylococcus hominis isolated from bloodstream infections. Antonie Leeuwenhoek 2018, 111, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Santoiemma, P.P.; Kalainov, D.M.; Mehta, M.P.; Bolon, M.K. An unusual case of Staphylococcus pasteuri osteomyelitis. Infect. Dis. Rep. 2020, 12, 8523. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Donat, E.; Ribes-Koninckx, C.; Fernández-Murga, M.L.; Sanz, Y. Duodenal-mucosal bacteria associated with celiac disease in children. Appl. Environ. Microbiol. 2013, 79, 5472–5479. [Google Scholar] [CrossRef]
- Pantůček, R.; Sedláček, I.; Indráková, A.; Vrbovská, V.; Mašlaňová, I.; Kovařovic, V.; Švec, P.; Králová, S.; Krištofová, L.; Kekláková, J.; et al. Staphylococcus edaphicus sp. nov., isolated in Antarctica, harbors the mecC gene and genomic islands with a suspected role in adaptation to extreme environments. Appl. Environ. Microbiol. 2018, 84, e01746-17. [Google Scholar] [CrossRef] [PubMed]
- de Alcântara Rodrigues, I.; Ferrari, R.G.; Panzenhagen, P.H.N.; Mano, S.B.; Conte-Junior, C.A. Antimicrobial resistance genes in bacteria from animal-based foods. Adv. Appl. Microbiol. 2020, 112, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.; Muurinen, J.; Virta, M.P.; Brandt, K.K.; Zhu, Y. Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Crit. Rev. Environ. Sci. 2021, 51, 2159–2196. [Google Scholar] [CrossRef]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A review of current bacterial resistance to antibiotics in food animals. Front. Microbiol. 2022, 13, 822689. [Google Scholar] [CrossRef] [PubMed]
- Ikwap, K.; Gertzell, E.; Hansson, I.; Dahlin, L.; Selling, K.; Magnusson, U.; Dione, M.; Jacobson, M. The presence of antibiotic-resistant Staphylococcus spp. and Escherichia coli in smallholder pig farms in Uganda. BMC Vet. Res. 2021, 17, 31. [Google Scholar] [CrossRef]
- Pimenta, L.K.L.; Rodrigues, C.A.; Filho, A.R.G.; Coelho, C.J.; Goes, V.; Estrela, M.; de Souza, P.; Avelino, M.A.G.; Vieira, J.D.G.; Carneiro, L. Staphylococcus spp. causatives of infections and carrier of blaZ, femA, and mecA genes associated with resistance. Antibiotics 2023, 12, 671. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). ECDC/EFSA/EMA Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals. EFSA J. 2017, 15, 4872. [Google Scholar]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.; Feil, E.J.; Lindsay, J.A.; Peacock, S.J.; Day, N.P.J.; Enright, M.C.; Foster, T.J.; Moore, C.E.; Hurst, L.; Atkin, R.; et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 2004, 101, 9786–9791. [Google Scholar] [CrossRef] [PubMed]
- Llarrull, L.I.; Mobashery, S. Dissection of events in the resistance to β-lactam antibiotics mediated by the protein BlaR1 from Staphylococcus aureus. Biochemistry 2012, 51, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Pence, M.A.; Haste, N.M.; Meharena, H.S.; Olson, J.; Gallo, R.L.; Nizet, V.; Kristian, S.A. Beta-Lactamase Repressor BlaI Modulates Staphylococcus aureus cathelicidin antimicrobial peptide resistance and virulence. PLoS ONE 2015, 10, e0136605. [Google Scholar] [CrossRef]
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Ponce, F.; Higuera-Llantén, S.; Parás-Silva, J.; Gamboa-Acuña, N.; Cortés, J.; Opazo-Capurro, A.; Ugalde, J.A.; Alcalde-Rico, M.; Olivares-Pacheco, J. Genetic characterization of clinically relevant class 1 integrons carried by multidrug resistant bacteria (MDRB) isolated from the gut microbiota of highly antibiotic treated Salmo salar. J. Glob. Antimicrob. Resist. 2022, 29, 55–62. [Google Scholar] [CrossRef]
- Gu, B.; Kelesidis, T.; Tsiodras, S.; Hindler, J.; Humphries, R.M. The emerging problem of linezolid-resistant Staphylococcus. J. Antimicrob. Chemother. 2013, 68, 4–11. [Google Scholar] [CrossRef]
- Dong, Y.; Miao, X.; Zheng, Y.D.; Liu, J.; He, Q.-Y.; Ge, R.; Sun, X. Ciprofloxacin-Resistant Staphylococcus aureus displays enhanced resistance and virulence in iron-restricted conditions. J. Proteome Res. 2021, 20, 2839–2850. [Google Scholar] [CrossRef]
- Udo, E.E.; Boswihi, S.S.; Mathew, B.; Noronha, B.; Verghese, T. Resurgence of chloramphenicol resistance in Methicillin-Resistant Staphylococcus aureus due to the acquisition of a variant florfenicol exporter (fexAv)-mediated chloramphenicol resistance in Kuwait hospitals. Antibiotics 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Mohammadi, A.; Hajikhani, B.; Abiri, P.; Fazeli, M.; Nasiri, M.J.; Dadashi, M.; Goudarzi, M.; Haghighi, M. Mupirocin-resistant Staphylococcus aureus in Iran: A biofilm production and genetic characteristics. Biomed. Res. Int. 2022, 2022, 7408029. [Google Scholar] [CrossRef] [PubMed]



| Fish | Isolates | Nº CoNS (%) | S. capitis | S. edaphicus | S. epidermidis | S. hominis | S. pasteuri | S. saprophyticus |
|---|---|---|---|---|---|---|---|---|
| Hake A | 17 | 7 (41) | 0 | 1 | 2 | 1 | 0 | 3 |
| Hake B | 11 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hake C | 4 | 4 (100) | 0 | 0 | 3 | 0 | 1 | 0 |
| Hake D | 13 | 7 (53) | 0 | 0 | 5 | 0 | 1 | 1 |
| Hake E | 6 | 1 (17) | 1 | 0 | 0 | 0 | 0 | 0 |
| Hake F | 3 | 2 (66) | 0 | 0 | 2 | 0 | 0 | 0 |
| Hake G | 7 | 6 (86) | 0 | 0 | 4 | 2 | 0 | 0 |
| Hake H | 5 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 66 | 27 (41) | 1 | 1 | 16 | 3 | 2 | 4 |
| CoNS | Antibiotic Resistance | Virulence Factors | |
|---|---|---|---|
| Phenotype | Genotype | ||
| S. saprophyticus MAI5 | FD, PEN | Nf | Nf |
| S. epidermidis MAI9 | ERY, FD, PEN | msr(A/B), vgaA, fusB, blaZ | hla, SCCmecV |
| S. epidermidis MAI11 | ERY, FD, PEN | mphC, msr(A/B) | hla |
| S. saprophyticus MAI15 | FD, KAN, PEN | Nf | Nf |
| S. edaphicus MAI16 | FD, PEN | Nf | Nf |
| S. saprophyticus MAI17 | PEN | Nf | Nf |
| S. hominis MAI20 | PEN | blaZ | Nf |
| S. epidermidis MCI6 | ERY, PEN, SXT | mphC, msr(A/B), blaZ, dfrA | hla, scn |
| S. epidermidis MCI8 | FD, PEN, TOB | fusB, blaZ | hla |
| S. pasteuri MCI10 | PEN | Nf | Nf |
| S. epidermidis MCH6 | PEN | blaZ | Nf |
| S. saprophyticus MDI3 | FD, PEN | blaZ | scn |
| S. epidermidis MDH2 | CN, DA, ERY, FD, FOX, KAN, PEN | mphC, msr(A/B), blaZ | hla |
| S. epidermidis MDH4 | ERY, PEN, SXT | mphC, msr(A/B), blaZ, dfrA | Nf |
| S. epidermidis MDH5 | CN, ERY, FD, KAN, PEN, TOB, SXT | mphC, msr(A/B), blaZ, dfrA | Nf |
| S. epidermidis MDH6 | ERY, FD, PEN | msr(A/B), vgaA, fusB, blaZ | hla, scn |
| S. epidermidis MDH7 | ERY, FD, PEN | msr(A/B), vgaA, fusB, blaZ | hla, scn |
| S. pasteuri MDH8 | PEN | blaZ | Nf |
| S. capitis MEH2 | FD | Nf | Nf |
| S. epidermidis MFH1 | Susceptible | Nd | Nf |
| S. epidermidis MFH8 | Susceptible | Nd | SCCmecIII |
| S. hominis MGI2 | ERY, PEN | msr(A/B), lnuA, blaZ | Nf |
| S. hominis MGI4 | ERY, PEN | msr(A/B), blaZ | Nf |
| S. epidermidis MGH2 | PEN | blaZ | scn |
| S. epidermidis MGH3 | PEN | blaZ | scn |
| S. epidermidis MGH4 | PEN | blaZ | scn |
| S. epidermidis MGH5 | PEN | blaZ | scn |
| Antibiotic a | S. epidermidis (n = 16) | S. saprophyticus (n = 4) | S. hominis (n = 3) | S. pasteuri (n = 2) | S. edaphicus (n = 1) | S. capitis (n = 1) | Total (n = 27) |
|---|---|---|---|---|---|---|---|
| Gentamycin | 12.5 (2) | 0 | 0 | 0 | 0 | 0 | 7.4 (2) |
| Clindamycin | 6.2 (1) | 0 | 0 | 0 | 0 | 0 | 3.7 (1) |
| Erythromycin | 50 (8) | 0 | 66.6 (2) | 0 | 0 | 0 | 37 (10) |
| Fusidic acid | 43.7 (7) | 50 (2) | 0 | 0 | 100 (1) | 100 (1) | 40.7 (11) |
| Cefoxitin | 6.2 (1) | 0 | 0 | 0 | 0 | 0 | 3.7 (1) |
| Kanamycin | 12.5 (2) | 25 (1) | 0 | 0 | 0 | 0 | 7.4 (2) |
| Penicillin | 87.5 (14) | 100 (4) | 100 (3) | 100 (2) | 100 (1) | 0 | 88.8 (24) |
| Trimethoprim-sulfamethoxazole | 18.7 (3) | 0 | 0 | 0 | 0 | 0 | 11.1 (3) |
| Tobramycin | 12.5 (2) | 0 | 0 | 0 | 0 | 0 | 7.4 (2) |
| Sensitive to all antibiotics | 12.5 (2) | 0 | 0 | 0 | 0 | 0 | 7.4 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Formoso, L.; Silva, V.; Contente, D.; Feito, J.; Hernández, P.E.; Borrero, J.; Igrejas, G.; del Campo, R.; Muñoz-Atienza, E.; Poeta, P.; et al. Antibiotic Resistance Genes, Virulence Factors, and Biofilm Formation in Coagulase-Negative Staphylococcus spp. Isolates from European Hakes (Merluccius merluccius, L.) Caught in the Northeast Atlantic Ocean. Pathogens 2023, 12, 1447. https://doi.org/10.3390/pathogens12121447
Díaz-Formoso L, Silva V, Contente D, Feito J, Hernández PE, Borrero J, Igrejas G, del Campo R, Muñoz-Atienza E, Poeta P, et al. Antibiotic Resistance Genes, Virulence Factors, and Biofilm Formation in Coagulase-Negative Staphylococcus spp. Isolates from European Hakes (Merluccius merluccius, L.) Caught in the Northeast Atlantic Ocean. Pathogens. 2023; 12(12):1447. https://doi.org/10.3390/pathogens12121447
Chicago/Turabian StyleDíaz-Formoso, Lara, Vanessa Silva, Diogo Contente, Javier Feito, Pablo E. Hernández, Juan Borrero, Gilberto Igrejas, Rosa del Campo, Estefanía Muñoz-Atienza, Patrícia Poeta, and et al. 2023. "Antibiotic Resistance Genes, Virulence Factors, and Biofilm Formation in Coagulase-Negative Staphylococcus spp. Isolates from European Hakes (Merluccius merluccius, L.) Caught in the Northeast Atlantic Ocean" Pathogens 12, no. 12: 1447. https://doi.org/10.3390/pathogens12121447
APA StyleDíaz-Formoso, L., Silva, V., Contente, D., Feito, J., Hernández, P. E., Borrero, J., Igrejas, G., del Campo, R., Muñoz-Atienza, E., Poeta, P., & Cintas, L. M. (2023). Antibiotic Resistance Genes, Virulence Factors, and Biofilm Formation in Coagulase-Negative Staphylococcus spp. Isolates from European Hakes (Merluccius merluccius, L.) Caught in the Northeast Atlantic Ocean. Pathogens, 12(12), 1447. https://doi.org/10.3390/pathogens12121447












