Ocular Manifestations of Flavivirus Infections
Abstract
:1. Introduction
2. General Aspects
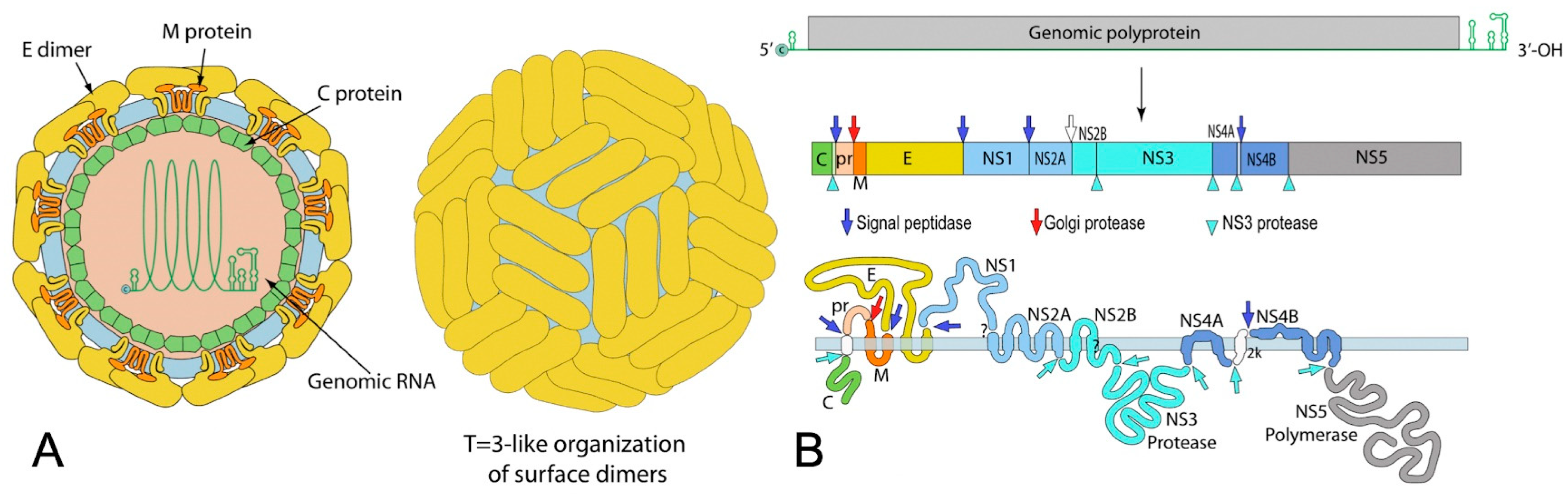
2.1. Virological Features
2.2. Transmission
2.3. Diagnosis
3. Ocular Complications of Flaviviruses
3.1. Dengue Fever (DF)
3.1.1. Epidemiology
3.1.2. Systemic Manifestations
3.1.3. Ocular Manifestations
- -
- Dengue-fever-associated maculopathy:DF-associated maculopathy, the most common ocular manifestation of acute DFV infection, is reported in 10% of hospitalized patients and is serotype-dependent [38,39]. Notably, one study linked maculopathy to serotype specificity, with a 10% incidence during DENV-1 epidemics but nothing during DENV-2 outbreaks [39]. Symptoms typically emerge 3–11 days after fever onset and improve over 2–4 weeks. Patients may present with sudden vision loss, central scotoma, or floaters. The lesions are typically asymmetric and often bilateral, primarily associated with intraretinal hemorrhages, manifesting as dots, blots, or flame-shaped hemorrhages. However, some patients remain asymptomatic, with lesions visible only through fluorescein or indocyanine green angiography (ICGA) [4,7,40,41]. Fluorescein angiography (FA) commonly reveals retinal vascular leakage (Figure 2) and occlusion, while ICGA identifies hypofluorescent spots corresponding to subretinal lesions and additional spots in areas without clinically evident dots [37]. OCT is valuable for detecting and monitoring dengue-induced inflammatory ischemic foveolitis and outer maculopathy, showing disruptions in outer retinal layers, conical foveal elevation, and focal thickening of the outer neurosensory retina RPE, aligning with round foveal yellowish lesions seen clinically (Figure 3). OCT is also instrumental in detecting and assessing associated serous retinal detachment (SRD) and macular edema. Teoh et al. used OCT to categorize patients into three groups: (1) diffuse retinal thickening, (2) cystoid macular edema, and (3) foveolitis. Their findings were correlated with visual acuity and prognosis [40]. DF-related foveolitis pertains to the yellow-orange central foveal lesion in patients with dengue maculopathy, visible on OCT as conical foveal elevation and focal outer neurosensory RPE thickening, often associated with prolonged scotomata persistence [40,42]. In dengue-related maculopathy, the prevalence of cystoid macular edema and foveolitis was evaluated to 24.6% and 33.7%, respectively [40]. More recently, OCT–angiography (OCTA) has revealed ischemia in the deep retinal capillary plexus [40,43].
- -
- Other posterior segment manifestations
- -
- Anterior segment manifestations
- -
- Other ocular manifestations
3.1.4. Diagnosis
3.1.5. Treatment and Prognosis
3.2. West Nile Virus (WNV) Infection
3.2.1. Epidemiology
3.2.2. Systemic Manifestations
3.2.3. Ocular Manifestations
3.2.4. Diagnosis
3.2.5. Treatment and Prognosis
3.3. Yellow Fever Virus
3.3.1. Epidemiology
3.3.2. Systemic Manifestations
3.3.3. Ocular Manifestations
3.3.4. Diagnosis
3.3.5. Treatment and Prognosis
3.4. Zika Virus
3.4.1. Epidemiology
3.4.2. Systemic Manifestations
3.4.3. Ocular Manifestations
3.4.4. Diagnosis
3.4.5. Treatment and Prognosis
3.5. Japanese Encephalitis Virus
3.5.1. Epidemiology
3.5.2. Systemic Manifestations
3.5.3. Ocular Manifestations
3.5.4. Diagnosis
3.5.5. Treatment and Prognosis
3.6. Kyasanur Forest Disease Virus
3.6.1. Epidemiology
3.6.2. Systemic Manifestations
3.6.3. Ocular Manifestations
3.6.4. Diagnosis
3.6.5. Treatment and Prognosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ludwig, G.V.; Iacono-Connors, L.C. Insect-Transmitted Vertebrate Viruses: Flaviviridae. In Vitro Cell. Dev. Biol. Anim. 1993, 29, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, M.-D.; Mazzon, M.; Jacobs, M.; Amara, A. Pathogenesis of Flavivirus Infections: Using and Abusing the Host Cell. Cell Host Microbe 2009, 5, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.S.; Doyle, M.M.; Smart, K.M.; Young, C.C.W.; Drape, G.W.; Johnson, C.K. Predicting Wildlife Reservoirs and Global Vulnerability to Zoonotic Flaviviruses. Nat. Commun. 2018, 9, 5425. [Google Scholar] [CrossRef] [PubMed]
- Merle, H.; Donnio, A.; Jean-Charles, A.; Guyomarch, J.; Hage, R.; Najioullah, F.; Césaire, R.; Cabié, A. Ocular Manifestations of Emerging Arboviruses: Dengue Fever, Chikungunya, Zika Virus, West Nile Virus, and Yellow Fever. J. Fr. Ophtalmol. 2018, 41, e235–e243. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Neto, F.D.; Falcão, L.F.M.; Moraes, E.C.d.S.; David, J.P.F.; Vieira-Junior, A.d.S.; Silva, C.C.; de Sousa, J.R.; Duarte, M.I.S.; Vasconcelos, P.F.d.C.; Quaresma, J.A.S. Dengue Fever Ophthalmic Manifestations: A Review and Update. Rev. Med. Virol. 2023, 33, e2422. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Mahendradas, P.; Curi, A.; Khochtali, S.; Cunningham, E.T. Emerging Viral Infections Causing Anterior Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; Patel, R.; Goyal, S.; Rajaratnam, T.; Sharma, A.; Hossain, P. Ocular Manifestations of Emerging Viral Diseases. Eye 2021, 35, 1117–1139. [Google Scholar] [CrossRef] [PubMed]
- Benzekri, R.; Belfort, R.; Ventura, C.V.; de Paula Freitas, B.; Maia, M.; Leite, M.; Labetoulle, M.; Rousseau, A. Ocular manifestations of Zika virus: What we do and do not know. J. Fr. Ophtalmol. 2017, 40, 138–145. [Google Scholar] [CrossRef]
- Marianneau, P.; Steffan, A.M.; Royer, C.; Drouet, M.T.; Jaeck, D.; Kirn, A.; Deubel, V. Infection of Primary Cultures of Human Kupffer Cells by Dengue Virus: No Viral Progeny Synthesis, but Cytokine Production Is Evident. J. Virol. 1999, 73, 5201–5206. [Google Scholar] [CrossRef]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef]
- Krishnan, M.N.; Sukumaran, B.; Pal, U.; Agaisse, H.; Murray, J.L.; Hodge, T.W.; Fikrig, E. Rab 5 Is Required for the Cellular Entry of Dengue and West Nile Viruses. J. Virol. 2007, 81, 4881–4885. [Google Scholar] [CrossRef] [PubMed]
- Hulo, C.; de Castro, E.; Masson, P.; Bougueleret, L.; Bairoch, A.; Xenarios, I.; Le Mercier, P. ViralZone: A Knowledge Resource to Understand Virus Diversity. Nucleic Acids Res. 2011, 39, D576–D582. [Google Scholar] [CrossRef] [PubMed]
- Alpert, S.G.; Fergerson, J.; Noël, L.P. Intrauterine West Nile Virus: Ocular and Systemic Findings. Am. J. Ophthalmol. 2003, 136, 733–735. [Google Scholar] [CrossRef]
- Iwamoto, M.; Jernigan, D.B.; Guasch, A.; Trepka, M.J.; Blackmore, C.G.; Hellinger, W.C.; Pham, S.M.; Zaki, S.; Lanciotti, R.S.; Lance-Parker, S.E.; et al. Transmission of West Nile Virus from an Organ Donor to Four Transplant Recipients. N. Engl. J. Med. 2003, 348, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Intrauterine West Nile Virus Infection—New York, 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1135–1136. [Google Scholar]
- Sbrana, E.; Tonry, J.H.; Xiao, S.-Y.; da Rosa, A.P.A.T.; Higgs, S.; Tesh, R.B. Oral Transmission of West Nile Virus in a Hamster Model. Am. J. Trop. Med. Hyg. 2005, 72, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Odelola, H.A.; Oduye, O.O. West Nile Virus Infection of Adult Mice by Oral Route. Arch. Virol. 1977, 54, 251–253. [Google Scholar] [CrossRef]
- Sabino, E.C.; Loureiro, P.; Lopes, M.E.; Capuani, L.; McClure, C.; Chowdhury, D.; Di-Lorenzo-Oliveira, C.; Oliveira, L.C.; Linnen, J.M.; Lee, T.-H.; et al. Transfusion-Transmitted Dengue and Associated Clinical Symptoms during the 2012 Epidemic in Brazil. J. Infect. Dis. 2016, 213, 694–702. [Google Scholar] [CrossRef]
- Slavov, S.N.; Cilião-Alves, D.C.; Gonzaga, F.A.C.; Moura, D.R.; de Moura, A.C.A.M.; de Noronha, L.A.G.; Cassemiro, É.M.; Pimentel, B.M.S.; Costa, F.J.Q.; da Silva, G.A.; et al. Dengue Seroprevalence among Asymptomatic Blood Donors during an Epidemic Outbreak in Central-West Brazil. PLoS ONE 2019, 14, e0213793. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Mathur, A.; Chandra, A.; Das, S.K.; Tandon, H.O.; Singh, U.K. Transplacental Infection with Japanese Encephalitis Virus. J. Infect. Dis. 1980, 141, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika Virus Intrauterine Infection Causes Fetal Brain Abnormality and Microcephaly: Tip of the Iceberg? Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Guérin, B.; Pozzi, N. Viruses in Boar Semen: Detection and Clinical as Well as Epidemiological Consequences Regarding Disease Transmission by Artificial Insemination. Theriogenology 2005, 63, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Gubern, C.G.; et al. Persistence of Zika Virus in Body Fluids—Final Report. N. Engl. J. Med. 2018, 379, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Serodiagnosis of Flaviviral Infections and Vaccinations in Humans. Adv. Virus Res. 2003, 61, 3–65. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Roberts, A.; Gandhi, S. Japanese Encephalitis Virus: A Review on Emerging Diagnostic Techniques. Front. Biosci. 2020, 25, 1875–1893. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue. Curr. Opin. Infect. Dis. 2002, 15, 471–476. [Google Scholar] [CrossRef]
- Murray, N.E.A.; Quam, M.B.; Wilder-Smith, A. Epidemiology of Dengue: Past, Present and Future Prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154787-1. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(St) Century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, H.K.; Bhai, S.; John, M.; Xavier, J. Ocular Manifestations of Dengue Fever in an East Indian Epidemic. Can. J. Ophthalmol. 2006, 41, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Srinivasan, R.; Setia, S.; Soundravally, R.; Pandian, D.G. Uveitis Following Dengue Fever. Eye 2009, 23, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Loh, B.-K.; Bacsal, K.; Chee, S.-P.; Cheng, B.C.-L.; Wong, D. Foveolitis Associated with Dengue Fever: A Case Series. Ophthalmologica 2008, 222, 317–320. [Google Scholar] [CrossRef]
- Su, D.H.-W.; Bacsal, K.; Chee, S.-P.; Flores, J.V.P.; Lim, W.-K.; Cheng, B.C.-L.; Jap, A.H.-E.; Dengue Maculopathy Study Group. Prevalence of Dengue Maculopathy in Patients Hospitalized for Dengue Fever. Ophthalmology 2007, 114, 1743–1747. [Google Scholar] [CrossRef]
- Chee, E.; Sims, J.L.; Jap, A.; Tan, B.H.; Oh, H.; Chee, S.-P. Comparison of Prevalence of Dengue Maculopathy during Two Epidemics with Differing Predominant Serotypes. Am. J. Ophthalmol. 2009, 148, 910–913. [Google Scholar] [CrossRef]
- Teoh, S.C.; Chee, C.K.; Laude, A.; Goh, K.Y.; Barkham, T.; Ang, B.S.; Eye Institute Dengue-related Ophthalmic Complications Workgroup. Optical Coherence Tomography Patterns as Predictors of Visual Outcome in Dengue-Related Maculopathy. Retina 2010, 30, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Bacsal, K.E.; Chee, S.-P.; Cheng, C.-L.; Flores, J.V.P. Dengue-Associated Maculopathy. Arch. Ophthalmol. 2007, 125, 501–510. [Google Scholar] [CrossRef]
- Yip, V.C.-H.; Sanjay, S.; Koh, Y.T. Ophthalmic Complications of Dengue Fever: A Systematic Review. Ophthalmol. Ther. 2012, 1, 2. [Google Scholar] [CrossRef]
- Agarwal, A.; Aggarwal, K.; Dogra, M.; Kumar, A.; Akella, M.; Katoch, D.; Bansal, R.; Singh, R.; Gupta, V.; OCTA Study Group. Dengue-Induced Inflammatory, Ischemic Foveolitis and Outer Maculopathy: A Swept-Source Imaging Evaluation. Ophthalmol. Retina 2019, 3, 170–177. [Google Scholar] [CrossRef]
- Khairallah, M.; Yahia, S.B.; Attia, S. Arthropod Vector-Borne Uveitis in the Developing World. Int. Ophthalmol. Clin. 2010, 50, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, K. Dengue Retinochoroiditis. Ann. Saudi Med. 2012, 32, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Somkijrungroj, T.; Kongwattananon, W. Ocular Manifestations of Dengue. Curr. Opin. Ophthalmol. 2019, 30, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, S.K. Bilateral Ciliochoroidal Effusion with Secondary Angle Closure and Myopic Shift in Dengue Fever. Ocul. Immunol. Inflamm. 2023, 31, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.; Teoh, S.C. Dengue Eye Disease. Surv. Ophthalmol. 2015, 60, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Saranappa, S.B.S.; Sowbhagya, H.N. Panophthalmitis in Dengue Fever. Indian Pediatr. 2012, 49, 760. [Google Scholar] [CrossRef] [PubMed]
- Arya, D.; Das, S.; Shah, G.; Gandhi, A. Panophthalmitis Associated with Scleral Necrosis in Dengue Hemorrhagic Fever. Indian J. Ophthalmol. 2019, 67, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.F.; Ooi, E.E. Diagnosis of Dengue: An Update. Expert Rev. Anti-Infect. Ther. 2012, 10, 895–907. [Google Scholar] [CrossRef]
- Teoh, B.-T.; Sam, S.-S.; Tan, K.-K.; Johari, J.; Abd-Jamil, J.; Hooi, P.-S.; AbuBakar, S. The Use of NS1 Rapid Diagnostic Test and qRT-PCR to Complement IgM ELISA for Improved Dengue Diagnosis from Single Specimen. Sci. Rep. 2016, 6, 27663. [Google Scholar] [CrossRef]
- Hirayama, T.; Mizuno, Y.; Takeshita, N.; Kotaki, A.; Tajima, S.; Omatsu, T.; Sano, K.; Kurane, I.; Takasaki, T. Detection of Dengue Virus Genome in Urine by Real-Time Reverse Transcriptase PCR: A Laboratory Diagnostic Method Useful after Disappearance of the Genome in Serum. J. Clin. Microbiol. 2012, 50, 2047–2052. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Dalugama, C. Dengue Infection: Global Importance, Immunopathology and Management. Clin. Med. 2022, 22, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-K.; Mathur, R.; Koh, A.; Yeoh, R.; Chee, S.-P. Ocular Manifestations of Dengue Fever. Ophthalmology 2004, 111, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.S.M.; Kamisah, Y. Potential Medicinal Plants for the Treatment of Dengue Fever and Severe Acute Respiratory Syndrome-Coronavirus. Biomolecules 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; De Silva, N.L.; Weeratunga, P.; Rodrigo, C.; Sigera, C.; Fernando, S.D. Carica Papaya Extract in Dengue: A Systematic Review and Meta-Analysis. BMC Complement. Altern. Med. 2019, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.H.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Scherwitzl, I.; Mongkolsapaja, J.; Screaton, G. Recent Advances in Human Flavivirus Vaccines. Curr. Opin. Virol. 2017, 23, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef]
- Biswal, S.; Borja-Tabora, C.; Martinez Vargas, L.; Velásquez, H.; Theresa Alera, M.; Sierra, V.; Johana Rodriguez-Arenales, E.; Yu, D.; Wickramasinghe, V.P.; Duarte Moreira, E.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children Aged 4-16 Years: A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Garg, S.; Jampol, L.M. Systemic and Intraocular Manifestations of West Nile Virus Infection. Surv. Ophthalmol. 2005, 50, 3–13. [Google Scholar] [CrossRef]
- Troupin, A.; Colpitts, T.M. Overview of West Nile Virus Transmission and Epidemiology. Methods Mol. Biol. 2016, 1435, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Zannoli, S.; Sambri, V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Gyure, K.A. West Nile Virus Infections. J. Neuropathol. Exp. Neurol. 2009, 68, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Ben Yahia, S.; Ladjimi, A.; Zeghidi, H.; Ben Romdhane, F.; Besbes, L.; Zaouali, S.; Messaoud, R. Chorioretinal Involvement in Patients with West Nile Virus Infection. Ophthalmology 2004, 111, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Limstrom, S.A.; Tarasewicz, D.G.; Lin, S.G. Ocular Features of West Nile Virus Infection in North America: A Study of 14 Eyes. Ophthalmology 2006, 113, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Winward, B.K.; Gottlieb, J.L.; Chang, J.S.; Bradbury, L.; Maganti, N.; Pathak, C.; Fowler, B.J. Ocular Findings Aid in Diagnosis of West Nile Virus. WMJ 2023, 122, 208–212. [Google Scholar] [PubMed]
- Abroug, F.; Ouanes-Besbes, L.; Letaief, M.; Ben Romdhane, F.; Khairallah, M.; Triki, H.; Bouzouiaia, N. A Cluster Study of Predictors of Severe West Nile Virus Infection. Mayo Clin. Proc. 2006, 81, 12–16. [Google Scholar] [CrossRef]
- Sivakumar, R.R.; Prajna, L.; Arya, L.K.; Muraly, P.; Shukla, J.; Saxena, D.; Parida, M. Molecular Diagnosis and Ocular Imaging of West Nile Virus Retinitis and Neuroretinitis. Ophthalmology 2013, 120, 1820–1826. [Google Scholar] [CrossRef]
- Dahal, U.; Mobarakai, N.; Sharma, D.; Pathak, B. West Nile Virus Infection and Diplopia: A Case Report and Review of Literature. Int. J. Gen. Med. 2013, 6, 369–373. [Google Scholar] [CrossRef]
- Khairallah, M.; Ben Yahia, S.; Attia, S.; Zaouali, S.; Ladjimi, A.; Messaoud, R. Linear Pattern of West Nile Virus-Associated Chorioretinitis Is Related to Retinal Nerve Fibres Organization. Eye 2007, 21, 952–955. [Google Scholar] [CrossRef]
- Learned, D.; Nudleman, E.; Robinson, J.; Chang, E.; Stec, L.; Faia, L.J.; Wolfe, J.; Williams, G.A. Multimodal Imaging of West Nile Virus Chorioretinitis. Retina 2014, 34, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Ben Yahia, S.; Attia, S.; Zaouali, S.; Jelliti, B.; Jenzri, S.; Ladjimi, A.; Messaoud, R. Indocyanine Green Angiographic Features in Multifocal Chorioretinitis Associated with West Nile Virus Infection. Retina 2006, 26, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Jelliti, B.; Jenzeri, S. Emergent Infectious Uveitis. Middle East Afr. J. Ophthalmol. 2009, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Kahloun, R. Ocular Manifestations of Emerging Infectious Diseases. Curr. Opin. Ophthalmol. 2013, 24, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Kahloun, R.; Gargouri, S.; Jelliti, B.; Sellami, D.; Ben Yahia, S.; Feki, J. Swept-Source Optical Coherence Tomography Angiography in West Nile Virus Chorioretinitis and Associated Occlusive Retinal Vasculitis. Ophthalmic. Surg. Lasers Imaging Retin. 2017, 48, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.; Saxena, D.; Rathinam, S.; Lalitha, P.; Joseph, C.R.; Sharma, S.; Soni, M.; Rao, P.V.L.; Parida, M. Molecular Detection and Characterization of West Nile Virus Associated with Multifocal Retinitis in Patients from Southern India. Int. J. Infect. Dis. 2012, 16, e53–e59. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Mannasse, B.; Koren, R.; Katz-Likvornik, S.; Hindiyeh, M.; Mandelboim, M.; Dovrat, S.; Sofer, D.; Mendelson, E. Superiority of West Nile Virus RNA Detection in Whole Blood for Diagnosis of Acute Infection. J. Clin. Microbiol. 2016, 54, 2294–2297. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Pinto, A.K.; Vogt, M.R.; Gale, M.; Diamond, M.S. Beta Interferon Controls West Nile Virus Infection and Pathogenesis in Mice. J. Virol. 2011, 85, 7186–7194. [Google Scholar] [CrossRef]
- Ben-Nathan, D.; Gershoni-Yahalom, O.; Samina, I.; Khinich, Y.; Nur, I.; Laub, O.; Gottreich, A.; Simanov, M.; Porgador, A.; Rager-Zisman, B.; et al. Using High Titer West Nile Intravenous Immunoglobulin from Selected Israeli Donors for Treatment of West Nile Virus Infection. BMC Infect. Dis. 2009, 9, 18. [Google Scholar] [CrossRef]
- Gorman, M.J.; Poddar, S.; Farzan, M.; Diamond, M.S. The Interferon-Stimulated Gene Ifitm3 Restricts West Nile Virus Infection and Pathogenesis. J. Virol. 2016, 90, 8212–8225. [Google Scholar] [CrossRef]
- Goh, V.S.L.; Mok, C.-K.; Chu, J.J.H. Antiviral Natural Products for Arbovirus Infections. Molecules 2020, 25, 2796. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Haigh, O.; Ksiaa, I.; Khairallah, M.; Labetoulle, M. Ocular Manifestations of West Nile Virus. Vaccines 2020, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.V.; Staples, J.E.; Huang, C.Y.-H.; Brault, A.C.; Nett, R.J. Combating West Nile Virus Disease—Time to Revisit Vaccination. N. Engl. J. Med. 2023, 388, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.J.; Shankar, M.B.; Fischer, M.; Meltzer, M.I.; Erin Staples, J.; Gould, C.V. Cost-Effectiveness and Impact of a Targeted Age- and Incidence-Based West Nile Virus Vaccine Strategy. Clin. Infect. Dis. 2021, 73, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.K.; Stoessel, K.M.; Adelman, R.A. Choroidal Neovascularization Associated with West Nile Virus Chorioretinitis. Semin. Ophthalmol. 2007, 22, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.R.; Hariprasad, S.M.; Jampol, L.M.; Sheth, V.S. Use of Intravitreous Bevacizumab to Treat Macular Edema in West Nile Virus Chorioretinitis. Arch. Ophthalmol. 2012, 130, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Sanz, G.; De Jesus Rodriguez, E.; Vila-Delgado, M.; Oliver, A.L. An Unusual Case of Unilateral Chorioretinitis and Blind Spot Enlargement Associated with Asymptomatic West Nile Virus Infection. Am. J. Ophthalmol. Case Rep. 2020, 18, 100723. [Google Scholar] [CrossRef]
- Khairallah, M.; Yahia, S.B.; Letaief, M.; Attia, S.; Kahloun, R.; Jelliti, B.; Zaouali, S.; Messaoud, R. A Prospective Evaluation of Factors Associated with Chorioretinitis in Patients with West Nile Virus Infection. Ocul. Immunol. Inflamm. 2007, 15, 435–439. [Google Scholar] [CrossRef]
- Khairallah, M.; Ben Yahia, S.; Attia, S.; Jelliti, B.; Zaouali, S.; Ladjimi, A. Severe Ischemic Maculopathy in a Patient with West Nile Virus Infection. Ophthalmic. Surg. Lasers Imaging 2006, 37, 240–242. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow Fever: An Update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Lucey, D.; Gostin, L.O. A Yellow Fever Epidemic: A New Global Health Emergency? JAMA 2016, 315, 2661–2662. [Google Scholar] [CrossRef] [PubMed]
- Douam, F.; Ploss, A. Yellow Fever Virus: Knowledge Gaps Impeding the Fight Against an Old Foe. Trends Microbiol. 2018, 26, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Brandão-de-Resende, C.; Cunha, L.H.M.; Oliveira, S.L.; Pereira, L.S.; Oliveira, J.G.F.; Santos, T.A.; Vasconcelos-Santos, D.V. Characterization of Retinopathy Among Patients With Yellow Fever During 2 Outbreaks in Southeastern Brazil. JAMA Ophthalmol. 2019, 137, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Vianello, S.; Silva de Souza, G.; Maia, M.; Belfort, R.; de Oliveira Dias, J.R. Ocular Findings in Yellow Fever Infection. JAMA Ophthalmol. 2019, 137, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.L.N.; Barros, R.S.; Silva, S.P.; Rodrigues, D.S.G.; Cruz, A.C.R.; Dos Santos, F.B.; Vasconcelos, P.F.C.; Tesh, R.B.; Nunes, B.T.D.; Medeiros, D.B.A. The Usefulness of a Duplex RT-qPCR during the Recent Yellow Fever Brazilian Epidemic: Surveillance of Vaccine Adverse Events, Epizootics and Vectors. Pathogens 2021, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- De Rezende, I.M.; Oliveira, G.F.G.; Costa, T.A.; Khan, A.; Pereira, L.S.; Santos, T.A.; Alves, P.A.; Calzavara-Silva, C.E.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; et al. Yellow Fever Molecular Diagnosis Using Urine Specimens during Acute and Convalescent Phases of the Disease. J. Clin. Microbiol. 2022, 60, e00254-22. [Google Scholar] [CrossRef] [PubMed]
- Kling, K.; Domingo, C.; Bogdan, C.; Duffy, S.; Harder, T.; Howick, J.; Kleijnen, J.; McDermott, K.; Wichmann, O.; Wilder-Smith, A.; et al. Duration of Protection After Vaccination Against Yellow Fever: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, A.L.; Moraes, H.V. de Anterior and Intermediate Uveitis Following Yellow Fever Vaccination with Fractional Dose: Case Reports. Ocul. Immunol. Inflamm. 2019, 27, 521–523. [Google Scholar] [CrossRef]
- Campos, W.R.; Cenachi, S.P.F.; Soares, M.S.; Gonçalves, P.F.; Vasconcelos-Santos, D.V. Vogt-Koyanagi-Harada-like Disease Following Yellow Fever Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 124–127. [Google Scholar] [CrossRef]
- Hansen, C.A.; Barrett, A.D.T. The Present and Future of Yellow Fever Vaccines. Pharmaceuticals 2021, 14, 891. [Google Scholar] [CrossRef]
- Vannice, K.; Wilder-Smith, A.; Hombach, J. Fractional-Dose Yellow Fever Vaccination—Advancing the Evidence Base. N. Engl. J. Med. 2018, 379, 603–605. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Child Health and Human Development. Yellow Fever Vaccine. In Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006. [Google Scholar]
- Low, J.G.; Ng, J.H.J.; Ong, E.Z.; Kalimuddin, S.; Wijaya, L.; Chan, Y.F.Z.; Ng, D.H.L.; Tan, H.-C.; Baglody, A.; Chionh, Y.-H.; et al. Phase 1 Trial of a Therapeutic Anti-Yellow Fever Virus Human Antibody. N. Engl. J. Med. 2020, 383, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Mello, C.; Casadio, L.V.B.; Avelino-Silva, V.I.; Yeh-Li, H.; Sztajnbok, J.; Joelsons, D.; Antonio, M.B.; Pinho, J.R.R.; Malta, F.d.M.; Gomes-Gouvêa, M.S.; et al. Efficacy of Sofosbuvir as Treatment for Yellow Fever: Protocol for a Randomised Controlled Trial in Brazil (SOFFA Study). BMJ Open 2019, 9, e027207. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, E.; Xiao, S.-Y.; Guzman, H.; Ye, M.; Travassos da Rosa, A.P.A.; Tesh, R.B. Efficacy of Post-Exposure Treatment of Yellow Fever with Ribavirin in a Hamster Model of the Disease. Am. J. Trop. Med. Hyg. 2004, 71, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, V.M.; Broult, J. Potential for Zika Virus Transmission through Blood Transfusion Demonstrated during an Outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014, 19, 20761. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus. I. Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- de Oliveira, W.K.; de França, G.V.A.; Carmo, E.H.; Duncan, B.B.; de Souza Kuchenbecker, R.; Schmidt, M.I. Infection-Related Microcephaly after the 2015 and 2016 Zika Virus Outbreaks in Brazil: A Surveillance-Based Analysis. Lancet 2017, 390, 861–870. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Barreras, P.; Pardo, C.A. Zika Virus-Associated Neurological Disease in the Adult: Guillain-Barré Syndrome, Encephalitis, and Myelitis. Semin. Reprod. Med. 2016, 34, 273–279. [Google Scholar] [CrossRef]
- Leonhard, S.E.; Bresani-Salvi, C.C.; Lyra Batista, J.D.; Cunha, S.; Jacobs, B.C.; Brito Ferreira, M.L.; P Militão de Albuquerque, M.d.F. Guillain-Barré Syndrome Related to Zika Virus Infection: A Systematic Review and Meta-Analysis of the Clinical and Electrophysiological Phenotype. PLoS Negl. Trop. Di.s 2020, 14, e0008264. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Troumani, Y.; Touhami, S.; Jackson, T.L.; Ventura, C.V.; Stanescu-Segall, D.M.; Errera, M.-H.; Rousset, D.; Bodaghi, B.; Cartry, G.; David, T.; et al. Association of Anterior Uveitis With Acute Zika Virus Infection in Adults. JAMA Ophthalmol. 2021, 139, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cerbino-Neto, J.; Mesquita, E.C.; Souza, T.M.L.; Parreira, V.; Wittlin, B.B.; Durovni, B.; Lemos, M.C.F.; Vizzoni, A.; Bispo de Filippis, A.M.; Sampaio, S.A.; et al. Clinical Manifestations of Zika Virus Infection, Rio de Janeiro, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Parke, D.W.; Almeida, D.R.P.; Albini, T.A.; Ventura, C.V.; Berrocal, A.M.; Mittra, R.A. Serologically Confirmed Zika-Related Unilateral Acute Maculopathy in an Adult. Ophthalmology 2016, 123, 2432–2433. [Google Scholar] [CrossRef] [PubMed]
- Kodati, S.; Palmore, T.N.; Spellman, F.A.; Cunningham, D.; Weistrop, B.; Sen, H.N. Bilateral Posterior Uveitis Associated with Zika Virus Infection. Lancet 2017, 389, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Ventura, L.O. Ophthalmologic Manifestations Associated with Zika Virus Infection. Pediatrics 2018, 141, S161–S166. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Maia, M.; Travassos, S.B.; Martins, T.T.; Patriota, F.; Nunes, M.E.; Agra, C.; Torres, V.L.; van der Linden, V.; Ramos, R.C.; et al. Risk Factors Associated with the Ophthalmoscopic Findings Identified in Infants with Presumed Zika Virus Congenital Infection. JAMA Ophthalmol. 2016, 134, 912–918. [Google Scholar] [CrossRef]
- Labib, B.A.; Chigbu, D.I. Pathogenesis and Manifestations of Zika Virus-Associated Ocular Diseases. Trop. Med. Infect. Dis. 2022, 7, 106. [Google Scholar] [CrossRef]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Góis, A.L.; Belfort, R. Zika Virus in Brazil and Macular Atrophy in a Child with Microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef] [PubMed]
- Marquezan, M.C.; Ventura, C.V.; Sheffield, J.S.; Golden, W.C.; Omiadze, R.; Belfort, R.; May, W. Ocular Effects of Zika Virus-a Review. Surv. Ophthalmol. 2018, 63, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Ventura, L.O.; Bravo-Filho, V.; Martins, T.T.; Berrocal, A.M.; Gois, A.L.; de Oliveira Dias, J.R.; Araújo, L.; Escarião, P.; van der Linden, V.; et al. Optical Coherence Tomography of Retinal Lesions in Infants with Congenital Zika Syndrome. JAMA Ophthalmol. 2016, 134, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Zin, A.A.; Tsui, I.; Rossetto, J.; Vasconcelos, Z.; Adachi, K.; Valderramos, S.; Halai, U.-A.; Pone, M.V.d.S.; Pone, S.M.; Silveira Filho, J.C.B.; et al. Screening Criteria for Ophthalmic Manifestations of Congenital Zika Virus Infection. JAMA Pediatr. 2017, 171, 847–854. [Google Scholar] [CrossRef] [PubMed]
- de Paula Freitas, B.; de Oliveira Dias, J.R.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R. Ocular Findings in Infants with Microcephaly Associated with Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef]
- Vasconcelos, G.C.; Macedo Pereira, C.M.; Toledo de Paula, C.H.; de Souza Haueisen Barbosa, P.; Machado de Souza, D.; Coelho, L.M. Corneal Ectasia and High Ametropia in an Infant with Microcephaly Associated with Presumed Zika Virus Congenital Infection: New Ocular Findings. J. AAPOS 2019, 23, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Yepez, J.B.; Murati, F.A.; Pettito, M.; Peñaranda, C.F.; de Yepez, J.; Maestre, G.; Arevalo, J.F.; Johns Hopkins Zika Center. Ophthalmic Manifestations of Congenital Zika Syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017, 135, 440–445. [Google Scholar] [CrossRef]
- Fernandez, M.P.; Parra Saad, E.; Ospina Martinez, M.; Corchuelo, S.; Mercado Reyes, M.; Herrera, M.J.; Parra Saavedra, M.; Rico, A.; Fernandez, A.M.; Lee, R.K.; et al. Ocular Histopathologic Features of Congenital Zika Syndrome. JAMA Ophthalmol. 2017, 135, 1163–1169. [Google Scholar] [CrossRef]
- Agrawal, R.; Oo, H.H.; Balne, P.K.; Ng, L.; Tong, L.; Leo, Y.S. Zika Virus and the Eye. Ocul. Immunol. Inflamm. 2018, 26, 654–659. [Google Scholar] [CrossRef]
- Gouel-Cheron, A.; Lumbard, K.; Hunsberger, S.; Arteaga-Cabello, F.J.; Beigel, J.; Belaunzarán-Zamudio, P.F.; Caballero-Sosa, S.; Escobedo-López, K.; Ibarra-González, V.; Nájera-Cancino, J.G.; et al. Serial Real-Time RT-PCR and Serology Measurements Substantially Improve Zika and Dengue Virus Infection Classification in a Co-Circulation Area. Antivir. Res. 2019, 172, 104638. [Google Scholar] [CrossRef]
- Brasil, P.; Calvet, G.A.; Siqueira, A.M.; Wakimoto, M.; de Sequeira, P.C.; Nobre, A.; Quintana, M.d.S.B.; de Mendonça, M.C.L.; Lupi, O.; de Souza, R.V.; et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl. Trop. Dis. 2016, 10, e0004636. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.M.; Cone, M.; Mock, V.; Heberlein-Larson, L.; Stanek, D.; Blackmore, C.; Likos, A. Comparison of Test Results for Zika Virus RNA in Urine, Serum, and Saliva Specimens from Persons with Travel-Associated Zika Virus Disease—Florida, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Gourinat, A.-C.; O’Connor, O.; Calvez, E.; Goarant, C.; Dupont-Rouzeyrol, M. Detection of Zika Virus in Urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef]
- Rabe, I.B.; Staples, J.E.; Villanueva, J.; Hummel, K.B.; Johnson, J.A.; Rose, L.; Hills, S.; Wasley, A.; Fischer, M.; Powers, A.M. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; El Kalamouni, C.; Khalid, A.; Abdalla, A.N.; Zengin, G.; Khoa Bao, L.V.; Mahomoodally, M.F. Secondary Metabolites as Potential Drug Candidates against Zika Virus, an Emerging Looming Human Threat: Current Landscape, Molecular Mechanism and Challenges Ahead. J. Infect. Public Health 2023, 16, 754–770. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, M.; Lee, E.M.; Gorshkov, K.; Shiryaev, S.A.; He, S.; Sun, W.; Cheng, Y.-S.; Hu, X.; Tharappel, A.M.; et al. Emetine Inhibits Zika and Ebola Virus Infections through Two Molecular Mechanisms: Inhibiting Viral Replication and Decreasing Viral Entry. Cell Discov. 2018, 4, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Burgomaster, K.E.; Foreman, B.M.; Aleshnick, M.A.; Larman, B.C.; Gordon, D.N.; Maciejewski, S.; Morabito, K.M.; Ledgerwood, J.E.; Gaudinski, M.R.; Chen, G.L.; et al. Limited Flavivirus Cross-Reactive Antibody Responses Elicited by a Zika Virus Deoxyribonucleic Acid Vaccine Candidate in Humans. J. Infect. Dis. 2021, 224, 1550–1555. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Haut Conseil de la Sante Publique. Statement on the Medical Care Provided for and the Monitoring of New-Borns and Infants Having Been Exposed to the Zika Virus in Utero or Present. 2017. Available online: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=675 (accessed on 2 August 2018).
- Adebanjo, T.; Godfred-Cato, S.; Viens, L.; Fischer, M.; Staples, J.E.; Kuhnert-Tallman, W.; Walke, H.; Oduyebo, T.; Polen, K.; Peacock, G.; et al. Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection—United States, October 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1089–1099. [Google Scholar] [CrossRef]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese Encephalitis Emergence in Australia: The Potential Population at Risk. Clin. Infect. Dis. 2023, 76, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T. Control of Japanese Encephalitis--within Our Grasp? N. Engl. J. Med. 2006, 355, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.L.; Scherer, W.F.; Rosenberg, M.Z.; Gresser, I.; Hardy, J.L.; Bullock, H.R. Ecologic Studies of Japanese Encephalitis Virus in Japan. II. Mosquito Infection. Am. J. Trop. Med. Hyg. 1959, 8, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Lindsey, N.; Staples, J.E.; Hills, S.; Centers for Disease Control and Prevention (CDC). Japanese Encephalitis Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–27. [Google Scholar] [PubMed]
- Hills, S.L.; Netravathi, M.; Solomon, T. Japanese Encephalitis among Adults: A Review. Am. J. Trop. Med. Hyg. 2023, 108, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Solomon, T. Japanese Encephalitis—The Prospects for New Treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese Encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-T.; Chu, S.-Y.; Lee, Y.-C. Ischaemic Maculopathy in Japanese Encephalitis. Eye 2006, 20, 1439–1441. [Google Scholar] [CrossRef]
- Van, K.; Korman, T.M.; Nicholson, S.; Troutbeck, R.; Lister, D.M.; Woolley, I. Case Report: Japanese Encephalitis Associated with Chorioretinitis after Short-Term Travel to Bali, Indonesia. Am. J. Trop. Med Hyg. 2020, 103, 1691–1693. [Google Scholar] [CrossRef]
- Chanama, S.; Sukprasert, W.; Sa-ngasang, A.; A-nuegoonpipat, A.; Sangkitporn, S.; Kurane, I.; Anantapreecha, S. Detection of Japanese Encephalitis (JE) Virus-Specific IgM in Cerebrospinal Fluid and Serum Samples from JE Patients. Jpn. J. Infect. Dis. 2005, 58, 294–296. [Google Scholar]
- Swami, R.; Ratho, R.K.; Mishra, B.; Singh, M.P. Usefulness of RT-PCR for the Diagnosis of Japanese Encephalitis in Clinical Samples. Scand. J. Infect. Dis. 2008, 40, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Taraphdar, D.; Mukhopadhyay, S.K.; Chakrabarti, S.; Chatterjee, S. Serological and Molecular Diagnosis of Japanese Encephalitis Reveals an Increasing Public Health Problem in the State of West Bengal, India. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 15–19. [Google Scholar] [CrossRef]
- Sarkar, A.; Datta, S.; Pathak, B.K.; Mukhopadhyay, S.K.; Chatterjee, S. Japanese Encephalitis Associated Acute Encephalitis Syndrome Cases in West Bengal, India: A Sero-Molecular Evaluation in Relation to Clinico-Pathological Spectrum. J. Med. Virol. 2015, 87, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A. Control of Japanese Encephalitis in Japan: Immunization of Humans and Animals, and Vector Control. Curr. Top. Microbiol. Immunol. 2002, 267, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-I.; Lee, Y.-M. Japanese Encephalitis: The Virus and Vaccines. Hum. Vaccin. Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, Present, and Future of Japanese Encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.; Jabbar, B.; Ahmed, N.; Rehman, A.; Nasir, H.; Nadeem, S.; Jabbar, I.; Rahman, Z.U.; Azam, S. Epidemiology, Pathogenesis, and Control of a Tick-Borne Disease-Kyasanur Forest Disease: Current Status and Future Directions. Front. Cell. Infect. Microbiol. 2018, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Work, T.H.; Trapido, H.; Murthy, D.P.N.; Rao, R.L.; Bhatt, P.N.; Kulkarni, K.G. Kyasanur Forest Disease. III. A Preliminary Report on the Nature of the Infection and Clinical Manifestations in Human Beings. Indian J. Med. Sci. 1957, 11, 619–645. [Google Scholar]
- Chakraborty, S.; Andrade, F.C.D.; Ghosh, S.; Uelmen, J.; Ruiz, M.O. Historical Expansion of Kyasanur Forest Disease in India From 1957 to 2017: A Retrospective Analysis. Geohealth 2019, 3, 44–55. [Google Scholar] [CrossRef]
- Sadanandane, C.; Gokhale, M.D.; Elango, A.; Yadav, P.; Mourya, D.T.; Jambulingam, P. Prevalence and Spatial Distribution of Ixodid Tick Populations in the Forest Fringes of Western Ghats Reported with Human Cases of Kyasanur Forest Disease and Monkey Deaths in South India. Exp. Appl. Acarol. 2018, 75, 135–142. [Google Scholar] [CrossRef]
- Pattnaik, P. Kyasanur Forest Disease: An Epidemiological View in India. Rev. Med. Virol. 2006, 16, 151–165. [Google Scholar] [CrossRef]
- Ocular Manifestations of Kyasanur Forest Disease (a Clinical Study). Indian J. Ophthalmol. 1983, 31, 700–702.
- Iyer, C.G.; Laxmana Rao, R.; Work, T.H.; Narasimha Murthy, D.P. Kyasanur Forest Disease VI. Pathological Findings in Three Fatal Human Cases of Kyasanur Forest Disease. Indian J. Med. Sci. 1959, 13, 1011–1022. [Google Scholar]
- Mourya, D.T.; Yadav, P.D.; Mehla, R.; Barde, P.V.; Yergolkar, P.N.; Kumar, S.R.P.; Thakare, J.P.; Mishra, A.C. Diagnosis of Kyasanur Forest Disease by Nested RT-PCR, Real-Time RT-PCR and IgM Capture ELISA. J. Virol. Methods 2012, 186, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.W.M.; Ranadheera, C.; Nikiforuk, A.M.; Cutts, T.A.; Kobasa, D.; Court, D.A.; Theriault, S.S. Limited Effects of Type I Interferons on Kyasanur Forest Disease Virus in Cell Culture. PLoS Negl. Trop. Dis. 2016, 10, e0004871. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.K.; Pasi, A.; Kumar, S.; Kasabi, G.S.; Gujjarappa, P.; Shrivastava, A.; Mehendale, S.; Chauhan, L.S.; Laserson, K.F.; Murhekar, M. Kyasanur Forest Disease Outbreak and Vaccination Strategy, Shimoga District, India, 2013–2014. Emerg. Infect. Dis. 2015, 21, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Kasabi, G.S.; Murhekar, M.V.; Sandhya, V.K.; Raghunandan, R.; Kiran, S.K.; Channabasappa, G.H.; Mehendale, S.M. Coverage and Effectiveness of Kyasanur Forest Disease (KFD) Vaccine in Karnataka, South India, 2005–2010. PLoS Negl. Trop. Dis. 2013, 7, e2025. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, U.G.K.; Marinaik, C.B.; Gomes, A.R.; Rathnamma, D.; Byregowda, S.M.; Isloor, S.; Munivenkatarayappa, A.; Venkatesha, M.D.; Rao, S.; Rizwan, A.; et al. Evaluation of Safety and Potency of Kyasanur Forest Disease (KFD) Vaccine Inactivated with Different Concentrations of Formalin and Comparative Evaluation of In Vitro and In Vivo Methods of Virus Titration in KFD Vaccine. Biomedicines 2023, 11, 1871. [Google Scholar] [CrossRef]
- Ogden, N.H.; Bigras-Poulin, M.; O’Callaghan, C.J.; Barker, I.K.; Lindsay, L.R.; Maarouf, A.; Smoyer-Tomic, K.E.; Waltner-Toews, D.; Charron, D. A Dynamic Population Model to Investigate Effects of Climate on Geographic Range and Seasonality of the Tick Ixodes Scapularis. Int. J. Parasitol. 2005, 35, 375–389. [Google Scholar] [CrossRef]
- Satish, K.V.; Saranya, K.R.L.; Reddy, C.S.; Krishna, P.H.; Jha, C.S.; Rao, P.V.V.P. Geospatial Assessment and Monitoring of Historical Forest Cover Changes (1920–2012) in Nilgiri Biosphere Reserve, Western Ghats, India. Environ. Monit. Assess. 2014, 186, 8125–8140. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zina, S.M.; Hoarau, G.; Labetoulle, M.; Khairallah, M.; Rousseau, A. Ocular Manifestations of Flavivirus Infections. Pathogens 2023, 12, 1457. https://doi.org/10.3390/pathogens12121457
Zina SM, Hoarau G, Labetoulle M, Khairallah M, Rousseau A. Ocular Manifestations of Flavivirus Infections. Pathogens. 2023; 12(12):1457. https://doi.org/10.3390/pathogens12121457
Chicago/Turabian StyleZina, Sourour Meziou, Gautier Hoarau, Marc Labetoulle, Moncef Khairallah, and Antoine Rousseau. 2023. "Ocular Manifestations of Flavivirus Infections" Pathogens 12, no. 12: 1457. https://doi.org/10.3390/pathogens12121457
APA StyleZina, S. M., Hoarau, G., Labetoulle, M., Khairallah, M., & Rousseau, A. (2023). Ocular Manifestations of Flavivirus Infections. Pathogens, 12(12), 1457. https://doi.org/10.3390/pathogens12121457





