Potential Use of Brazilian Green Propolis Extracts as New Photosensitizers for Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Brazilian Green Propolis Extracts
2.2. Extraction of Water-Soluble Substances
Fractionation of Crude Extracts
2.3. Light Sources
2.4. Photodegradation Assay
2.5. Bacterial Strain and Growth Conditions
2.6. Antimicrobial Photodynamic Therapy (aPDT) on Single-Species Biofilm
2.7. Antimicrobial Photodynamic Therapy (aPDT) on Dual-Species Biofilm
2.8. Reactive Oxygen Species (ROS) Detection Cell-Free System (Solution)
2.9. Cytotoxicity Assessment
2.10. Effect of BGP-Mediated Antimicrobial Photodynamic Therapy on the Color Stability of Restorative Dental Materials
2.10.1. Preparation of Specimens
2.10.2. Color Stability
2.11. Statistical Analysis
3. Results
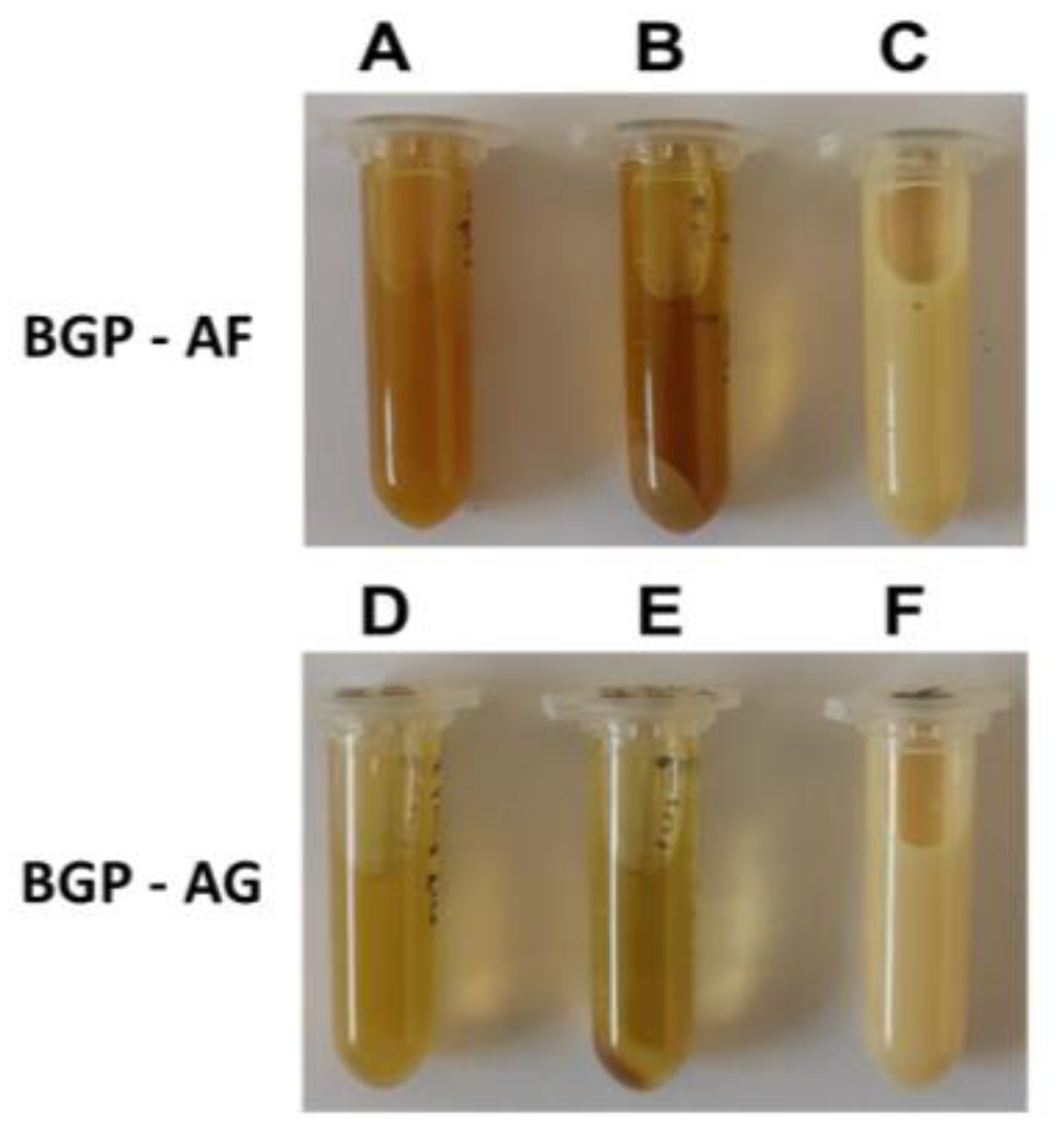
3.1. Physicochemical Characterization of Brazilian Green propolis Extracts and Preparation of the Crude Extract Fraction
Spectrophotometric Profile of the Extracts
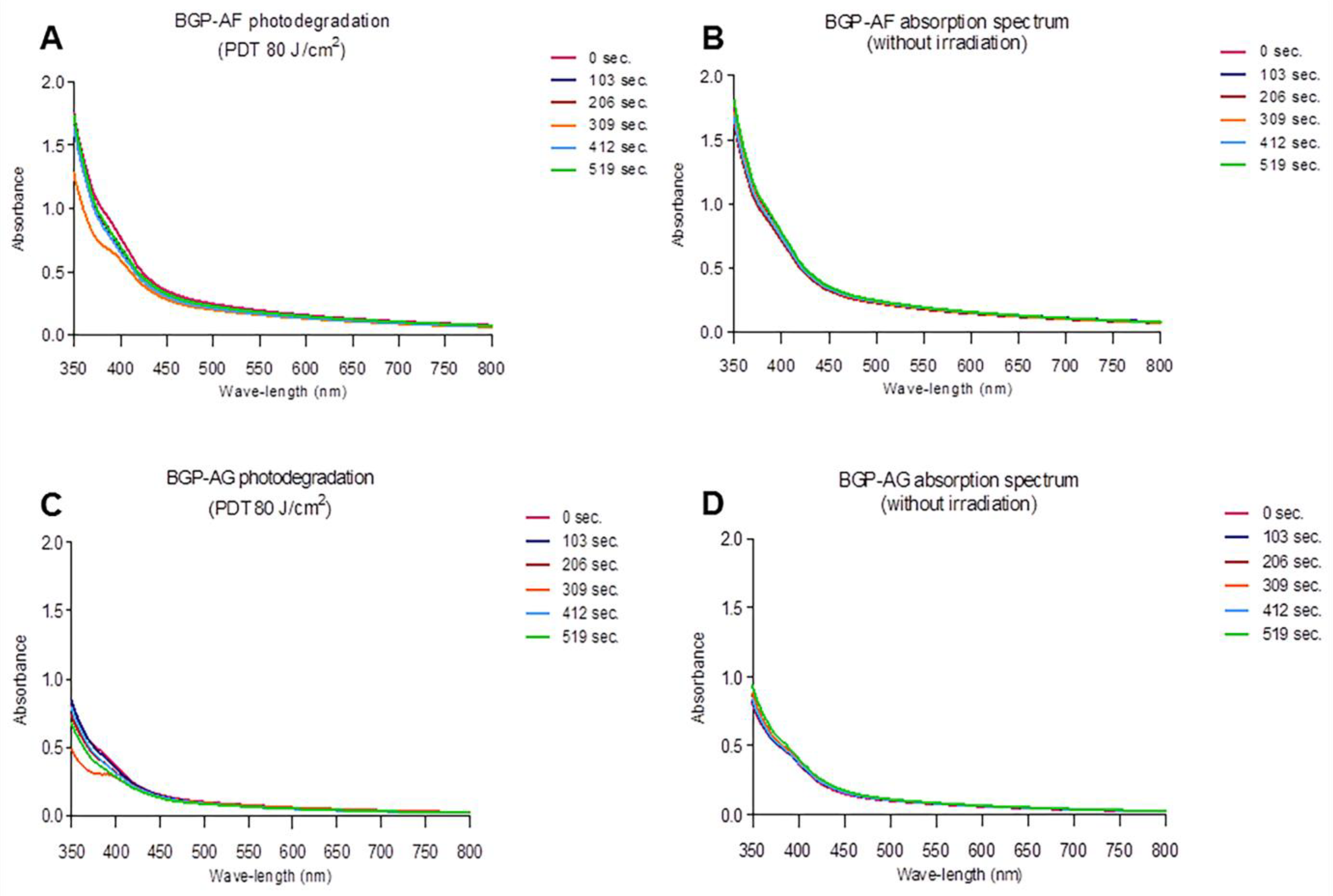
3.2. Visible Light Absorption Spectrum
3.3. Photodegradation
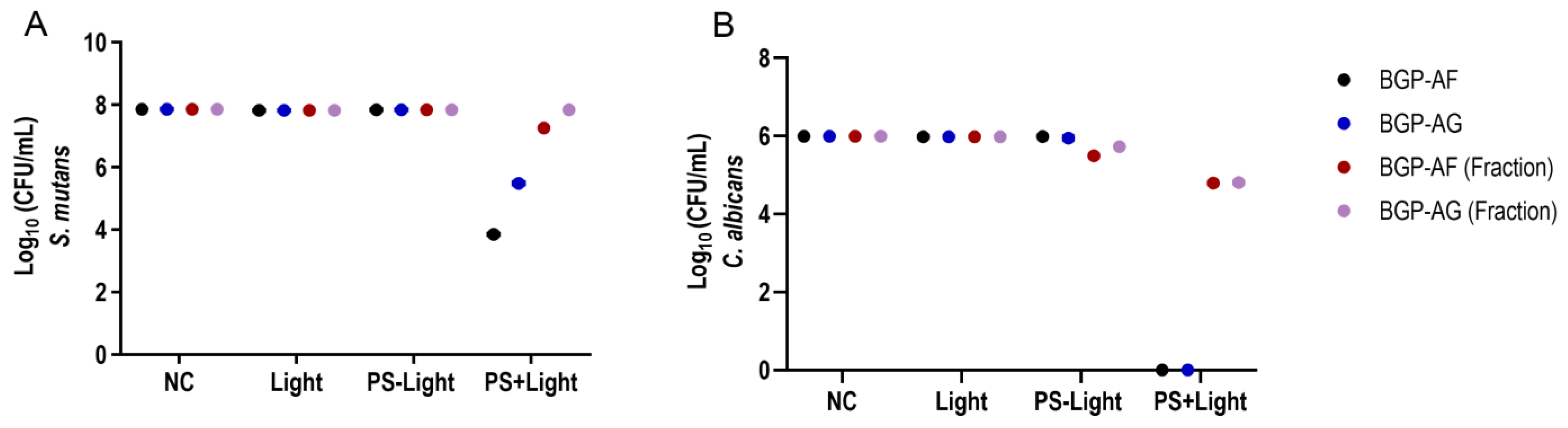
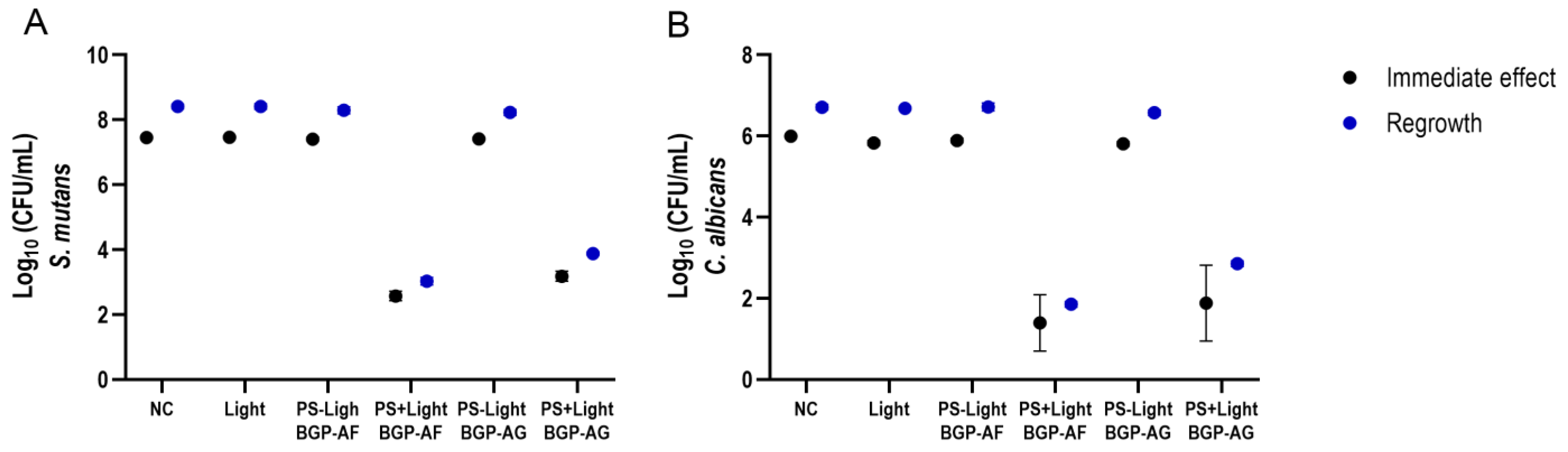
3.4. Antimicrobial Photodynamic Therapy (aPDT) on Single-Species Biofilm
3.5. Antimicrobial Photodynamic Therapy (aPDT) on Dual-Species Biofilm
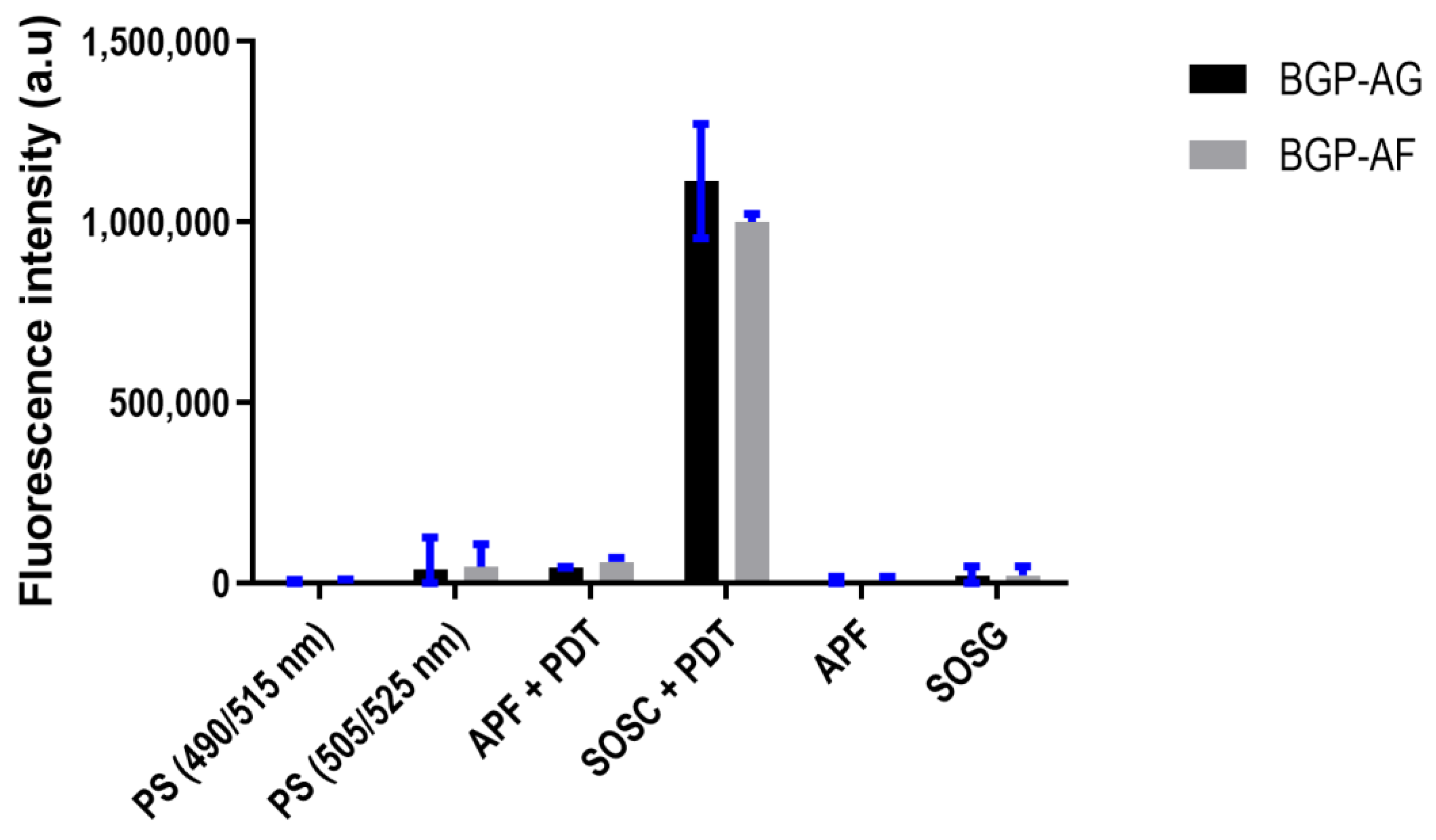
3.6. Reactive Oxygen Species (ROS) Detection
3.7. Pearson’s Correlation
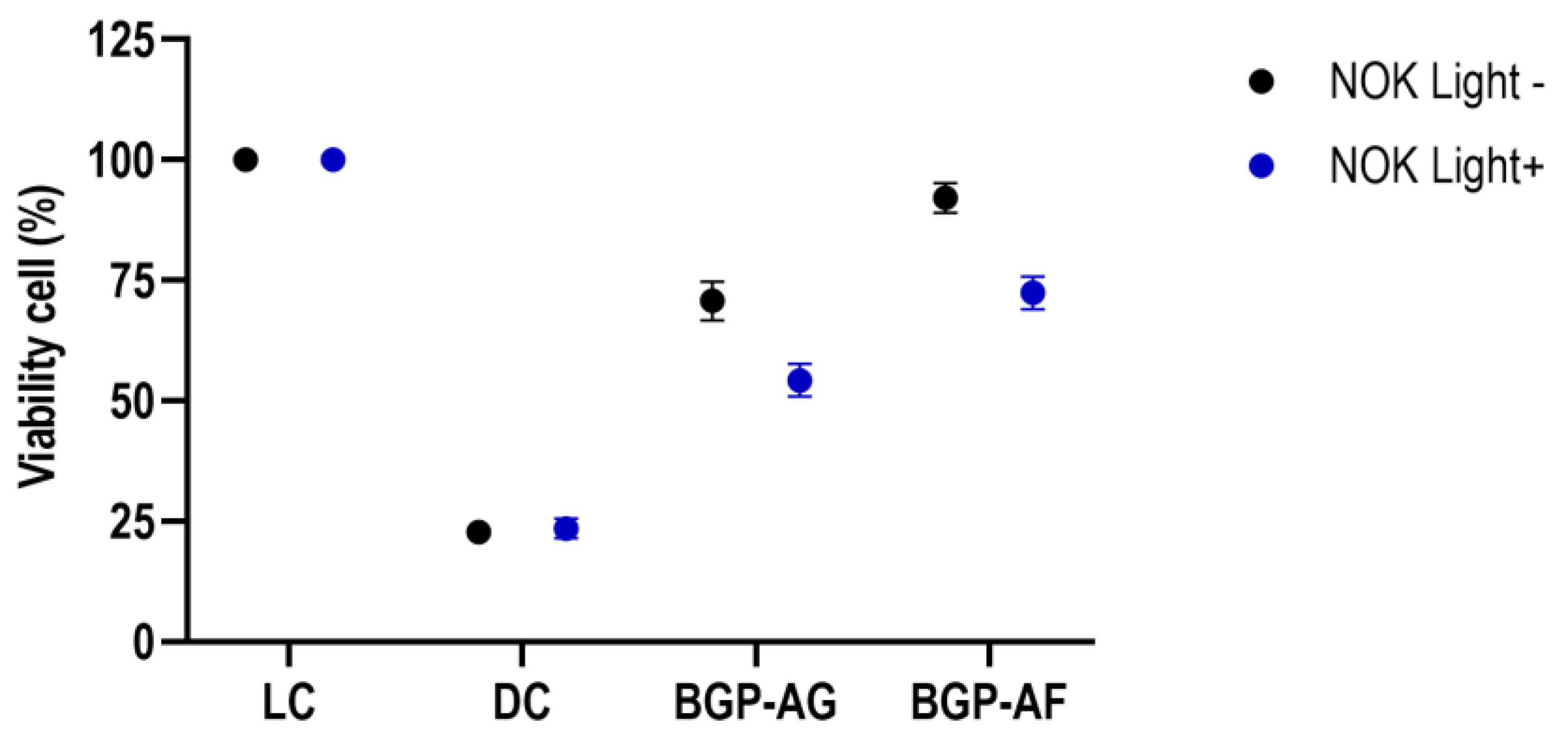
3.8. Cytotoxicity Assessment
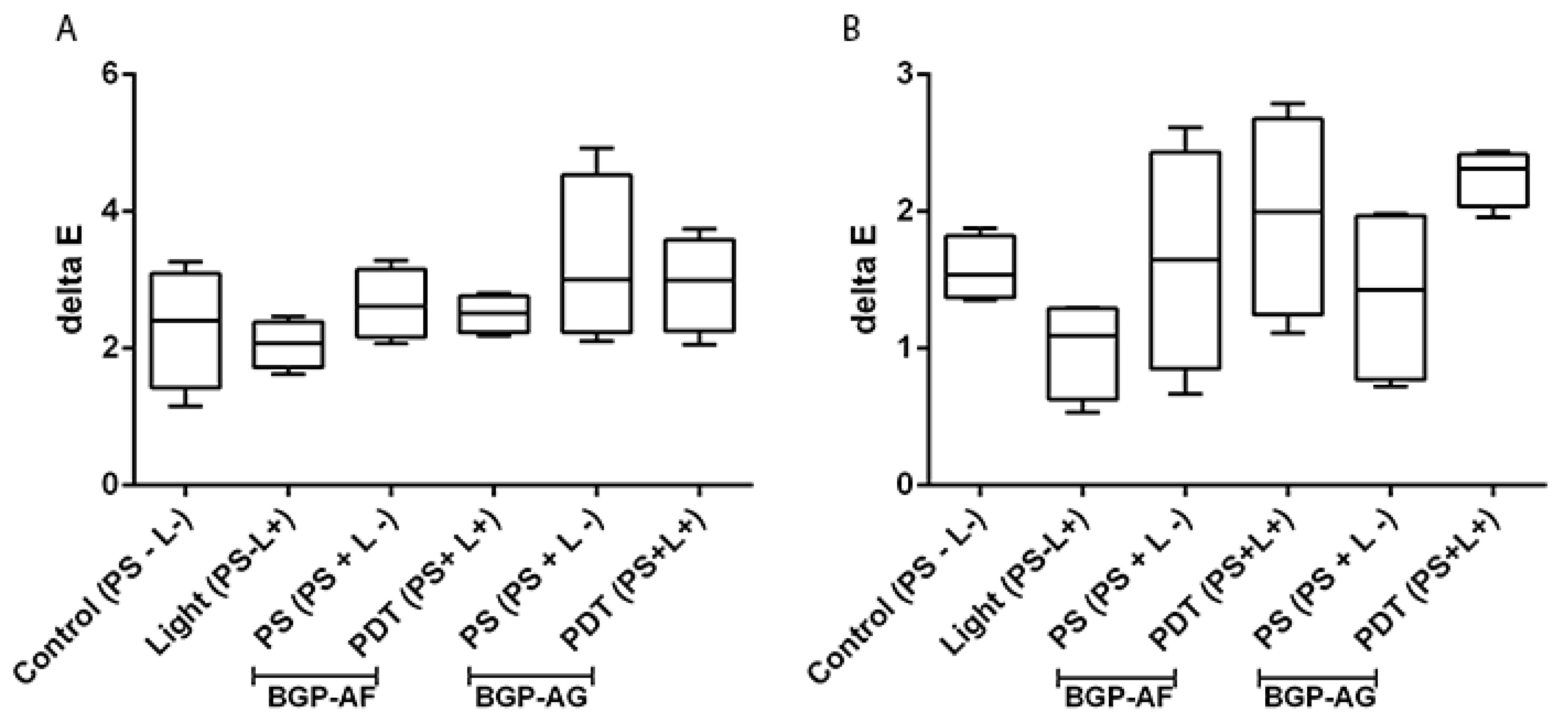
3.9. Effect of BGP-Mediated Antimicrobial Photodynamic Therapy on the Color Stability of Restorative Dental Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uribe, S.E.; Innes, N.; Maldupa, I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Paediatr. Dent. 2021, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Contemporary perspective on plaque control. Br. Dent. J. 2012, 212, 601–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkenburg, C.; Slot, D.E.; Bakker, E.W.; Van der Weijden, F.A. Does dentifrice use help to remove plaque? A systematic review. J. Clin. Periodontol. 2016, 43, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; Paschoal, M.A.B.; Diniz, R.S.; Lage, L.M.; Gonçalves, L.M. Antimicrobial action of chlorhexidine digluconate in self-ligating and conventional metal brackets infected with Streptococcus mutans biofilm. Clin. Cosmet. Investig. Dent. 2018, 19, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Tokubo, L.M.; Rosalen, P.L.; de Cássia, O.S.J.; Freires, I.A.; Fujimaki, M.; Umeda, J.E.; Barbosa, P.M.; Tecchio, G.O.; Hioka, N.; de Freitas, C.F.; et al. Antimicrobial effect of photodynamic therapy using erythrosine/methylene blue combination on Streptococcus mutans biofilm. Photodiagn. Photodyn. Ther. 2018, 23, 94–98. [Google Scholar] [CrossRef]
- Orasmo, E.M.W.; Otani, C.; Khouri, S. In vitro AFM evaluation of Streptococcus mutans membrane exposed to two mouthwashes. J. Appl. Pharm. Sci. 2013, 3, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef] [Green Version]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.-H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.A.; Pearson, G.J.; Colles, M.J.; Wilson, M. The photo-activated antibacterial action of toluidine blue O in a collagen matrix and in carious dentine. Caries Res. 2004, 38, 530–536. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Huang, S.; Wang, S.; Wang, Y.; Lei, L.; Zhang, C.; Huang, X. Antimicrobial Photodynamic Effect of Cross-Kingdom Microorganisms with Toluidine Blue O and Potassium Iodide. Int. J. Mol. Sci. 2022, 23, 11373. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Tonon, C.C.; Spolidório, D.M.; Bagnato, V.S.; Giusti, J.S.; Santos-Pinto, L. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagn. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef]
- Araújo, P.V.; Correia-Silva, J.F.; Gomez, R.S.; Massara, M.L.; Cortes, M.E.; Poletto, L.T. Antimicrobial effect of photodynamic therapy in carious lesions in vivo, using culture and real-time PCR methods. Photodiagn. Photodyn. Ther. 2015, 12, 401–407. [Google Scholar] [CrossRef]
- de Oliveira, A.B.; Ferrisse, T.M.; Marques, R.S.; de Annunzio, S.R.; Brighenti, F.L.; Fontana, C.R. Effect of Photodynamic Therapy on Microorganisms Responsible for Dental Caries: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 3585. [Google Scholar] [CrossRef] [Green Version]
- Marotti, J.; Aranha, A.C.; Eduardo Cde, P.; Ribeiro, M.S. Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomed. Laser Surg. 2009, 27, 357–363. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [Green Version]
- Gasparetto, A.; Lapinski, T.F.; Zamuner, S.R.; Khouri, S.; Alves, L.P.; Munin, E.; Salvador, M.J. Extracts from Alternanthera maritima as natural photosensitizers in photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B 2010, 99, 15–20. [Google Scholar] [CrossRef]
- Andreazza, N.L.; Caramano de Lourenço, C.; Hernandez-Tasco, Á.J.; Pinheiro, M.L.; Alves Stefanello, M.É.; Vilaça Costa, E.; Salvador, M.J. Antimicrobial photodynamic effect of extracts and oxoaporphine alkaloid isomoschatoline from Guatteria blepharophylla. J. Photochem. Photobiol. B 2016, 160, 154–162. [Google Scholar] [CrossRef]
- Barroso, A.; Fittipaldi, M.; Agustí, G.; Morató, J.; Codony, F. Preliminary evaluation of photodynamic activity of manuka honey. Photodiagn. Photodyn. Ther. 2016, 14, 25–26. [Google Scholar] [CrossRef]
- Marqués-Calvo, M.S.; Codony, F.; Agustí, G.; Lahera, C. Visible light enhances the antimicrobial effect of some essential oils. Photodiagn. Photodyn. Ther. 2017, 17, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef]
- Bankova, V.; Marcucci, M.C.; Simova, S.; Nikolova, N.; Kujumgiev, A.; Popov, S. Antibacterial diterpenic acids from Brazilian propolis. Z. Nat. C 1996, 51, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.L.; Assunção, A.K.M.; da Silva, M.C.P.; dos Reis, A.S.; Costa, G.C.; Arruda, D.D.S.; Rocha, B.A.; Vaz, M.M.D.O.L.L.; Paes, A.M.D.A.; Guerra, R.N.M.; et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid. Based Complement. Alternat. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway. J. Pharm. Pharmacol. 2015, 67, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wang, Y.X.; Yu, N.Q.; Hu, D.; Wang, X.-Y.; Chen, X.-G.; Liao, Y.-W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules 2017, 22, 732. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.M.F.; Carvalho, J.C.T.; Caputo, L.R.G.; Patricio, K.C.M.; Barbosa, M.V.J.; Chieff, A.L.; Bastos, J.K. Atividade antiinflamatória, anti-úlcera gástrica e toxicidade subcrônica do extrato etanólico de própolis. Rev. Bras. Farmacogn. 2000, 9–10, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, characterization and pre-clinical trials of an innovative wound healing dressing based on propolis (EPP-AF®) -containing self-microemulsifying formulation incorporated in biocellulose membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fontana, C.R.; Abernethy, A.D.; Som, S.; Ruggiero, K.; Doucette, S.; Marcantonio, R.C.; Boussios, C.I.; Kent, R.; Goodson, J.M.; Tanner, A.C.; et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J. Periodontal. Res. 2009, 44, 751–759. [Google Scholar] [CrossRef]
- de Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; Zago, L.H.P.; Uliana, M.P.; de Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic therapy enhanced by the peptide aurein 1.2. Sci. Rep. 2018, 8, 4212. [Google Scholar] [CrossRef]
- Farias, A.L.; Carvalho, L.P.F.; Méndez, D.A.C.; Cruvinel, T.; Brighenti, F.L. Characterization of polymicrobial biofilms obtained from saliva or carious lesions in dentin. Biofouling 2020, 36, 877–887. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Silva, R.C.; Zuanon, A.C.C. Surface roughness of glass ionomer cements indicated for atraumatic restorative treatment (ART). Braz. Dent. J. 2006, 17, 106–109. [Google Scholar] [CrossRef] [Green Version]
- de Moraes Rego Roselino, L.; Chinelatti, M.A.; Alandia-Román, C.C.; Pires-de-Souza, F.d.C.P. Effect of Brushing Time and Dentifrice Abrasiveness on Color Change and Surface Roughness of Resin Composites. Braz. Dent. J. 2015, 26, 507–513. [Google Scholar] [CrossRef]
- Johnston, W.M.; Kao, E.C. Assessment of appearance match by visual observation and clinical colorimetry. J. Dent. Res. 1989, 68, 819–822. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Prado, J.M. Natural Product Extraction: Principles and Applications; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Cannell, R.J.P. Natural Products Isolation; Springer Science & Business Media: Totowa, NJ, USA, 1998. [Google Scholar]
- Kennes, L.N. Methodological Aspects in Studies Based on Clinical Routine Data. Adv. Ther. 2017, 34, 2199–2209. [Google Scholar] [CrossRef] [Green Version]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Sanches, C.V.G.; Sardi, J.C.O.; Terada, R.S.S.; Lazarini, J.G.; Freires, I.A.; Polaquini, C.R.; Torrezan, G.S.; Regasini, L.O.; Fujimaki, M.; Rosalen, P.L. Diacetylcurcumin: A new photosensitizer for antimicrobial photodynamic therapy in Streptococcus mutans biofilms. Biofouling 2019, 35, 340–349. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Pena Ribeiro, V.; Oliveira Almeida, M.; Aldana Mejía, J.A.; Casoti, R.; Kenupp Bastos, J. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis. J. Pharm. Biomed. Anal. 2020, 178, 112922. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.C.; de Menezes, R.F.; Carneiro, V.S.M.; Dos Santos-Neto, A.P.; Fontana, C.R.; Bagnato, V.S.; Harvey, C.M.; Gerbi, M.E.M. Photodynamic Inactivation of Cariogenic Pathogens Using Curcumin as Photosensitizer. Photomed. Laser Surg. 2017, 35, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simón-Soro, Á.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef] [Green Version]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. Efficacy of photodynamic therapy against Streptococcus mutans biofilm: Role of singlet oxygen. J. Photochem. Photobiol. B 2018, 183, 16–21. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Nardini, E.F.; Almeida, T.S.; Yoshimura, T.M.; Ribeiro, M.S.; Cardoso, R.J.; Garcez, A.S. The potential of commercially available phytotherapeutic compounds as new photosensitizers for dental antimicrobial PDT: A photochemical and photobiological in vitro study. Photodiagn. Photodyn. Ther. 2019, 27, 248–254. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties, and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73 (Suppl. S1), S1–S6. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. Photodynamic therapy: Update 2006. Part 1: Photochemistry and photobiology. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 293–302. [Google Scholar] [CrossRef]
- Baptista, A.; Gonçalves, R.V.; Bressan, J.; Pelúzio, M.D.C.G. Antioxidant and Antimicrobial Activities of Crude Extracts and Fractions of Cashew (Anacardium occidentale L.), Cajui (Anacardium microcarpum), and Pequi (Caryocar brasiliense C.): A Systematic Review. Oxid. Med. Cell. Longev. 2018, 2018, 3753562. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.S.; McGaw, L.J.; Elgorashi, E.E.; Naidoo, V.; Eloff, J.N. Polarity of extracts and fractions of four Combretum (Combretaceae) species used to treat infections and gastrointestinal disorders in southern African traditional medicine has a major effect on different relevant in vitro activities. J. Ethnopharmacol. 2014, 154, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, F.L.; Salvador, M.J.; Gontijo, A.; Delbem, A.; Delbem, Á.; Soares, C.P.; de Oliveira, M.; Girondi, C.M.; Koga-Ito, C.Y. Plant extracts: Initial screening, identification of bioactive compounds and effect against Candida albicans biofilms. Future Microbiol. 2017, 12, 15–27. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Khouri, S.; Gontijo, A.V.L.; Pascoal, A.C.; Salvador, M.J.; Koga-Ito, C.Y. Antifungal activity of extracts and isolated compounds from Buchenavia tomentosa on Candida albicans and non-albicans. Future Microbiol. 2015, 10, 917–927. [Google Scholar] [CrossRef]
- Bonnet, R.; Martínez, G. Photobleaching of sensitizers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.; Vaz, M.M.; Marchetti, J.M. Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Deluca, M.C.C.; Scarparo, R.K.; Aspesi, M.; Matte, B.F.; Brand, L.M.; Grecca, F.S.; Casagrande, L.; Kopper, P.M.P. Cytotoxic, Migration, and Angiogenic Effects of Photodynamic Therapy and Photobiomodulation Associated with a Revascularization Protocol. J. Endod. 2021, 47, 69–77. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.


| Experimental Group | BGP Concentration | Wavelength (nm) | Irradiance Dose (J/cm2) | Intensity (mW/cm2) | Pre-Irradiation Time (m) | Irradiation Time in Fractionated Mode (m) |
|---|---|---|---|---|---|---|
| Biofilm | ||||||
| Negative control | N/A | 0 | 0 | 0 | 0 | 0 |
| Light | N/A | 450 | 80 | 151 | 0 | 0 |
| BGP (crude/fraction) | 1% | 0 | 0 | 0 | 15 | 18 |
| PDT (crude/ fraction) | 1% | 450 | 80 | 15 | 15 | 18 |
| BGP-AF (A450) | BGP-AG (A450) | ||
|---|---|---|---|
| Ethanolic | Aqueous | Ethanolic | Aqueous |
| 0.0542 | 0.2469 | 0.0432 | 0.0695 |
| Protein (mg/mL) | |
|---|---|
| BGP-AF | 2.01 |
| BGP-AG | 1.51 |
| Source | df | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| S. mutans (single biofilm) | |||||
| Groups | 3 | 176.8 | 58.92 | 42,701 | <0.0001 |
| Treatment | 3 | 495.6 | 165.2 | 119,706 | <0.0001 |
| Groups*Treatment | 9 | 437.6 | 48.62 | 35,235 | <0.0001 |
| S. mutans (dual biofilm) | |||||
| Groups | 1 | 18.9 | 18.9 | 2328 | <0.0001 |
| Treatment | 5 | 599.1 | 119.8 | 14,758 | <0.0001 |
| Groups*Treatment | 5 | 0.9306 | 0.1861 | 22.92 | <0.0001 |
| C. albicans (single biofilm) | |||||
| Groups | 3 | 49.46 | 16.49 | 6556 | <0.0001 |
| Treatment | 3 | 373.4 | 124.5 | 40,184 | <0.0001 |
| Groups*Treatment | 9 | 182.8 | 20.31 | 5322 | <0.0001 |
| C. albicans (dual biofilm) | |||||
| Groups | 1 | 17.58 | 17.58 | 77.49 | <0.0001 |
| Treatment | 5 | 494.4 | 98.87 | 435.9 | <0.0001 |
| Groups*Treatment | 5 | 0.759 | 0.1518 | 0.6692 | 0.6476 |
| ROS | |||||
| Probe | 5 | 1.067 × 1013 | 2.2134 × 1012 | 721.2 | <0.0001 |
| Natural | 1 | 3.904 × 109 | 3.904 × 109 | 1.319 | 0.2553 |
| Probe*Natural | 5 | 3.487 × 1010 | 6.975 × 109 | 2.357 | 0.0510 |
| Cytotoxicity | |||||
| Light | 1 | 2508 | 2508 | 105.3 | <0.0001 |
| Groups | 3 | 104,510 | 34,837 | 1462 | <0.0001 |
| Light * Groups | 3 | 2782 | 927.5 | 39.93 | <0.0001 |
| Groups | Variables | p-Value | r (Pearson) | R2 | |
|---|---|---|---|---|---|
| S. mutans—immediate effect | |||||
| BGP-AG | PS + Light (log reduction) | SOSG | 0.0215 | 0.8776 | 0.7701 |
| BGP-AF | PS + Light (log reduction) | SOSG | 0.0047 | 0.9432 | 0.8896 |
| S. mutans—regrowth | |||||
| BGP-AG | PS + Light (log reduction) | SOSG | 0.0257 | 0.866 | 0.7499 |
| BGP-AF | PS + Light (log reduction) | SOSG | 0.0023 | 0.9602 | 0.922 |
| C. albicans—immediate effect | |||||
| BGP-AG | PS + Light (log reduction) | SOSG | 0.0076 | 0.9277 | 0.8606 |
| BGP-AF | PS + Light (log reduction) | SOSG | 0.0259 | 0.8656 | 0.7493 |
| C. albicans—regrowth | |||||
| BGP-AG | PS + Light (log reduction) | SOSG | 0.0198 | 0.8828 | 0.7794 |
| BGP-AF | PS + Light (log reduction) | SOSG | 0.0565 | 0.799 | 0.6384 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, A.B.; Ferrisse, T.M.; França, G.G.; de Annunzio, S.R.; Kopp, W.; Fontana, C.R.; Brighenti, F.L. Potential Use of Brazilian Green Propolis Extracts as New Photosensitizers for Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pathogens 2023, 12, 155. https://doi.org/10.3390/pathogens12020155
de Oliveira AB, Ferrisse TM, França GG, de Annunzio SR, Kopp W, Fontana CR, Brighenti FL. Potential Use of Brazilian Green Propolis Extracts as New Photosensitizers for Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pathogens. 2023; 12(2):155. https://doi.org/10.3390/pathogens12020155
Chicago/Turabian Stylede Oliveira, Analú Barros, Túlio Morandin Ferrisse, Gabriela Gomes França, Sarah Raquel de Annunzio, Willian Kopp, Carla Raquel Fontana, and Fernanda Lourenção Brighenti. 2023. "Potential Use of Brazilian Green Propolis Extracts as New Photosensitizers for Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms" Pathogens 12, no. 2: 155. https://doi.org/10.3390/pathogens12020155





