Comparison of Analytical Sensitivity (Limit of Detection) of Xpert MTB/RIF and Xpert MTB/RIF Ultra for Non-Sputum Specimens
Abstract
:1. Background
2. Methods
2.1. Preparation of MTB Stock
2.2. Sample Preparation
2.3. Xpert MTB/RIF US-IVD and Xpert MTB/RIF Ultra Tests
2.4. Statistical Analysis
3. Results
3.1. Limit of Detection (LoD)
3.2. Linearity for US-IVD and Ultra
3.3. Semi-Quantitative Results by Ultra
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dheda, K.; Perumal, T.; Moultrie, H.; Perumal, R.; Esmail, A.; Scott, A.J.; Udwadia, Z.; Chang, K.C.; Peter, J.; Pooran, A.; et al. The intersecting pandemics of tuberculosis and COVID-19: Population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 2022, 10, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Schiller, I.; Horne, D.J.; Pai, M.; Boehme, C.C.; Dendukuri, N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2014, CD009593. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.J.; Kohli, M.; Zifodya, J.S.; Schiller, I.; Dendukuri, N.; Tollefson, D.; Schumacher, S.G.; Ochodo, E.A.; Pai, M.; Steingart, K.R. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2019, 6, CD009593. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241565714. [Google Scholar]
- Aisenberg, G.M.; Jacobson, K.; Chemaly, R.F.; Rolston, K.V.; Raad, I.I.; Safdar, A. Extrapulmonary tuberculosis active infection misdiagnosed as cancer: Mycobacterium tuberculosis disease in patients at a Comprehensive Cancer Center (2001–2005). Cancer 2005, 104, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, D.; Abubakar, I.; Potts, H.; Hunter, P.R.; Kruijshaar, M.E.; Kon, O.M.; Southern, J. Risk factors for the misdiagnosis of tuberculosis in the UK, 2001–2011: TABLE 1. Eur. Respir. J. 2015, 46, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.A.; Pinsky, B.A.; Liu, A.; Banaei, N. Delayed Diagnosis of Tuberculous Meningitis Misdiagnosed as Herpes Simplex Virus-1 Encephalitis with the FilmArray Syndromic Polymerase Chain Reaction Panel. Open Forum Infect. Dis. 2017, 4, ofw245. [Google Scholar] [CrossRef]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. MBio 2017, 8, e00812-17. [Google Scholar] [CrossRef] [Green Version]
- Cepheid|Package Inserts. Available online: https://www.cepheid.com/en_US/package-inserts/1608 (accessed on 15 October 2022).
- Alemu, A.; Tadesse, M.; Seid, G.; Mollalign, H.; Eshetu, K.; Sinshaw, W.; Abebaw, Y.; Amare, M.; Dagne, B.; Diriba, G.; et al. Does Xpert® MTB/RIF assay give rifampicin resistance results without identified mutation? Review of cases from Addis Ababa, Ethiopia. BMC Infect. Dis. 2020, 20, 87. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Parmar, H.; Simmons, A.M.; Kale, D.; Tong, K.; Lieu, D.; Persing, D.; Kwiatkowski, R.; Alland, D.; Chakravorty, S. Automatic Identification of Individual rpoB Gene Mutations Responsible for Rifampin Resistance in Mycobacterium tuberculosis by Use of Melting Temperature Signatures Generated by the Xpert MTB/RIF Ultra Assay. J. Clin. Microbiol. 2019, 58, e00907-19. [Google Scholar] [CrossRef] [Green Version]
- Cepheid|MTB/RIF Molecular Test—Xpert MTB/RIF Ultra. Available online: https://www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-RIF-Ultra (accessed on 15 October 2022).
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Study team Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- Zifodya, J.S.; Kreniske, J.S.; Schiller, I.; Kohli, M.; Dendukuri, N.; Schumacher, S.G.; Ochodo, E.A.; Haraka, F.; Zwerling, A.A.; Pai, M.; et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst. Rev. 2021, 2, CD009593. [Google Scholar] [CrossRef]
- Marlowe, E.M.; Novak-Weekley, S.M.; Cumpio, J.; Sharp, S.E.; Momeny, M.A.; Babst, A.; Carlson, J.S.; Kawamura, M.; Pandori, M. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J. Clin. Microbiol. 2011, 49, 1621–1623. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas-Urquides, K.; Villarreal-Treviño, L.; Silva-Ramírez, B.; Rivadeneyra-Espinoza, L.; Said-Fernández, S.; de León, M.B. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz. J. Microbiol. 2013, 44, 287–289. [Google Scholar] [CrossRef] [Green Version]
- Bahr, N.C.; Nuwagira, E.; Evans, E.E.; Cresswell, F.V.; Bystrom, P.V.; Byamukama, A.; Bridge, S.C.; Bangdiwala, A.S.; Meya, D.B.; Denkinger, C.M.; et al. ASTRO-CM Trial Team Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: A prospective cohort study. Lancet Infect. Dis. 2018, 18, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Le Palud, P.; Cattoir, V.; Malbruny, B.; Magnier, R.; Campbell, K.; Oulkhouir, Y.; Zalcman, G.; Bergot, E. Retrospective observational study of diagnostic accuracy of the Xpert® MTB/RIF assay on fiberoptic bronchoscopy sampling for early diagnosis of smear-negative or sputum-scarce patients with suspected tuberculosis. BMC Pulm. Med. 2014, 14, 137. [Google Scholar] [CrossRef] [Green Version]
- Denkinger, C.M.; Schumacher, S.G.; Boehme, C.C.; Dendukuri, N.; Pai, M.; Steingart, K.R. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2014, 44, 435–446. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Meeting Report of a Technical Expert Consultation: Non-Inferiority Analysis of Xpert MTB/RIF Ultra Compared to Xpert MTB/RIF; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Carnevale, G.G.; Vargas, F.S.; Caiaffa-Filho, H.H.; Acencio, M.M.P.; Marçal, L.J.; Sales, R.K.B.; Teixeira, L.R.; Antonangelo, L. Preanalytical conditions can interfere with M. tuberculosis detection by PCR in respiratory samples. Clinics 2018, 73, e410. [Google Scholar] [CrossRef]
- WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis: Rapid Diagnostics for Tuberculosis Detection. 2021 Update. Available online: https://www.who.int/publications/i/item/9789240029415 (accessed on 23 October 2022).
- Donovan, J.; Thu, D.D.A.; Phu, N.H.; Dung, V.T.M.; Quang, T.P.; Nghia, H.D.T.; Oanh, P.K.N.; Nhu, T.B.; Chau, N.V.V.; Ha, V.T.N.; et al. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for the diagnosis of tuberculous meningitis: A prospective, randomised, diagnostic accuracy study. Lancet Infect. Dis. 2020, 20, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.B.; Theron, G.; Lenders, L.; Matinyena, B.; Connolly, C.; Singh, R.; Coovadia, Y.; Ndung’u, T.; Dheda, K. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: A prospective study. PLoS Med. 2013, 10, e1001536. [Google Scholar] [CrossRef] [Green Version]
- Amedeo, A.; Beci, G.; Giglia, M.; Lombardi, G.; Bisognin, F.; Chiarucci, F.; Corsini, I.; Dal Monte, P.; Tadolini, M. Evaluation of trace calls by Xpert MTB/RIF ultra for clinical management in low TB burden settings. PLoS ONE 2022, 17, e0272997. [Google Scholar] [CrossRef]
- Biswas, S.; Uddin, M.K.M.; Paul, K.K.; Ather, M.F.; Ahmed, S.; Nasrin, R.; Kabir, S.; Heysell, S.K.; Banu, S. Xpert MTB/RIF Ultra assay for the detection of Mycobacterium tuberculosis in people with negative conventional Xpert MTB/RIF but chest imaging suggestive of tuberculosis in Dhaka, Bangladesh. Int. J. Infect. Dis. 2022, 114, 244–251. [Google Scholar] [CrossRef] [PubMed]
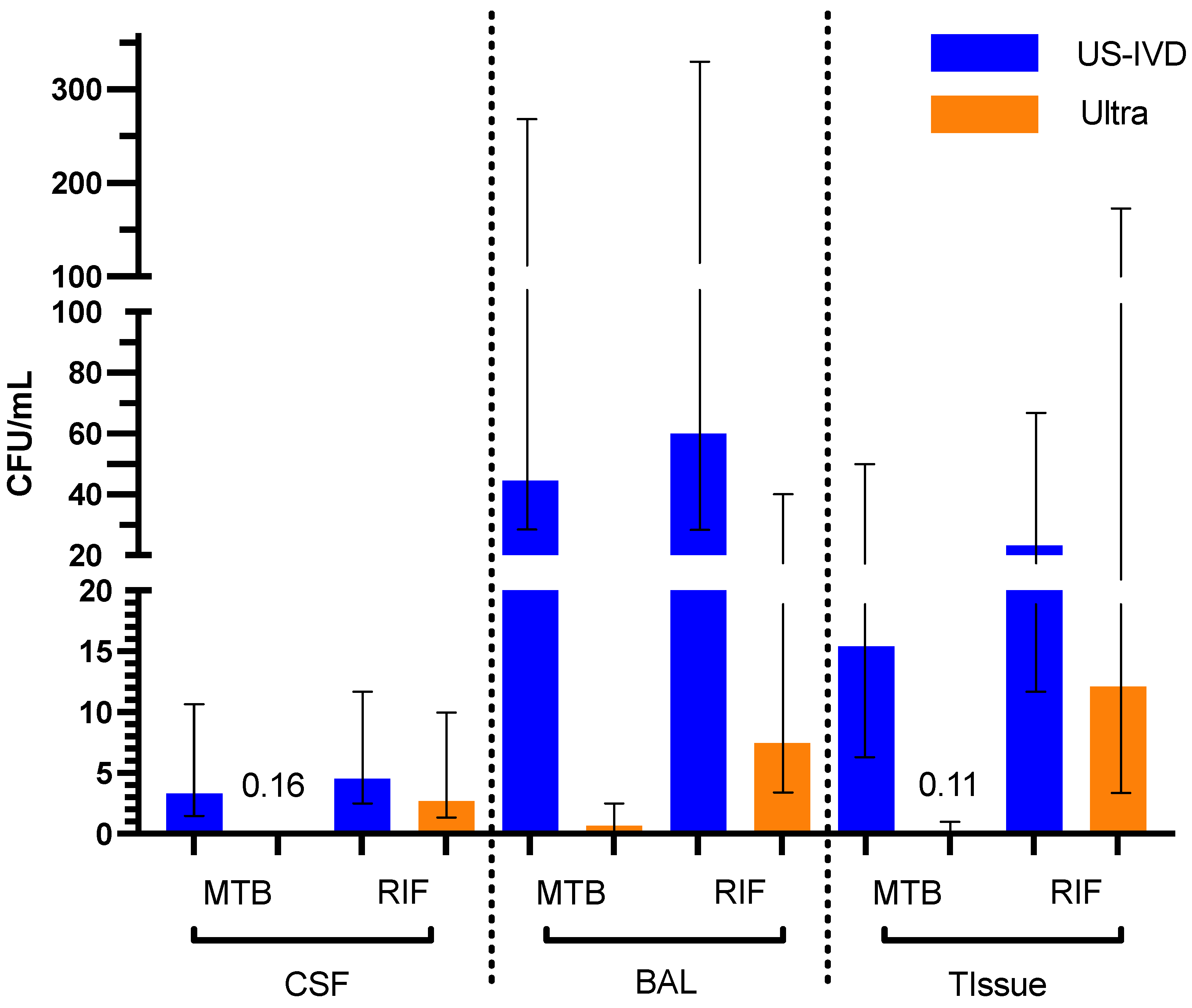


| Sample Type | US-IVD (95% CI) | Ultra (95% CI) | |
|---|---|---|---|
| CSF | MTB | 3.3 (2.2–11) | 0.16 (0.095–0.52) |
| RIF | 4.6 (2.9–14) | 2.7 (1.4–10) | |
| BAL | MTB | 45 (22–260) | 0.65 (0.39–2.3) |
| RIF | 60 (28– 353) | 7.5 (3.6–46) | |
| Tissue | MTB | 15 (8.0–49) | 0.11 (0.077–0.29) |
| RIF | 23 (12–70) | 12 (3.7–151) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, M.C.; Clarner, P.; Paroha, R.; Lee, S.; Thwe, P.M.; Ren, P. Comparison of Analytical Sensitivity (Limit of Detection) of Xpert MTB/RIF and Xpert MTB/RIF Ultra for Non-Sputum Specimens. Pathogens 2023, 12, 157. https://doi.org/10.3390/pathogens12020157
Nielsen MC, Clarner P, Paroha R, Lee S, Thwe PM, Ren P. Comparison of Analytical Sensitivity (Limit of Detection) of Xpert MTB/RIF and Xpert MTB/RIF Ultra for Non-Sputum Specimens. Pathogens. 2023; 12(2):157. https://doi.org/10.3390/pathogens12020157
Chicago/Turabian StyleNielsen, Marisa C., Paula Clarner, Ruchi Paroha, Sunhee Lee, Phyu M. Thwe, and Ping Ren. 2023. "Comparison of Analytical Sensitivity (Limit of Detection) of Xpert MTB/RIF and Xpert MTB/RIF Ultra for Non-Sputum Specimens" Pathogens 12, no. 2: 157. https://doi.org/10.3390/pathogens12020157
APA StyleNielsen, M. C., Clarner, P., Paroha, R., Lee, S., Thwe, P. M., & Ren, P. (2023). Comparison of Analytical Sensitivity (Limit of Detection) of Xpert MTB/RIF and Xpert MTB/RIF Ultra for Non-Sputum Specimens. Pathogens, 12(2), 157. https://doi.org/10.3390/pathogens12020157







