Rational Development of Live-Attenuated Zika Virus Vaccines
Abstract
:1. Introduction
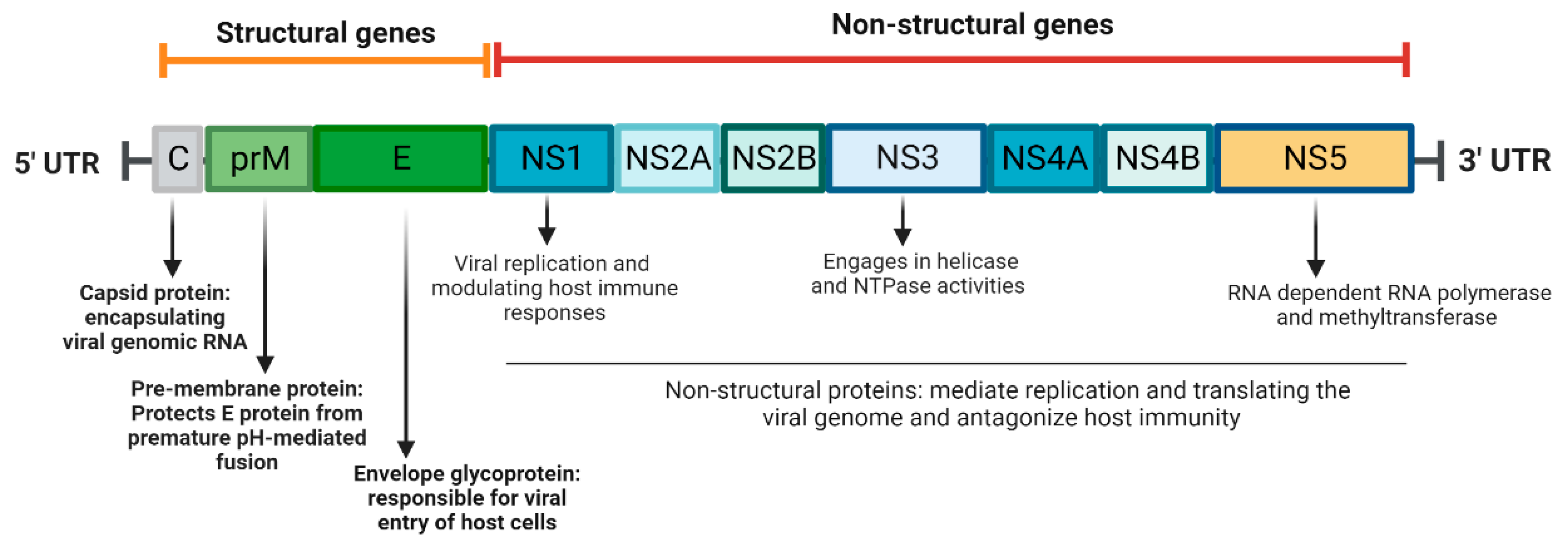
2. ZIKV Biology
3. Animal Models of ZIKV Infection
4. Adaptive Immunity to ZIKV Infection and Vaccination
5. ZIKV LAVs
5.1. Live Chimeric Vaccines
5.1.1. Chimeric LAVs with Mammalian-Specific Flavivirus Backbone
5.1.2. Chimeric LAVs with Insect-Specific Flavivirus Backbone
5.2. LAV Mutants
5.2.1. UTR Deletion Mutants
5.2.2. LAVs with Mutations in the Structural Gene(s)
5.2.3. LAVs with Mutations in the NS Gene(s)
5.2.4. LAVs with Combinatorial Mutations in Both Structural and NS Genes
5.3. Codon Pair Deoptimization
6. Other Platforms of ZIKV Vaccines
| Vaccine Platform/Status | Strengths | Weaknesses | References |
|---|---|---|---|
| LAV: Preclinical studies | Induce strong immune response Long term immunity Adjuvant not required Preservation of native antigen Rapid and durable immunity Single dose | Consideration for genetic stability | [47,48,49,58,59,60,61,62,63,64,65,66,67,68,74,75,76] |
| Purified inactivated vaccine: Preclinical studies, phase 1 clinical trials | Induce strong immune response to multiple viral antigens Easy to prepare | Potential epitope alteration by inactivation process Adjuvant and multiple doses are required | [78,79,80] |
| Recombinant DNA vaccine: Preclinical studies, clinical trials | Non-infectious Easier to design, inexpensive Low risk Stable at ambient temperature | Induce lower immunogenicity May need special device/technology for administration Potential risk of integration into the host genome | [44,81,82,83,84,85] |
| mRNA vaccines: Preclinical studies, phase 1 clinical trials | Highly safe Easy and fast to produce Induce strong neutralizing antibodies | Induce lower immunogenicity Potential risk of RNA-induced interferon response Need low temperature for storage Multiple doses are required | [45,46] |
| Viral vector vaccines: Preclinical studies, clinical trials | Induce strong immune response No adjuvant required | Risk of genomic integration Multiple doses are required Host immunity against the vector may negatively affect the effectiveness of the vaccine | [18,78,86,87,88,89,90] |
7. Summary and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Giannakopoulos, X.; Sofikitis, N.; Papadopoulou, C. An Update on Sexual Transmission of Zika Virus. Pathogens 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.H.; Wilson, M.E. Update on non-vector transmission of dengue: Relevant studies with Zika and other flaviviruses. Trop. Dis. Travel Med. Vaccines 2016, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Adam, A.; Jassoy, C. Epidemiology and Laboratory Diagnostics of Dengue, Yellow Fever, Zika, and Chikungunya Virus Infections in Africa. Pathogens 2021, 10, 1324. [Google Scholar] [CrossRef]
- Alonso-Palomares, L.A.; Moreno-Garcia, M.; Lanz-Mendoza, H.; Salazar, M.I. Molecular Basis for Arbovirus Transmission by Aedes aegypti Mosquitoes. Intervirology 2018, 61, 255–264. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect. Dis. 2016, 16, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef] [Green Version]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastere, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef] [Green Version]
- Ghaffar, K.A.; Ng, L.F.P.; Renia, L. Fast Tracks and Roadblocks for Zika Vaccines. Vaccines 2018, 6, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Hui, L.; Nie, Y.; Tefsen, B.; Wu, Y. ZIKV viral proteins and their roles in virus-host interactions. Sci. China Life Sci. 2021, 64, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Kurup, D.; Wirblich, C.; Lambert, R.; Diba, L.Z.; Leiby, B.E.; Schnell, M.J. Measles-based Zika vaccine induces long-term immunity and requires NS1 antibodies to protect the female reproductive tract. NPJ Vaccines 2022, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Bos, S.; Tsetsarkin, K.A.; Pletnev, A.G.; Despres, P.; Gadea, G.; Zhao, R.Y. The Roles of prM-E Proteins in Historical and Epidemic Zika Virus-mediated Infection and Neurocytotoxicity. Viruses 2019, 11, 157. [Google Scholar] [CrossRef]
- Sirohi, D.; Kuhn, R.J. Zika Virus Structure, Maturation, and Receptors. J. Infect. Dis. 2017, 216, S935–S944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, A.P.; Moraes, A.H. Zika virus proteins at an atomic scale: How does structural biology help us to understand and develop vaccines and drugs against Zika virus infection? J. Venom. Anim. Toxins. Incl. Trop. Dis. 2019, 25, e20190013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [Green Version]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimaraes, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Adams Waldorf, K.M.; Stencel-Baerenwald, J.E.; Kapur, R.P.; Studholme, C.; Boldenow, E.; Vornhagen, J.; Baldessari, A.; Dighe, M.K.; Thiel, J.; Merillat, S.; et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med. 2016, 22, 1256–1259. [Google Scholar] [CrossRef]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef] [Green Version]
- Koide, F.; Goebel, S.; Snyder, B.; Walters, K.B.; Gast, A.; Hagelin, K.; Kalkeri, R.; Rayner, J. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front. Microbiol. 2016, 7, 2028. [Google Scholar] [CrossRef]
- Li, X.F.; Dong, H.L.; Huang, X.Y.; Qiu, Y.F.; Wang, H.J.; Deng, Y.Q.; Zhang, N.N.; Ye, Q.; Zhao, H.; Liu, Z.Y.; et al. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine 2016, 12, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuna, C.E.; Lim, S.Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.O.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliota, M.T.; Dudley, D.M.; Newman, C.M.; Mohr, E.L.; Gellerup, D.D.; Breitbach, M.E.; Buechler, C.R.; Rasheed, M.N.; Mohns, M.S.; Weiler, A.M.; et al. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS Negl. Trop. Dis. 2016, 10, e0005168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219. [Google Scholar] [CrossRef] [Green Version]
- Bassi, M.R.; Kongsgaard, M.; Steffensen, M.A.; Fenger, C.; Rasmussen, M.; Skjodt, K.; Finsen, B.; Stryhn, A.; Buus, S.; Christensen, J.P.; et al. CD8+ T cells complement antibodies in protecting against yellow fever virus. J. Immunol. 2015, 194, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Larena, M.; Regner, M.; Lee, E.; Lobigs, M. Pivotal role of antibody and subsidiary contribution of CD8+ T cells to recovery from infection in a murine model of Japanese encephalitis. J. Virol. 2011, 85, 5446–5455. [Google Scholar] [CrossRef] [Green Version]
- Mathews, J.H.; Roehrig, J.T.; Brubaker, J.R.; Hunt, A.R.; Allan, J.E. A synthetic peptide to the E glycoprotein of Murray Valley encephalitis virus defines multiple virus-reactive T- and B-cell epitopes. J. Virol. 1992, 66, 6555–6562. [Google Scholar] [CrossRef] [Green Version]
- Akondy, R.S.; Johnson, P.L.; Nakaya, H.I.; Edupuganti, S.; Mulligan, M.J.; Lawson, B.; Miller, J.D.; Pulendran, B.; Antia, R.; Ahmed, R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 3050–3055. [Google Scholar] [CrossRef]
- Halstead, S.B. Achieving safe, effective, and durable Zika virus vaccines: Lessons from dengue. Lancet Infect. Dis. 2017, 17, e378–e382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aid, M.; Abbink, P.; Larocca, R.A.; Boyd, M.; Nityanandam, R.; Nanayakkara, O.; Martinot, A.J.; Moseley, E.T.; Blass, E.; Borducchi, E.N.; et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell 2017, 169, 610–620.e14. [Google Scholar] [CrossRef] [Green Version]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and Role of the CD8(+) T Cell Response during Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef] [Green Version]
- Adam, A.; Fontes-Garfias, C.R.; Sarathy, V.V.; Liu, Y.; Luo, H.; Davis, E.; Li, W.; Muruato, A.E.; Wang, B.; Ahatov, R.; et al. A genetically stable Zika virus vaccine candidate protects mice against virus infection and vertical transmission. NPJ Vaccines 2021, 6, 27. [Google Scholar] [CrossRef]
- Li, G.; Adam, A.; Luo, H.; Shan, C.; Cao, Z.; Fontes-Garfias, C.R.; Sarathy, V.V.; Teleki, C.; Winkelmann, E.R.; Liang, Y.; et al. An attenuated Zika virus NS4B protein mutant is a potent inducer of antiviral immune responses. NPJ Vaccines 2019, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef]
- Cohen, J. Zika rewrites maternal immunization ethics. Science 2017, 357, 241. [Google Scholar] [CrossRef]
- Holland, J.; Spindler, K.; Horodyski, F.; Grabau, E.; Nichol, S.; VandePol, S. Rapid evolution of RNA genomes. Science 1982, 215, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.L.; Volk, S.M.; Pandya, J.; Wang, E.; Liang, X.; Weaver, S.C. Stability of RNA virus attenuation approaches. Vaccine 2011, 29, 2230–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.J.; Monath, T.P. Chimeric flaviviruses: Novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis. Adv. Virus Res. 2003, 61, 469–509. [Google Scholar] [CrossRef]
- Chin, W.X.; Lee, R.C.H.; Kaur, P.; Lew, T.S.; Yogarajah, T.; Kong, H.Y.; Teo, Z.Y.; Salim, C.K.; Zhang, R.R.; Li, X.F.; et al. A single-dose live attenuated chimeric vaccine candidate against Zika virus. NPJ Vaccines 2021, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Silengo, S.J.; Whiteman, M.C.; Kinney, R.M. Chimeric dengue 2 PDK-53/West Nile NY99 viruses retain the phenotypic attenuation markers of the candidate PDK-53 vaccine virus and protect mice against lethal challenge with West Nile virus. J. Virol. 2005, 79, 7300–7310. [Google Scholar] [CrossRef] [Green Version]
- Kum, D.B.; Boudewijns, R.; Ma, J.; Mishra, N.; Schols, D.; Neyts, J.; Dallmeier, K. A chimeric yellow fever-Zika virus vaccine candidate fully protects against yellow fever virus infection in mice. Emerg. Microbes Infect. 2020, 9, 520–533. [Google Scholar] [CrossRef]
- Mishra, N.; Boudewijns, R.; Schmid, M.A.; Marques, R.E.; Sharma, S.; Neyts, J.; Dallmeier, K. A Chimeric Japanese Encephalitis Vaccine Protects against Lethal Yellow Fever Virus Infection without Inducing Neutralizing Antibodies. mBio 2020, 11, e02494-19. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, A.S.; Pattnaik, A.; Sahoo, B.R.; Guinn, Z.P.; Bullard, B.L.; Weaver, E.A.; Steffen, D.; Natarajan, S.K.; Petro, T.M.; Pattnaik, A.K. An Attenuated Zika Virus Encoding Non-Glycosylated Envelope (E) and Non-Structural Protein 1 (NS1) Confers Complete Protection against Lethal Challenge in a Mouse Model. Vaccines 2019, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, A.S.; Pattnaik, A.; Sahoo, B.R.; Muthukrishnan, E.; Natarajan, S.K.; Steffen, D.; Vu, H.L.X.; Delhon, G.; Osorio, F.A.; Petro, T.M.; et al. Zika Virus Encoding Nonglycosylated Envelope Protein Is Attenuated and Defective in Neuroinvasion. J. Virol. 2017, 91, e01348-17. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Yang, Y.; Muruato, A.E.; Zou, J.; Shan, C.; Nunes, B.T.; Medeiros, D.B.; Vasconcelos, P.F.; Weaver, S.C.; Rossi, S.L.; et al. Understanding Zika Virus Stability and Developing a Chimeric Vaccine through Functional Analysis. mBio 2017, 8, e02134-16. [Google Scholar] [CrossRef]
- Available online: https://www.niaid.nih.gov/diseases-conditions/zika-vaccines (accessed on 26 January 2023).
- Giel-Moloney, M.; Goncalvez, A.P.; Catalan, J.; Lecouturier, V.; Girerd-Chambaz, Y.; Diaz, F.; Maldonado-Arocho, F.; Gomila, R.C.; Bernard, M.C.; Oomen, R.; et al. Chimeric yellow fever 17D-Zika virus (ChimeriVax-Zika) as a live-attenuated Zika virus vaccine. Sci. Rep. 2018, 8, 13206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Dong, H.L.; Wang, H.J.; Huang, X.Y.; Qiu, Y.F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazlewood, J.E.; Tang, B.; Yan, K.; Rawle, D.J.; Harrison, J.J.; Hall, R.A.; Hobson-Peters, J.; Suhrbier, A. The Chimeric Binjari-Zika Vaccine Provides Long-Term Protection against ZIKA Virus Challenge. Vaccines 2022, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Langsjoen, R.M.; Porier, D.L.; Erasmus, J.H.; Bergren, N.A.; Bolling, B.G.; Luo, H.; Singh, A.; Guzman, H.; Popov, V.L.; et al. Isolation of a novel insect-specific flavivirus with immunomodulatory effects in vertebrate systems. Virology 2021, 562, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Porier, D.L.; Wilson, S.N.; Auguste, D.I.; Leber, A.; Coutermarsh-Ott, S.; Allen, I.C.; Caswell, C.C.; Budnick, J.A.; Bassaganya-Riera, J.; Hontecillas, R.; et al. Enemy of My Enemy: A Novel Insect-Specific Flavivirus Offers a Promising Platform for a Zika Virus Vaccine. Vaccines 2021, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.P.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes-Garfias, C.R.; Shan, C.; Luo, H.; Muruato, A.E.; Medeiros, D.B.A.; Mays, E.; Xie, X.; Zou, J.; Roundy, C.M.; Wakamiya, M.; et al. Functional Analysis of Glycosylation of Zika Virus Envelope Protein. Cell Rep. 2017, 21, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Jordan, J.L.; Laurent-Rolle, M.; Ashour, J.; Martinez-Sobrido, L.; Ashok, M.; Lipkin, W.I.; Garcia-Sastre, A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005, 79, 8004–8013. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Wang, X.J.; Mokhonov, V.V.; Shi, P.Y.; Randall, R.; Khromykh, A.A. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J. Virol. 2005, 79, 1934–1942. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D.; Seeger, C. Differential effects of mutations in NS4B on West Nile virus replication and inhibition of interferon signaling. J. Virol. 2007, 81, 11809–11816. [Google Scholar] [CrossRef]
- Munoz-Jordan, J.L.; Sanchez-Burgos, G.G.; Laurent-Rolle, M.; Garcia-Sastre, A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundin, M.; Monne, M.; Widell, A.; Von Heijne, G.; Persson, M.A. Topology of the membrane-associated hepatitis C virus protein NS4B. J. Virol. 2003, 77, 5428–5438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicker, J.A.; Whiteman, M.C.; Beasley, D.W.; Davis, C.T.; Zhang, S.; Schneider, B.S.; Higgs, S.; Kinney, R.M.; Barrett, A.D. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology 2006, 349, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Liu, X.; Shu, T.; Deng, W.; Liao, M.; Zheng, Y.; Zheng, X.; Zhang, X.; Li, T.; Fan, W.; et al. A Live-Attenuated Zika Virus Vaccine with High Production Capacity Confers Effective Protection in Neonatal Mice. J. Virol. 2021, 95, e0038321. [Google Scholar] [CrossRef]
- Li, P.; Ke, X.; Wang, T.; Tan, Z.; Luo, D.; Miao, Y.; Sun, J.; Zhang, Y.; Liu, Y.; Hu, Q.; et al. Zika Virus Attenuation by Codon Pair Deoptimization Induces Sterilizing Immunity in Mouse Models. J. Virol. 2018, 92, e00701-18. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.R.; Walsh, E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J. Clin. Microbiol. 1988, 26, 1595–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbink, P.; Stephenson, K.E.; Barouch, D.H. Zika virus vaccines. Nat. Rev. Microbiol. 2018, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Lecouturier, V.; Pavot, V.; Berry, C.; Donadieu, A.; de Montfort, A.; Boudet, F.; Rokbi, B.; Jackson, N.; Heinrichs, J. An optimized purified inactivated Zika vaccine provides sustained immunogenicity and protection in cynomolgus macaques. NPJ Vaccines 2020, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Han, H.H.; Diaz, C.; Acosta, C.J.; Liu, M.; Borkowski, A. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: An observer-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1282–1292. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine—Preliminary Report. N. Engl. J. Med. 2017, 385, e35. [Google Scholar] [CrossRef] [PubMed]
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. NS1 DNA vaccination protects against Zika infection through T cell-mediated immunity in immunocompetent mice. Sci. Adv. 2019, 5, eaax2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Y.; Pang, Z.; Du, Y.; Wang, W.; Yang, Y.; Wang, W.; Gao, G.F.; Huang, B.; Deng, Y.; Tan, W. NS1-based DNA vaccination confers mouse protective immunity against ZIKV challenge. Infect. Genet. Evol. 2020, 85, 104521. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Duehr, J.; Dulin, H.; Broecker, F.; Brown, J.A.; Arumemi, F.O.; Bermudez Gonzalez, M.C.; Leyva-Grado, V.H.; Evans, M.J.; Simon, V.; et al. Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model. Nat. Commun. 2018, 9, 4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Bullard, B.L.; Corder, B.N.; Gordon, D.N.; Pierson, T.C.; Weaver, E.A. Characterization of a Species E Adenovirus Vector as a Zika virus vaccine. Sci. Rep. 2020, 10, 3613. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [Green Version]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Betancourt, D.; de Queiroz, N.M.; Xia, T.; Ahn, J.; Barber, G.N. Cutting Edge: Innate Immune Augmenting Vesicular Stomatitis Virus Expressing Zika Virus Proteins Confers Protective Immunity. J. Immunol. 2017, 198, 3023–3028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, A.; Lee, C.; Wang, T. Rational Development of Live-Attenuated Zika Virus Vaccines. Pathogens 2023, 12, 194. https://doi.org/10.3390/pathogens12020194
Adam A, Lee C, Wang T. Rational Development of Live-Attenuated Zika Virus Vaccines. Pathogens. 2023; 12(2):194. https://doi.org/10.3390/pathogens12020194
Chicago/Turabian StyleAdam, Awadalkareem, Christy Lee, and Tian Wang. 2023. "Rational Development of Live-Attenuated Zika Virus Vaccines" Pathogens 12, no. 2: 194. https://doi.org/10.3390/pathogens12020194
APA StyleAdam, A., Lee, C., & Wang, T. (2023). Rational Development of Live-Attenuated Zika Virus Vaccines. Pathogens, 12(2), 194. https://doi.org/10.3390/pathogens12020194







