Lysine-Derived Maillard Reaction Products Inhibit the Growth of Salmonella enterica Serotype Typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Crude MRPs
2.2. Determination of Crude MRP Stock Absorbance at 420 nm
2.3. Bacterial Strain and Growth Conditions
2.4. Growth of S. Typhimurium with MRP under Acidic and Thermal Stress Conditions
2.5. Efficacy of GL MRP against S. Typhimurium
2.6. Growth of S. Typhimurium with GL MRP at 37 °C and 55 °C
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sugar-Amino Acid Combinations to Generate HMW MRP Products
3.2. Effect of MRPs on the Survival of S. Typhimurium under Sublethal Acidic and Thermal Stress Conditions
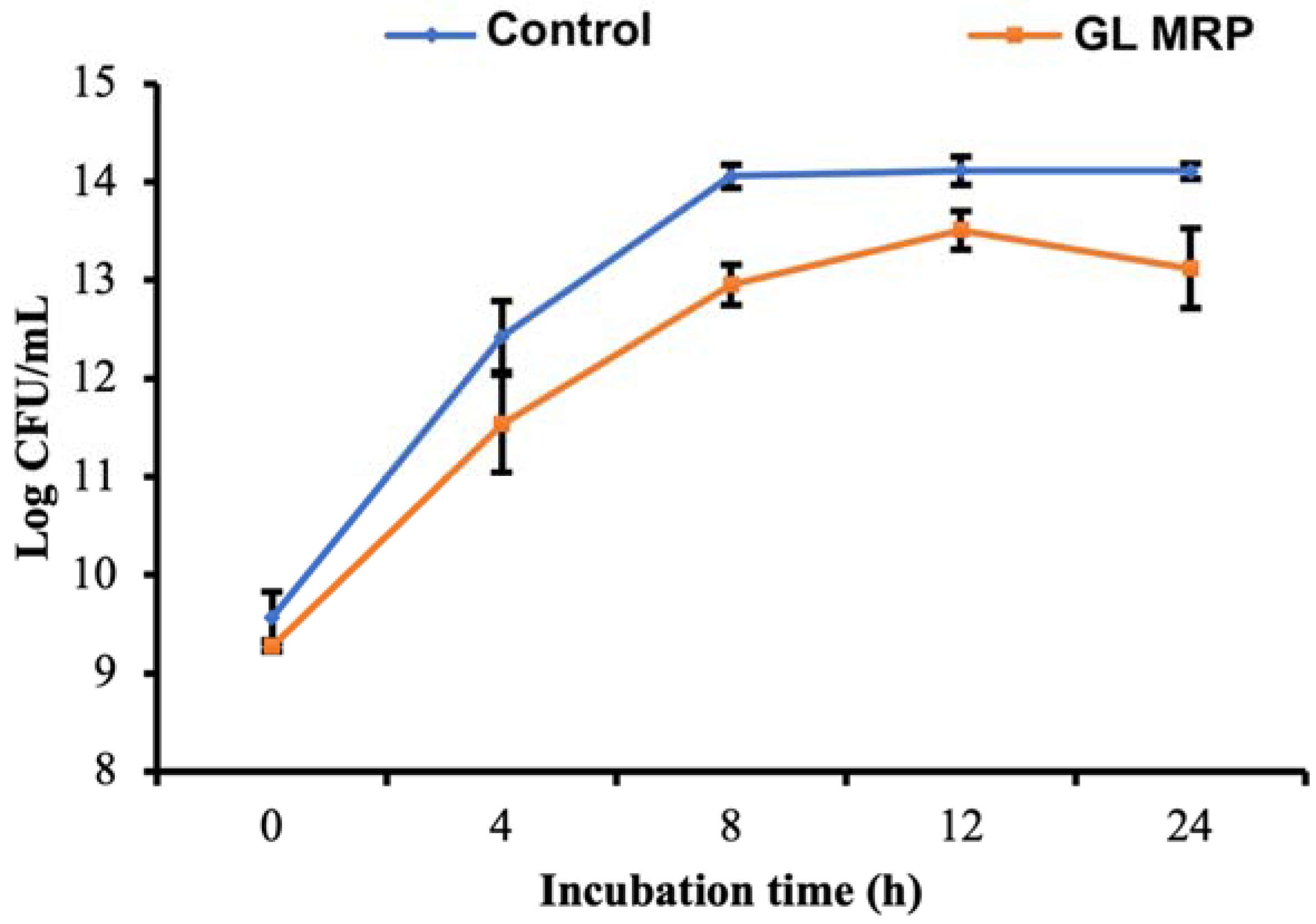
3.3. GL MRP Inhibits the Growth of S. Typhimurium at Optimal Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.v.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Reports of Selected Salmonella Outbreak Investigations. Available online: https://www.cdc.gov/salmonella/outbreaks.html (accessed on 3 February 2019).
- Pradhan, D.; Devi Negi, V. Stress-Induced Adaptations in Salmonella: A Ground for Shaping Its Pathogenesis. Microbiol. Res. 2019, 229, 126311. [Google Scholar] [CrossRef] [PubMed]
- Berk, P.A.; de Jonge, R.; Zwietering, M.H.; Abee, T.; Kieboom, J. Acid Resistance Variability Among Isolates of Salmonella Enterica Serovar Typhimurium DT104. J. Appl. Microbiol. 2005, 99, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Madruga, M.; Steele, E.M.; Reynolds, C.; Levy, R.B.; Rauber, F. Trends in Food Consumption According to the Degree of Food Processing Among the UK Population Over 11 Years. Br. J. Nutr. 2022, 1–8. [Google Scholar] [CrossRef]
- Błaszczyk, I.; Iciek, J. Selection of Heat Treatment Conditions and Prevention of Secondary Microbial Contamination of Liquid Sugar: Practical Remarks. J. Food Sci. Technol. 2021, 58, 2842–2846. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Food Safety and Inspection Service Safe Minimum Internal Temperature Chart. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/safe-temperature-chart (accessed on 25 December 2022).
- Kilibarda, N.; Brdar, I.; Baltic, B.; Markovic, V.; Mahmutovic, H.; Karabasil, N.; Stanisic, S. The Safety and Quality of Sous Vide Food. Meat Technol. 2018, 59, 38–45. [Google Scholar] [CrossRef]
- Government of Canada Safe Cooking Temperatures. Available online: https://www.canada.ca/en/health-canada/services/general-food-safety-tips/safe-internal-cooking-temperatures.html (accessed on 3 January 2023).
- McIntyre, L.; Jorgenson, V.; Ritson, M. Sous Vide Style Cooking Practices Linked to Salmonella Enteritidis Illnesses. Environ. Health Rev. 2017, 60, 42–49. [Google Scholar] [CrossRef]
- Public Health Ontario Case Study: Sous Vide. Available online: https://www.publichealthontario.ca/-/media/Documents/C/2016/case-study-sous-vide.pdf?rev=bf5d650a459d431aabf5493d4cdd2e03&sc_lang=en (accessed on 26 December 2022).
- Einarsson, H.; Snygg, B.G.; Eriksson, C. Inhibition of Bacterial Growth by Maillard Reaction Products. J. Agric. Foodchem. 1983, 31, 1043–1047. [Google Scholar] [CrossRef]
- Kundinger, M.M.; Zabala-Díaz, I.B.; Chalova, V.I.; Ricke, S.C. Effects of Maillard Reaction Products on HilA Expression in Salmonella Typhimurium. J. Food Sci. 2008, 73, M32–M35. [Google Scholar] [CrossRef]
- Sheikh-Zeinoddin, M.; Perehinec, T.M.; Hill, S.E.; Rees, C.E.D. Maillard Reaction Causes Suppression of Virulence Gene Expression in Listeria Monocytogenes. Int. J. Food Microbiol. 2000, 61, 41–49. [Google Scholar] [CrossRef]
- Gu, F.L.; Kim, J.M.; Abbas, S.; Zhang, X.M.; Xia, S.Q.; Chen, Z.X. Structure and Antioxidant Activity of High Molecular Weight Maillard Reaction Products from Casein-Glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Iijima, T.; Watanabe, T.; Nakazawa, H. Antioxidative Effect of Maillard Reaction Products Using Glucose-Glycine Model System. J. Agric. Food Chem. 1997, 45, 4106–4109. [Google Scholar] [CrossRef]
- Ramonaityte, D.T.; Keršiene, M.; Adams, A.; Tehrani, K.A.; Kimpe, N. de The Interaction of Metal Ions with Maillard Reaction Products in a Lactose-Glycine Model System. Food Res. Int. 2009, 42, 331–336. [Google Scholar] [CrossRef]
- Hirano, M.; Miura, M.; Gomyo, T. A Tentative Measurement of Brown Pigments in Various Processed Foods. Biosci. Biotechnol. Biochem. 1996, 60, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D. Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions. Antioxidants 2021, 10, 1840. [Google Scholar] [CrossRef]
- Yates, G.T.; Smotzer, T. On the Lag Phase and Initial Decline of Microbial Growth Curves. J. Theor. Biol. 2007, 244, 511–517. [Google Scholar] [CrossRef]
- Westoby, M.; Nielsen, D.A.; Gillings, M.R.; Litchman, E.; Madin, J.S.; Paulsen, I.T.; Tetu, S.G. Cell Size, Genome Size, and Maximum Growth Rate Are Near-Independent Dimensions of Ecological Variation Across Bacteria and Archaea. Ecol. Evol. 2021, 11, 3956–3976. [Google Scholar] [CrossRef]
- Mikami, Y.; Murata, M. Effects of Sugar and Buffer Types, and PH on Formation of Maillard Pigments in the Lysine Model System. Food Sci. Technol. Res. 2015, 21, 813–819. [Google Scholar] [CrossRef]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.S.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag Phase Is a Distinct Growth Phase That Prepares Bacteria for Exponential Growth and Involves Transient Metal Accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Wang, S.; D Kitts, D. Evidence to Indicate That Maillard Reaction Products Can Provide Selective Antimicrobial Activity. Integr. Food Nutr. Metab. 2016, 3, 330–335. [Google Scholar] [CrossRef]
- Shiroda, M.; Pratt, Z.L.; Döpfer, D.; Wong, A.C.L.; Kaspar, C.W. RpoS Impacts the Lag Phase of Salmonella Enterica During Osmotic Stress. Fems Microbiol. Lett. 2014, 357, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Soo Lee, I.; Lin, J.; Hall, H.K.; Bearson, B.; Foster, J.W. The Stationary-phase Sigma Factor ΣS (RpoS) Is Required for a Sustained Acid Tolerance Response in Virulent Salmonella Typhimurium. Mol. Microbiol. 1995, 17, 155–167. [Google Scholar] [CrossRef]
- Fang, F.C.; Libby, S.J.; Buchmeiert, N.A.; Loewen, P.C.; Switala, J.; Harwood, J.; Guiney, D.G. The Alternative (a Factor KatF (RpoS) Regulates Salmonella Virulence. Proc. Natl. Acad. Sci. USA 1992, 89, 11978–11982. [Google Scholar] [CrossRef]
- Chalova, V.I.; Hernández-Hernández, O.; Muthaiyan, A.; Sirsat, S.A.; Natesan, S.; Sanz, M.L.; Moreno, F.J.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Growth and Transcriptional Response of Salmonella Typhimurium LT2 to Glucose-Lysine-Based Maillard Reaction Products Generated Under Low Water Activity Conditions. Food Res. Int. 2012, 45, 1044–1053. [Google Scholar] [CrossRef]
- Kukuminato, S.; Koyama, K.; Koseki, S. Antibacterial Properties of Melanoidins Produced from Various Combinations of Maillard Reaction against Pathogenic Bacteria. Am. Soc. Microbiol. 2021, 9, e01142-21. [Google Scholar] [CrossRef] [PubMed]
- Hegele, J.; Münch, G.; Pischetsrieder, M. Identification of Hydrogen Peroxide as a Major Cytotoxic Component in Maillard Reaction Mixtures and Coffee. Mol. Nutr. Food Res. 2009, 53, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roca, B.; Delgado-Andrade, C.; Pilar Navarro, M.; Seiquer, I. Effects of Maillard Reaction Products from Glucose-Lysine Model Systems on Oxidative Stress Markers and Against Oxidative Induction by Hydrogen Peroxide in Caco-2 Cells. J. Food Nutr. Res. 2011, 50, 237–248. [Google Scholar]
- Ünlütürk, A.; Turantasl, F. Bactericidal Effect of Hydrogen Peroxide on Salmonella Typhimurium in Liquid Whole Egg. J. Appl. Bacteriol. 1987, 62, 25–28. [Google Scholar] [CrossRef]
- Ukuku, D.O.; Pilizota, V.; Sapers, G.M. Effect of Hot Water and Hydrogen Peroxide Treatments on Survival of Salmonella and Microbial Quality of Whole and Fresh-Cut Cantaloupe †. J. Food Prot. 2004, 67, 432–437. [Google Scholar] [CrossRef]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard Reaction Products as Antimicrobial Components for Packaging Films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef]
- Rufian-Henares, J.A.; de La Cueva, S.P. Antimicrobial Activity of Coffee Melanoidins—A Study of Their Metal-Chelating Properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Rurián-Henares, J.A.; Morales, F.J. Antimicrobial Activity of Melanoidins against Escherichia Coli Is Mediated by a Membrane-Damage Mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.K.; Mehta, B.S.; Akukwe, B. Maillard Reaction Products Inhibit the Periodontal Pathogen Aggregatibacter Actinomycetemcomitans by Chelating Oron. Arch. Oral. Biol. 2021, 122, 104989. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, Y.; Song, W.; Cai, L.; Wang, Y.; Guo, J. Analysis on Antibacterial Activities and Volatile Compounds of Maillard Reaction Products Derived From Squid Skin. E3s Web Conf. 2020, 145, 1–6. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef]
- Morales, F.J.; Fernández-Fraguas, C.; Jiménez-Pérez, S. Iron-Binding Ability of Melanoidins from Food and Model Systems. Food Chem. 2005, 90, 821–827. [Google Scholar] [CrossRef]

| Crude MRP | Absorbance at 420 nm 1,2 |
|---|---|
| FL | 1.030 ± 0.006 b |
| GL | 1.690 ± 0.048 a |
| RL | 0.578 ± 0.011 d |
| XL | 0.813 ± 0.037 c |
| Crude MRP | λ at 37 °C | λ at 42 °C | |
|---|---|---|---|
| pH 5.5 2 | pH 7.2 | pH 7.2 2 | |
| Control (No MRP) | 5.54 ± 0.11 a | 5.06 ± 0.05 a | 5.54 ± 0.29 a |
| FL | 5.23 ± 0.30 a | 4.70 ± 0.26 a | 4.95 ± 0.40 a,b |
| GL | 4.51 ± 0.31 b | 4.78 ± 0.29 a | 4.79 ± 0.26 b |
| RL | 5.18 ± 0.57 a | 4.59 ± 0.31 a | 4.83 ± 0.19 b |
| XL | 5.09 ± 0.33 a,b | 4.60 ± 0.28 a | 4.93 ± 0.45 b |
| Crude MRP | µ at 37 °C | µ at 42 °C | |
|---|---|---|---|
| pH 5.5 2 | pH 7.2 2 | pH 7.2 2 | |
| Control (No MRP) | 0.128 ± 0.004 a | 0.230 ± 0.011 a | 0.163 ± 0.010 a |
| FL | 0.105 ± 0.005 b | 0.160 ± 0.042 b | 0.116 ± 0.046 b |
| GL | 0.087 ± 0.013 b | 0.160 ± 0.060 b | 0.097 ± 0.008 b |
| RL | 0.094 ± 0.017 b | 0.144 ± 0.032 b | 0.092 ± 0.004 b |
| XL | 0.095 ± 0.009 b | 0.127 ± 0.007 b | 0.101 ± 0.015 b |
| µmax 2 | No 2 | Nmax 2 | ΔN 2 | |
|---|---|---|---|---|
| Control | 1.653 ± 0.059 a | 9.569 ± 0.261 a | 14.108 ± 0.084 a | 4.538 ± 0.205 a |
| GL MRP | 1.323 ± 0.251 b | 9.268 ± 0.068 b | 13.263 ± 0.125 b | 3.996 ± 0.132 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.W.Y.; Mu, K.; Kitts, D.D.; Wang, S. Lysine-Derived Maillard Reaction Products Inhibit the Growth of Salmonella enterica Serotype Typhimurium. Pathogens 2023, 12, 215. https://doi.org/10.3390/pathogens12020215
Wong CWY, Mu K, Kitts DD, Wang S. Lysine-Derived Maillard Reaction Products Inhibit the Growth of Salmonella enterica Serotype Typhimurium. Pathogens. 2023; 12(2):215. https://doi.org/10.3390/pathogens12020215
Chicago/Turabian StyleWong, Catherine W. Y., Kaiwen Mu, David D. Kitts, and Siyun Wang. 2023. "Lysine-Derived Maillard Reaction Products Inhibit the Growth of Salmonella enterica Serotype Typhimurium" Pathogens 12, no. 2: 215. https://doi.org/10.3390/pathogens12020215
APA StyleWong, C. W. Y., Mu, K., Kitts, D. D., & Wang, S. (2023). Lysine-Derived Maillard Reaction Products Inhibit the Growth of Salmonella enterica Serotype Typhimurium. Pathogens, 12(2), 215. https://doi.org/10.3390/pathogens12020215





