Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Endophytic Bacteria
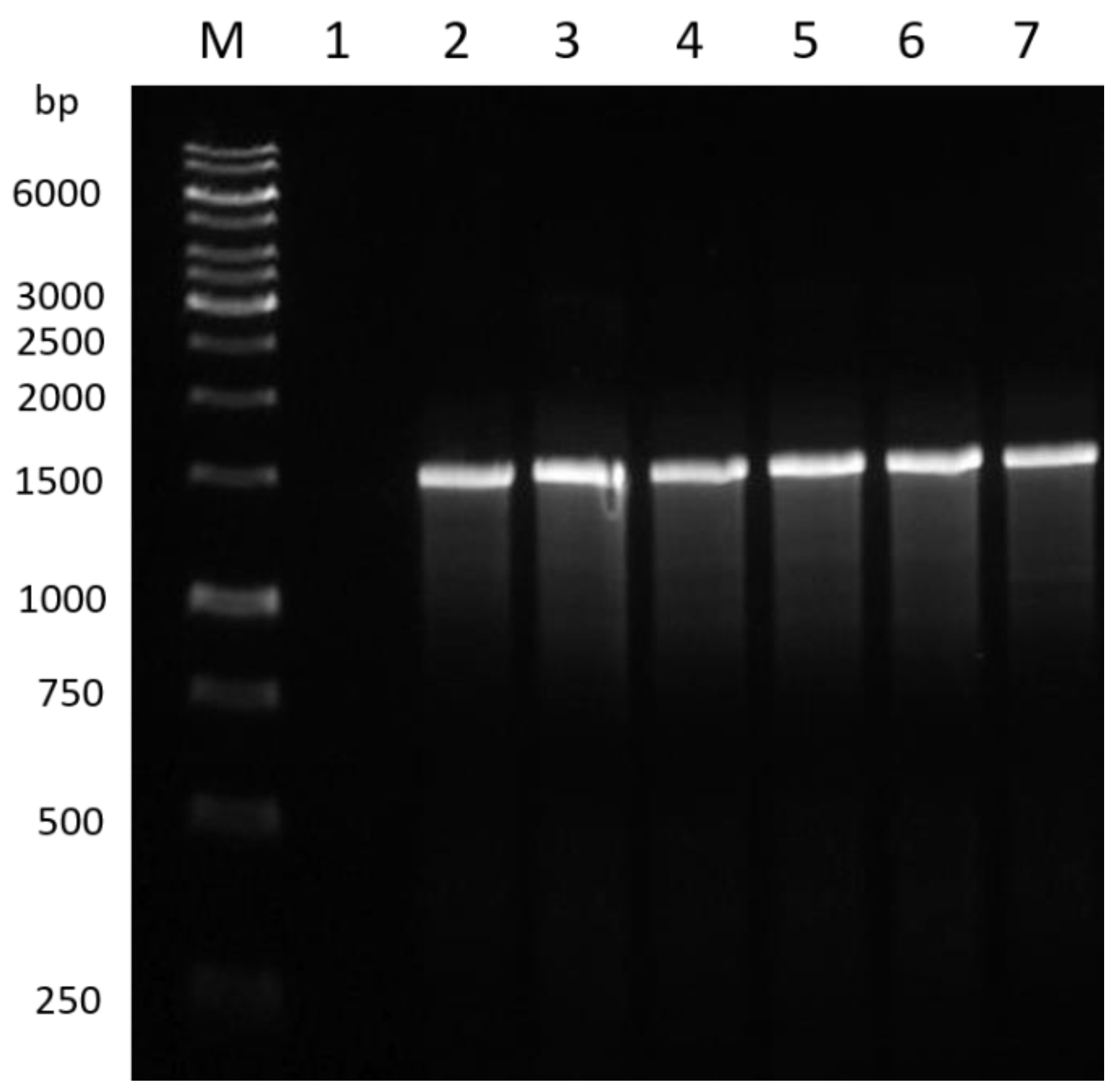
2.2. DNA Extraction and PCR Amplification of the 16S rRNA Gene
2.3. DNA Sequencing and Data Analysis
2.4. Gram Stain and Bacterial Morphology
2.5. Catalase Test
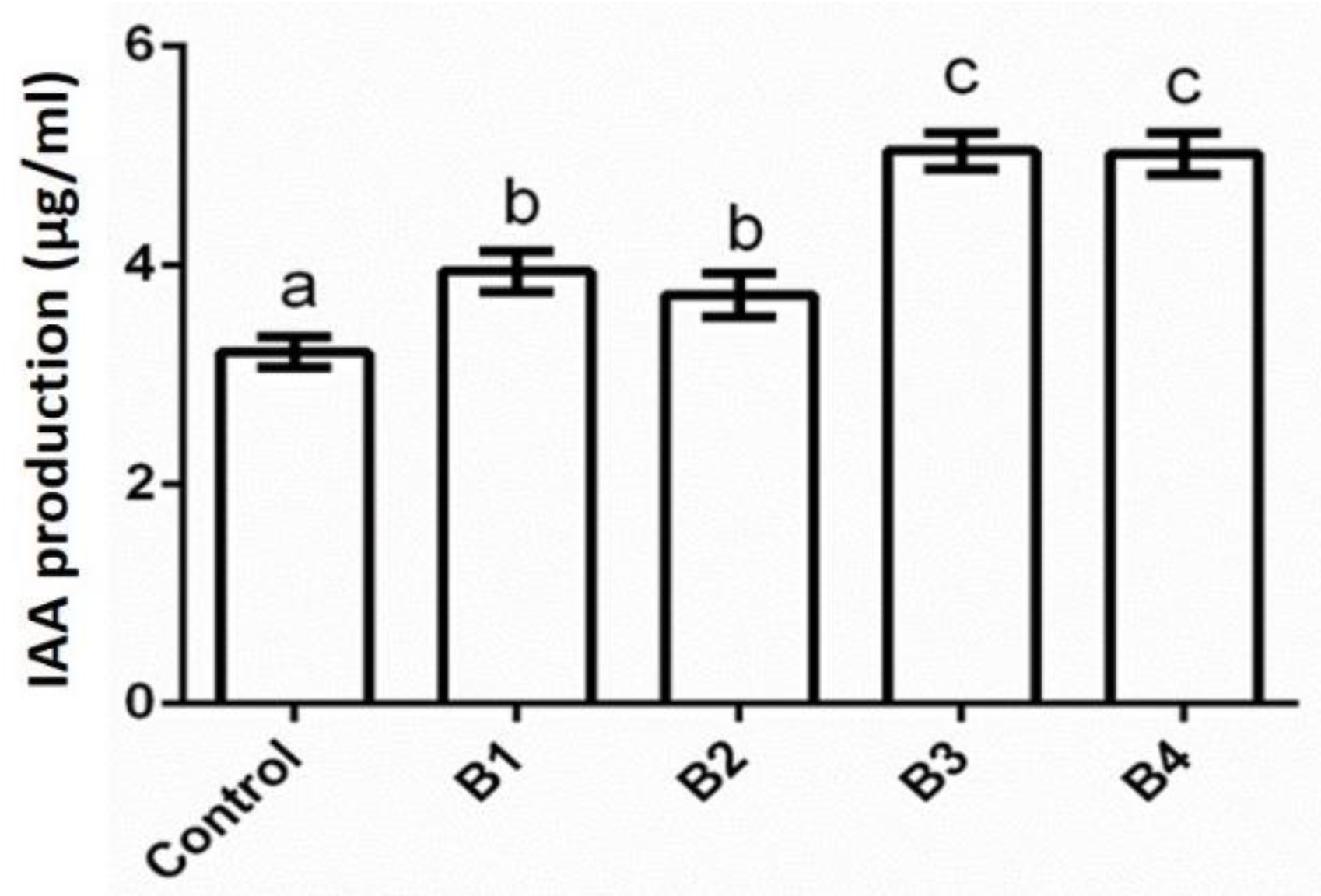
2.6. Indole Acetic Acid (IAA) Production Test
2.7. Phosphate Solubilization Test
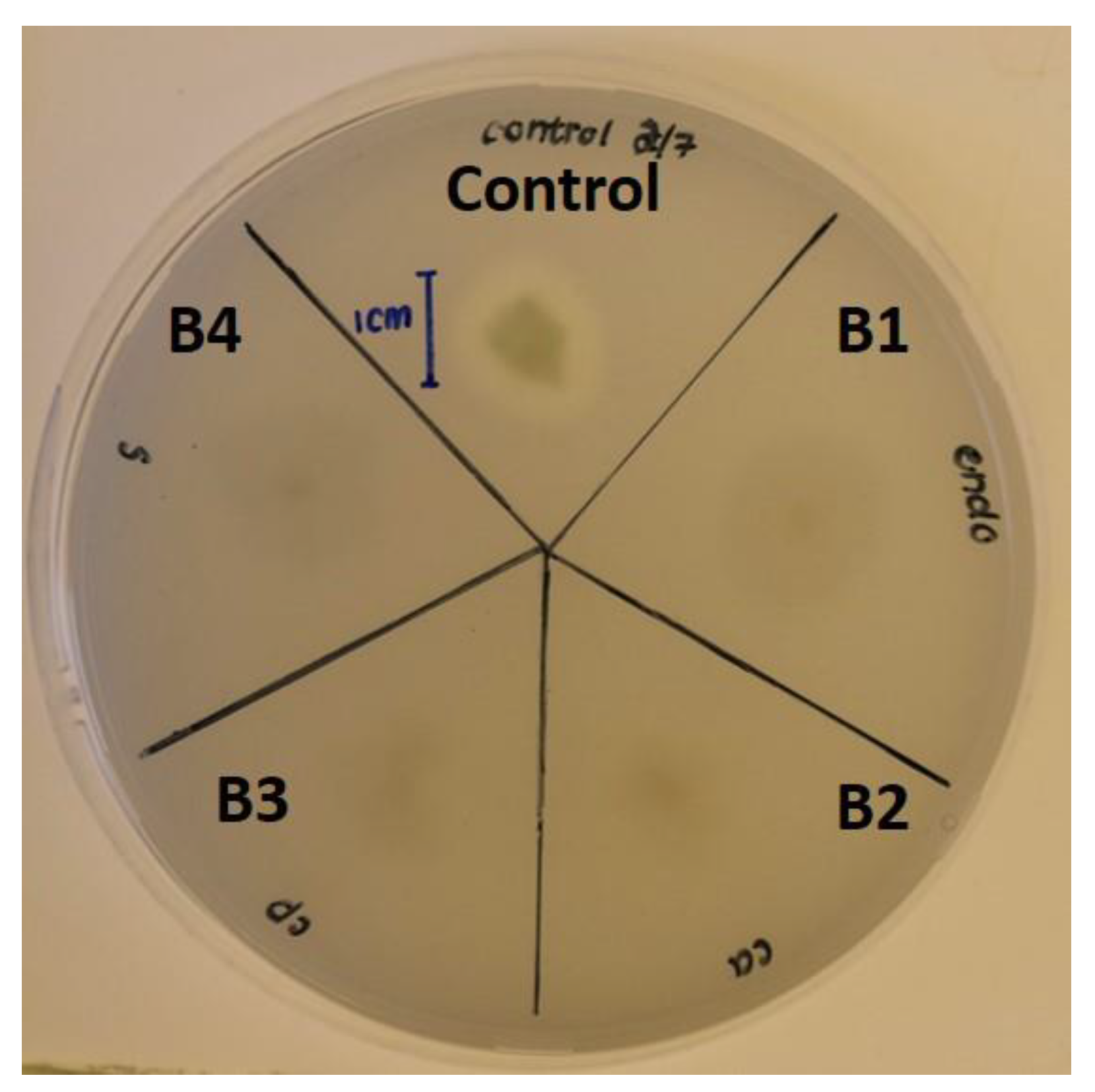
2.8. Siderophore Plate Assay
2.9. Protease Activity Test
2.10. Chitinase Activity Test
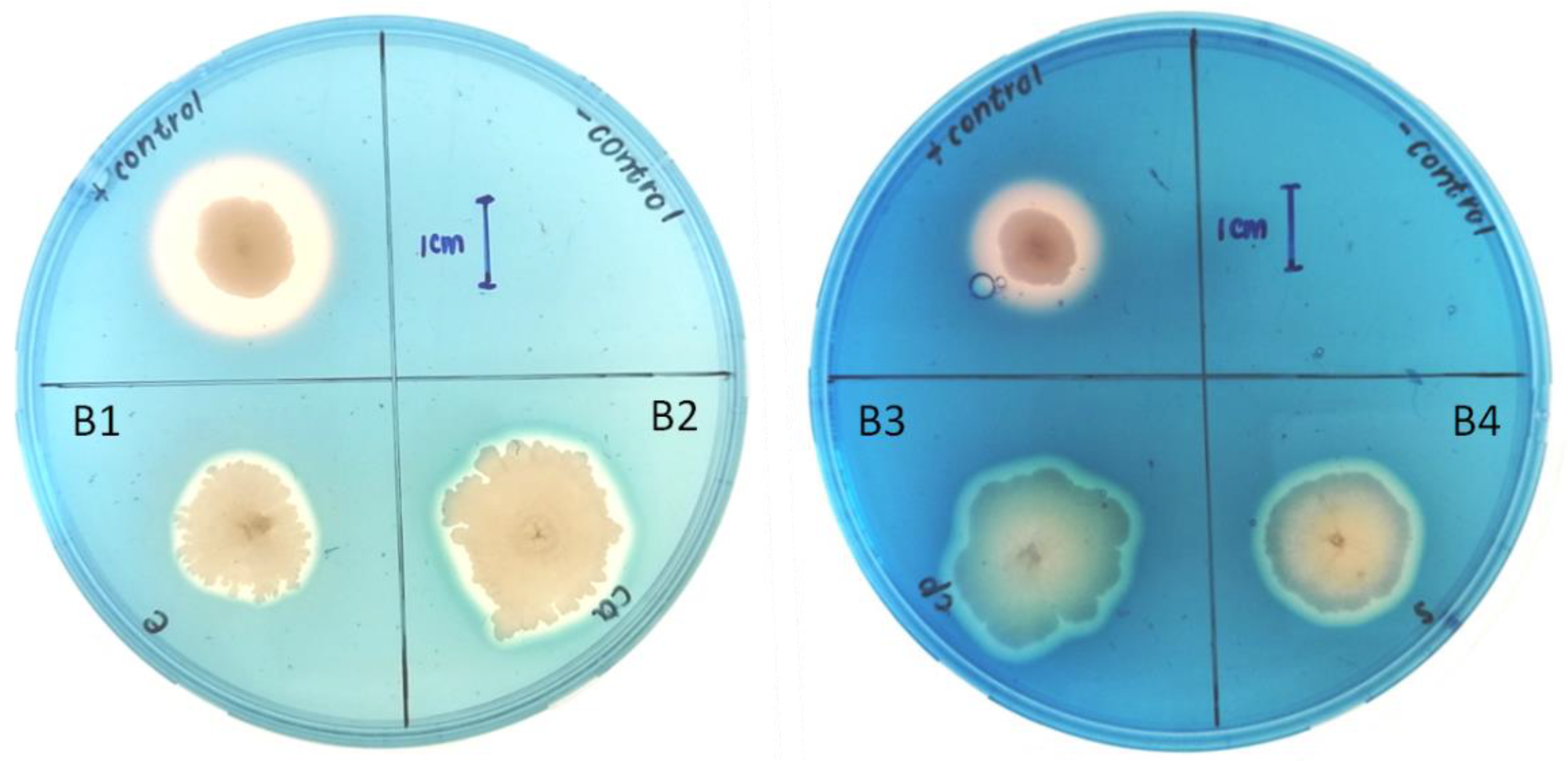
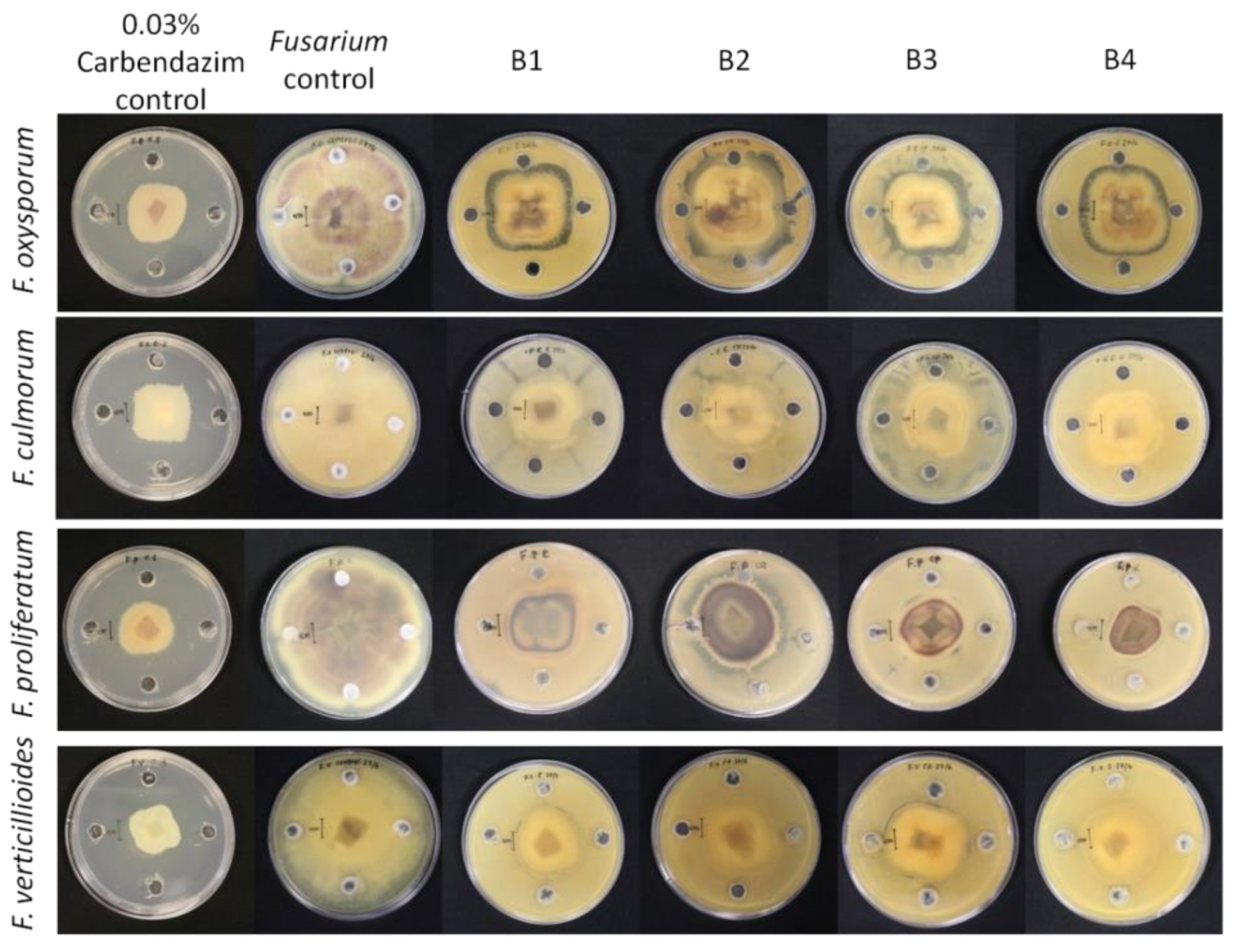
2.11. Antifungal Activity of Bacterial Isolates
2.12. Statistical Analysis
3. Results
3.1. PCR Amplification of the Bacterial Isolates
3.2. Molecular Identification and Phylogenetic Analysis of Bacterial Isolates
3.3. Biochemical Characterization of Bacterial Isolates
3.4. Biocontrol Activity of the Bacterial Isolates against Fusarium Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Latz, M.A.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Beukes, I.; Rose, L.J.; Shephard, G.S.; Flett, B.C.; Viljoen, A. Mycotoxigenic Fusarium species associated with grain crops in South Africa-A review. South Afr. J. Sci. 2017, 113, 1–12. [Google Scholar] [CrossRef]
- Ohike, T.; Makuni, K.; Okanami, M.; Ano, T. Screening of endophytic bacteria against fungal plant pathogens. J. Environ. Sci. 2013, 25, S122–S126. [Google Scholar] [CrossRef]
- Dinolfo, M.I.; Castañares, E.; Stenglein, S.A. Fusarium-plant interaction: State of the art-a review. Plant Protect. Sci. 2017, 53, 61–70. [Google Scholar] [CrossRef]
- Hogg, A.; Johnston, R.; Johnston, J.; Klouser, L.; Kephart, K.; Dyer, A. Monitoring Fusarium crown rot populations in spring wheat residues using quantitative real-time polymerase chain reaction. Phytopathology 2010, 100, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Cumagun, C.J.R.; Ramos, J.S.; Dimaano, A.O.; Munaut, F.; Van Hove, F. Genetic characteristics of Fusarium verticillioides from corn in the Philippines. J. Gen. Plant Pathol. 2009, 75, 405–412. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Esawi, M.A.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- PalmEro, D.; De Cara, M.; Nosir, W.; GálvEz, L.; Cruz, A.; WooDWarD, S.; GoNzálEz-JaéN, M.T.; TEllo, J.C. Fusarium proliferatum isolated from garlic in Spain: Identification, toxigenic potential and pathogenicity on related Allium species. Phytopathol. Mediterr. 2012, 51, 207–218. [Google Scholar]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases–review and future prospects. Biol. Control. 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Alberts, J.F.; Van Zyl, W.H.; Gelderblom, W.C. Biologically based methods for control of fumonisin-producing Fusarium species and reduction of the fumonisins. Front. Microbiol. 2016, 7, 548. [Google Scholar] [CrossRef]
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, N. Environmental risks of fungicides used in horticultural production systems. Fungicides 2010, 1, 273–304. [Google Scholar]
- Arjumend, T.; Sarıhan, E.O.; Yıldırım, M.U. Plant-Bacterial Symbiosis: An Ecologically Sustainable Agriculture Production Alternative to Chemical Fertilizers. In Revisiting Plant Biostimulants; IntechOpen: London, UK, 2022. [Google Scholar]
- Lin, T.; Zhao, L.; Yang, Y.; Guan, Q.; Gong, M. Potential of endophytic bacteria isolated from ‘Sophora alopecuroides’ nodule inbiological control against Verticillium wilt disease. Aust. J. Crop Sci. 2013, 7, 139–146. [Google Scholar]
- Mousa, W.K.; Shearer, C.R.; Limay-Rios, V.; Zhou, T.; Raizada, M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 2015, 6, 805. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Goyal, S. Co-evolution of secondary metabolites during biological competition for survival and advantage: An overview. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–17. [Google Scholar]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, fiw114. [Google Scholar] [CrossRef]
- Fravel, D.R.; Connick, W.J.; Lewis, J.A. Formulation of microorganisms to control plant diseases. In Formulation of Microbial Biopesticides; Burges, H.D., Ed.; Springer: Dordrecht, Netherlands, 1998; pp. 187–202. [Google Scholar]
- Khan, M.R. Beneficial bacteria for biological control of fungal pathogens of cereals. In Bacteria in Agrobiology: Disease Management; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–165. [Google Scholar]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Stecher, G.; Li, M.; Knyaz, C.; Kumar, K. MEGA X: Molecular Evolutionary Genetics Analysis (MEGA) across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Moyes, R.B.; Reynolds, J.; Breakwell, D.P. Differential staining of bacteria: Gram stain. Curr. Protoc. Microbiol. 2009, 15, A.3C.1–A.3C.8. [Google Scholar] [CrossRef] [PubMed]
- Reiner, K. Catalase test protocol. Am. Soc. Microbiol. 2010, 1–6. Available online: https://asm.org/getattachment/72a871fc-ba92-4128-a194-6f1bab5c3ab7/Catalase (accessed on 20 November 2022).
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef]
- Rao, W.S.; Sinha, M. Phosphate dissolving organisms in the soil and rhizosphere. Indian J. Agric. Sci. 1963, 33, 272–278. [Google Scholar]
- Alexander, D.; Zuberer, D. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Etminani, F.; Harighi, B. Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol. J. 2018, 34, 208. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.; Fazeli, M.; Yazdi, M.T.; Adrangi, S.; Al-Ahmadi, K.J.; Tasharrofi, N.; Mohseni, F.A. Optimization of cultural conditions for production of chitinase by a soil isolate of Massilia timonae. Biotechnology 2009, 8, 93–99. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef]
- Ma, Z.; Bielenberg, D.; Brown, K.; Lynch, J. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Acuña, J.J.; Jorquera, M.A.; Martínez, O.A.; Menezes-Blackburn, D.; Fernández, M.T.; Marschner, P.; Greiner, R.; Mora, M. Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J. Soil Sci. Plant Nutr. 2011, 11, 1–12. [Google Scholar]
- Almoneafy, A.A.; Xie, G.; Tian, W.; Xu, L.; Zhang, G.; Ibrahim, M. Characterization and evaluation of Bacillus isolates for their potential plant growth and biocontrol activities against tomato bacterial wilt. Afr. J. Biotechnol. 2012, 11, 7193–7201. [Google Scholar]
- Aramsirirujiwet, Y.; Gumlangmak, C.; Kitpreechavanich, V. Studies on antagonistic effect against plant pathogenic fungi from endophytic fungi isolated from Hottuynia cordata Thunb and screening for Siderophore and indole-3-acetic acid production. Asia-Pac. J. Sci. Technol. 2016, 21, 55–66. [Google Scholar]
- Ramyabharathi, S.; Raguchander, T. Mode of action of Bacillus subtilis EPCO16 against tomato Fusarium wilt. Biochem. Cell. Arch. 2014, 14, 47–50. [Google Scholar]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Isolation and Characterization of Bacillus sp. with In-vitro Antagonistic Activity against Fusarium oxysporum from Rhizosphere of Tomato. J. Agric. Sci. Technol. 2018, 15, 1501–1512. [Google Scholar]
- Rendón-Ramírez, A.; Shukla, M.; Oda, M.; Chakraborty, S.; Minda, R.; Dandekar, A.M.; Ásgeirsson, B.; Goñi, F.M.; Rao, B.J. A computational module assembled from different protease family motifs identifies PI PLC from Bacillus cereus as a putative prolyl peptidase with a serine protease scaffold. PLoS ONE 2013, 8, e70923. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Lugtenberg, B.J.J. Biotechnological applications of bacterial endophytes. Curr. Biotechnol. 2014, 3, 60–75. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Bacon, C.W.; Yates, I.E.; Hinton, D.M.; Meredith, F. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 2001, 109, 325–332. [Google Scholar]

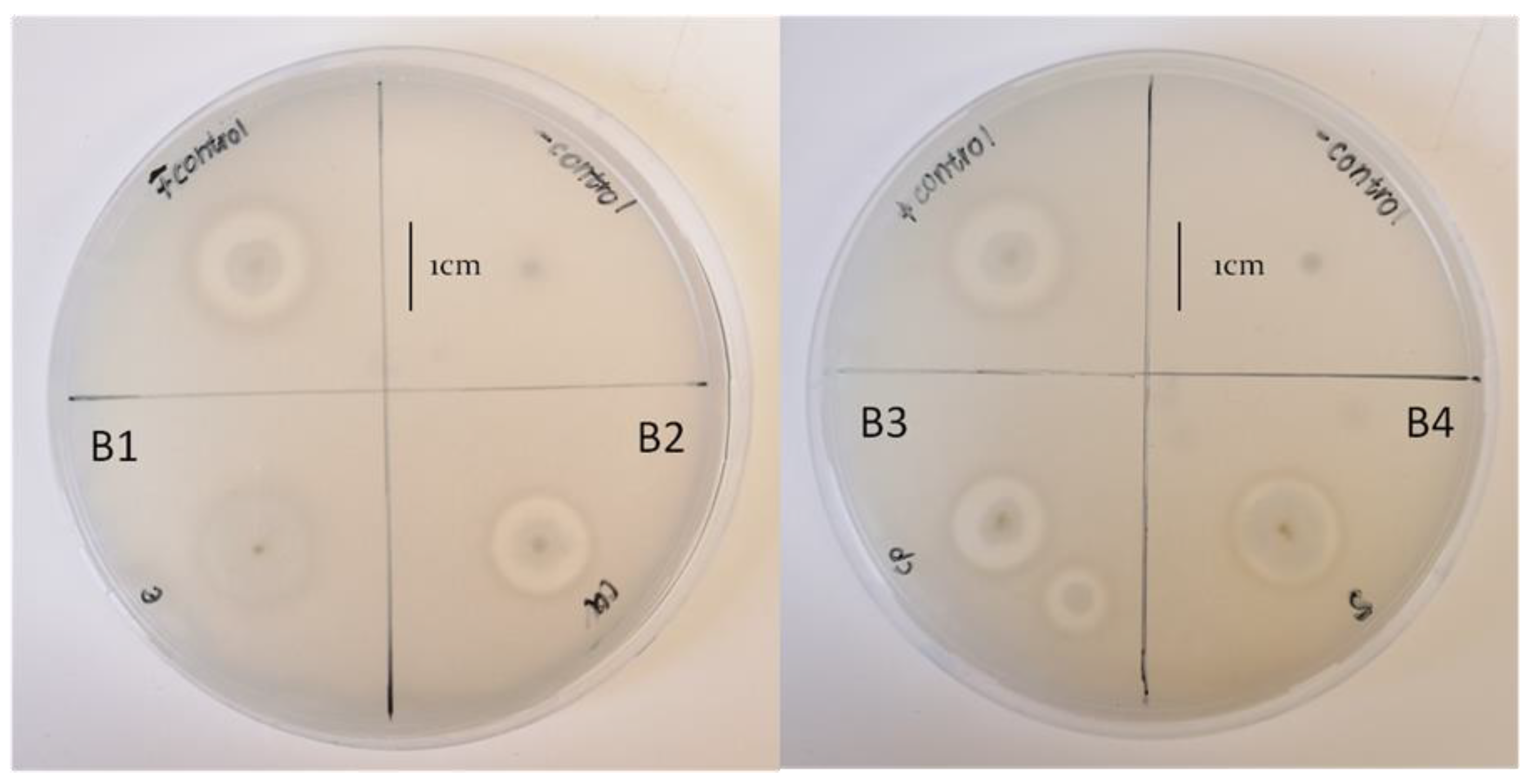

| Bacterial Isolates | Host | Tissue | Top BLAST Hit | Accession Number | Sequence Identity (%) |
|---|---|---|---|---|---|
| B1 | Glycine max L. | leaves | Bacillus subtilis strain S-8 | MT588731.1 | 98.35 |
| B2 | Brassica napus L. | seeds | Bacillus tequilensis strain 127 | MW228161.1 | 98.69 |
| B3 | Vigna unguiculata | seeds | Bacillus tequilensis strain km24 | JF411247.1 | 97.84 |
| B4 | Glycine max L. | seeds | Bacillus tequilensis strain A37 | OP435764.1 | 98.50 |
| Bacterial Isolates | Catalase Activity | Gram Staining | Shape/Type | Phosphate Solubilization | Siderophores Activity | Protease Activity | Chitinase Activity |
|---|---|---|---|---|---|---|---|
| Control | + | + | Rod | 20.22 ± 0.51 | 21.20 ± 0.10 b | 15.30 ± 0.75 b | − |
| B1 | + | + | Rod | − | 16.50 ± 0.25 a | 15.50 ± 0.43 b | − |
| B2 | + | + | Rod | − | 21.20 ± 1.27 b | 12.30 ± 0.81 a | 5.10 ± 0.42 a |
| B3 | + | + | Rod | − | 20.90 ± 0.93 b | 11.60 ± 0.42 a | 6.13 ± 0.71 a |
| B4 | + | + | Rod | − | 18.00 ± 0.97 ab | 12.80 ± 0.30 a | − |
| Fungal Species | B1 | B2 | B3 | B4 | Carbendazim |
|---|---|---|---|---|---|
| F. oxysporum | 40.64 ± 0.38 b | 35.54 ± 0.18 a | 44.36 ± 0.48 b | 39.48 ± 0.46 ab | 59.66 ± 0.40 c |
| F. culmorum | 54.06 ± 0.05 ab | 55.65 ± 0.38 ab | 51.94 ± 0.52 a | 50.49 ± 0.16 a | 56.45 ± 0.73 b |
| F. proliferatum | 58.74 ± 0.09 b | 45.67 ± 0.99 b | 60.75 ± 0.21 b | 64.79 ± 0.40 b | 60.89 ± 0.34 b |
| F. verticillioides | 57.49 ± 0.23 c | 51.73 ± 0.26 b | 43.29 ± 0.23 a | 53.39 ± 0.02 c | 64.68 ± 0.13 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baard, V.; Bakare, O.O.; Daniel, A.I.; Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species. Pathogens 2023, 12, 254. https://doi.org/10.3390/pathogens12020254
Baard V, Bakare OO, Daniel AI, Nkomo M, Gokul A, Keyster M, Klein A. Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species. Pathogens. 2023; 12(2):254. https://doi.org/10.3390/pathogens12020254
Chicago/Turabian StyleBaard, Vejonepher, Olalekan Olanrewaju Bakare, Augustine Innalegwu Daniel, Mbukeni Nkomo, Arun Gokul, Marshall Keyster, and Ashwil Klein. 2023. "Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species" Pathogens 12, no. 2: 254. https://doi.org/10.3390/pathogens12020254
APA StyleBaard, V., Bakare, O. O., Daniel, A. I., Nkomo, M., Gokul, A., Keyster, M., & Klein, A. (2023). Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species. Pathogens, 12(2), 254. https://doi.org/10.3390/pathogens12020254








