Immunotherapy Using Immunogenic Mimotopes Selected by Phage Display plus Amphotericin B Inducing a Therapeutic Response in Mice Infected with Leishmania amazonensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Parasites
2.3. Amplification of the Phage Clones
2.4. Preparation of the Parasites and Infection
2.5. Immunotherapeutics Schedules
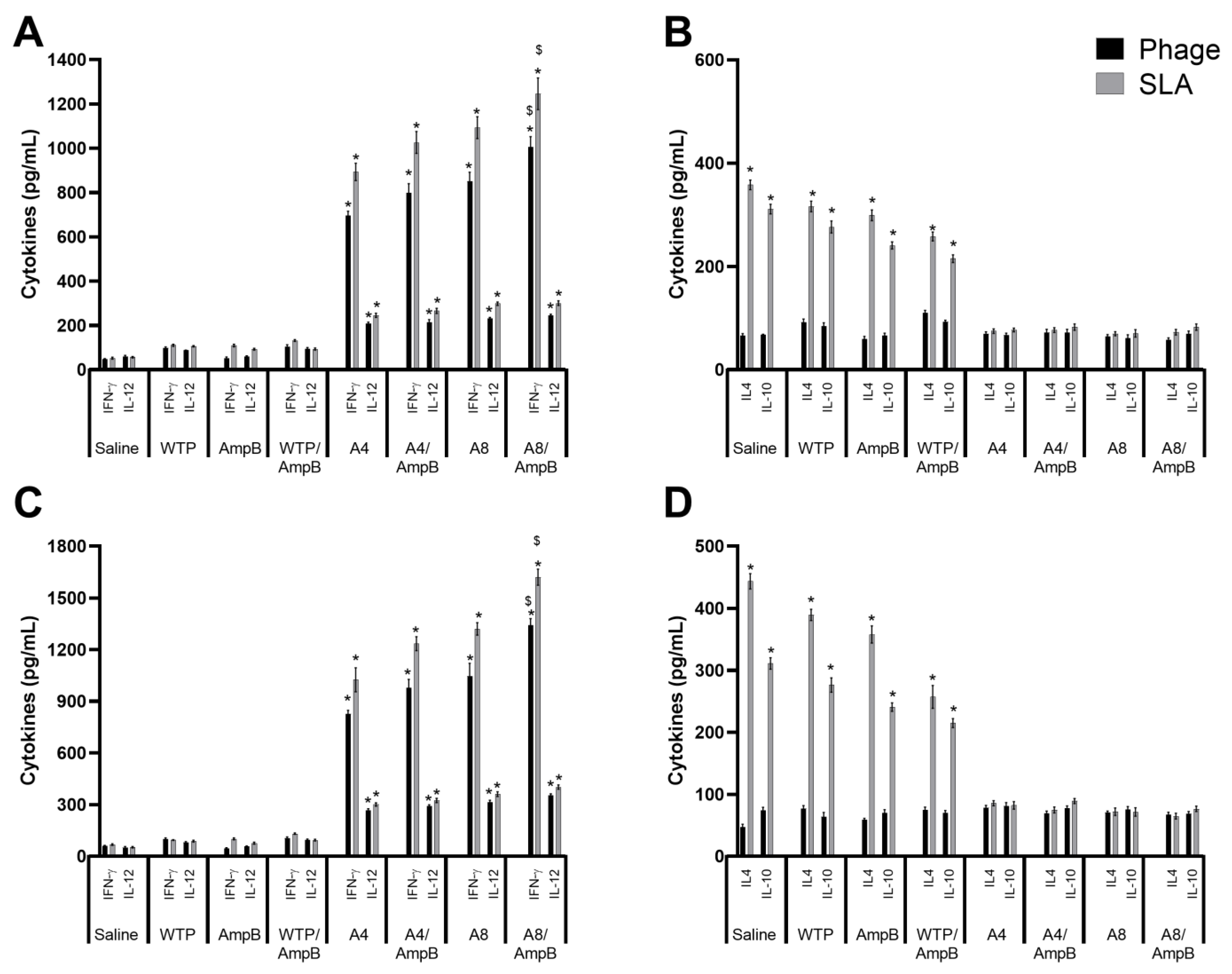
2.6. Evaluation of Cell Response
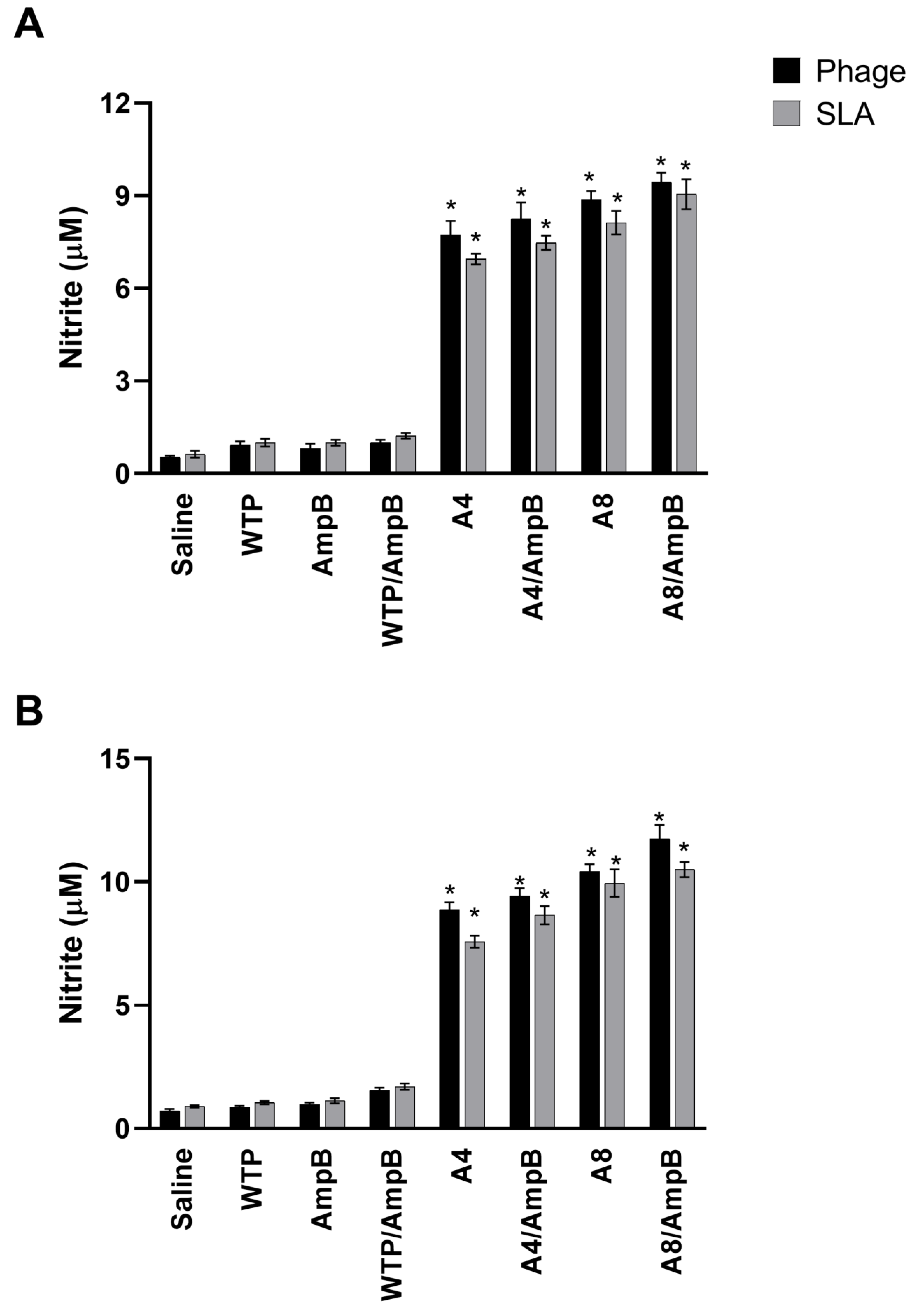
2.6.1. Capture ELISA and Nitrite Production
2.6.2. Polyfunctional T-cell Analysis by Flow Cytometry
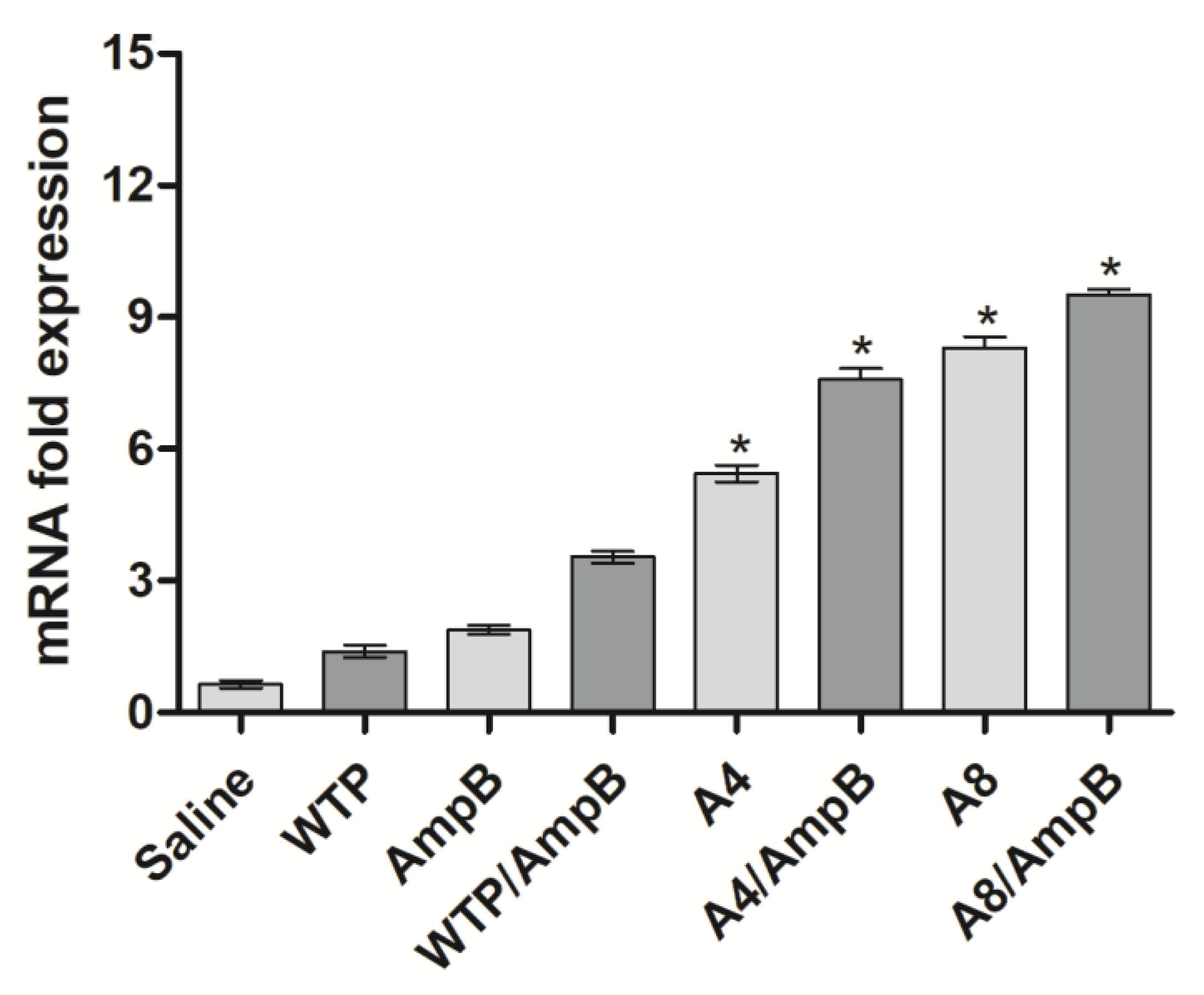
2.6.3. RNA Extraction and Real-Time-qPCR (RT-qPCR)
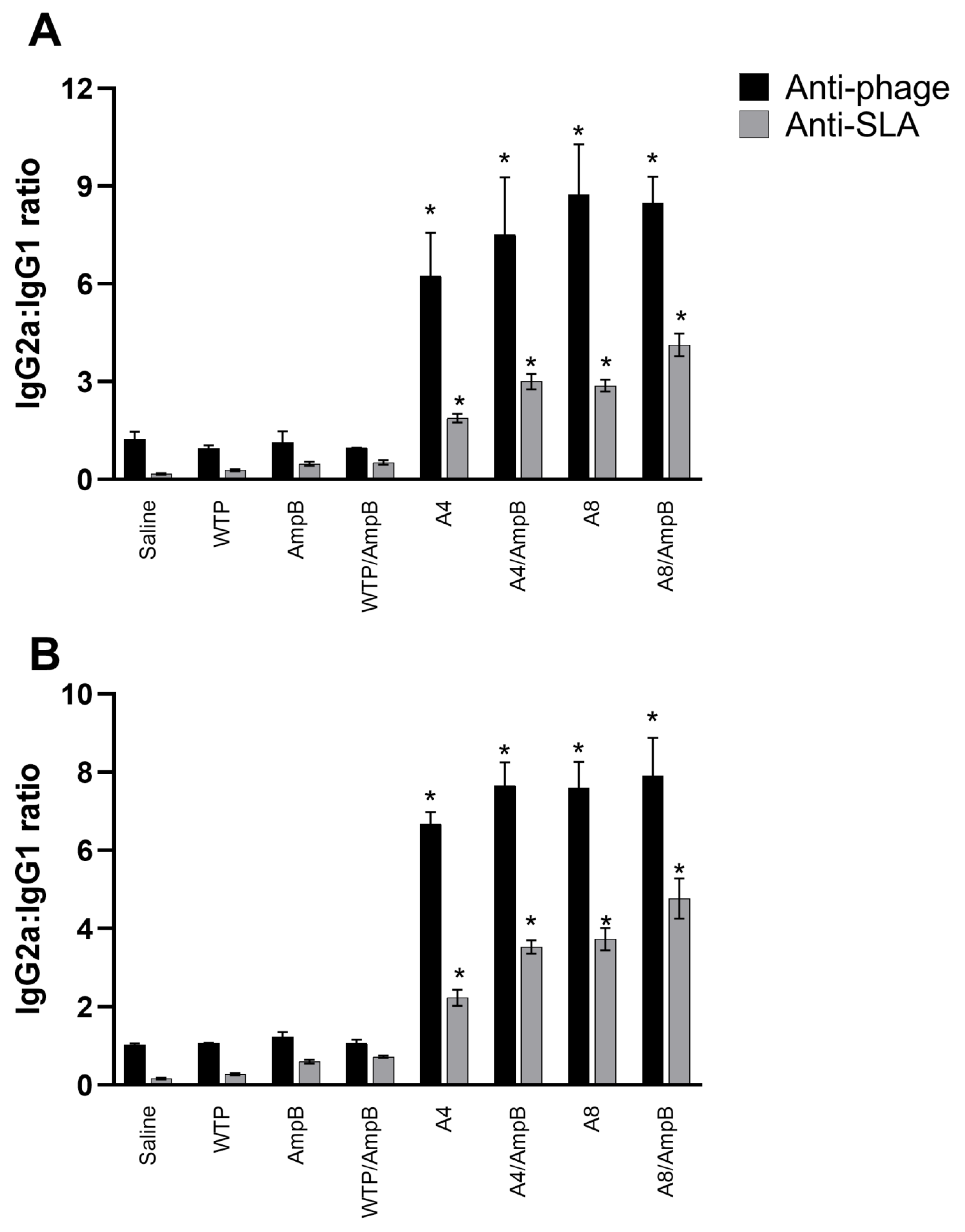
2.7. Humoral Response
2.8. In Vivo Toxicity
2.9. Evaluation of Parasite Burden
2.10. Statistical Analysis
3. Results
3.1. Cell Response Generated after the Immunotherapeutics Procedures
3.2. Humoral Response Developed after the Immunotherapeutics
3.3. In Vivo Toxicity Evaluated in the Treated and Infected Mice
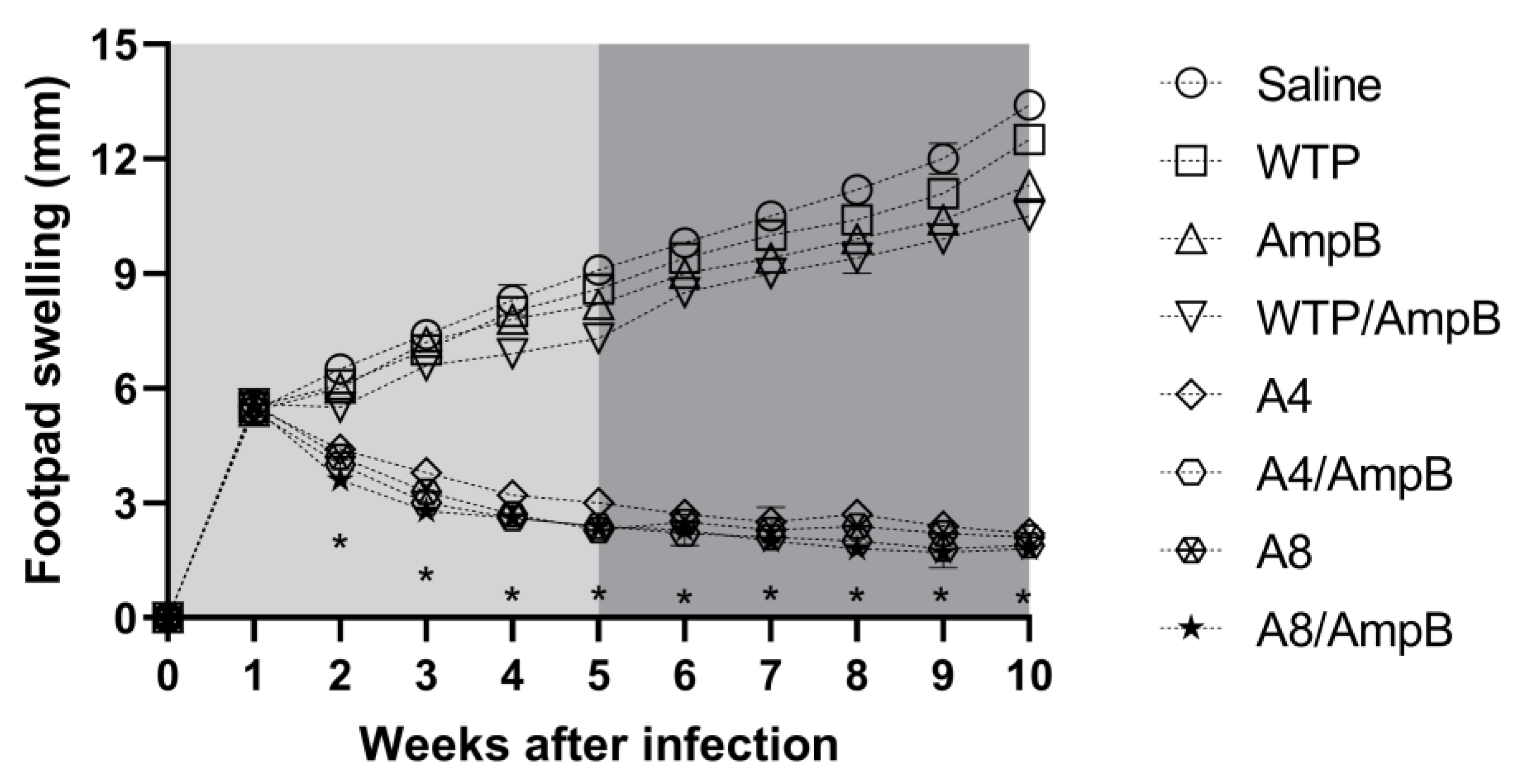
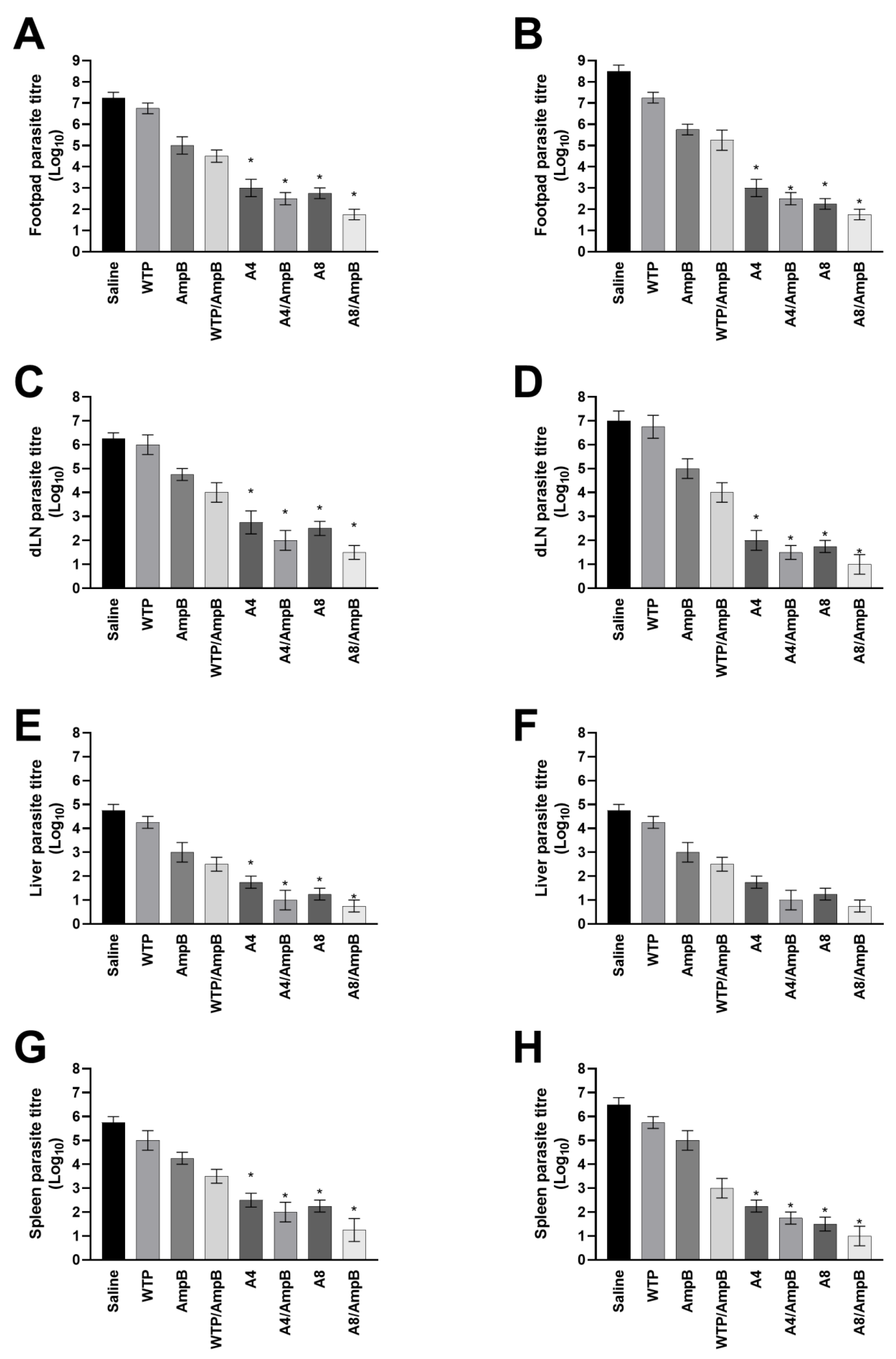
3.4. Evolution of L. amazonensis Infection in the Treated Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 26, 750. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G., Jr.; Tesh, R.B. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef]
- Barral, A.; Badaro, R.; Carvalho, E.M.; Barral-Netto, M.; De Jesus, A.R.; Momen, H.; Almeida, R.; McMahon-Pratt, D.; Pedral-Sampaio, D. Leishmaniasis in Bahia, Brazil: Evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am. J. Trop. Med. Hyg. 1991, 44, 536–546. [Google Scholar] [CrossRef]
- Abreu-Silva, A.L.; Calabrese, K.S.; Cupolilo, S.M.N.; Cardoso, F.O.; Souza, C.S.F.; Da Costa, S.G. Histopathological studies of visceralized Leishmania (Leishmania) amazonensis in mice experimentally infected. Vet. Parasitol. 2004, 121, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Porto, V.B.G.; Carvalho, L.B.; Buzo, B.F.; Litvoc, M.N.; Santos, A.C.S.; Rocci, R.A.; Soares, S.R.C.; Zampieri, R.A.; Duarte, M.I.S.; Lindoso, J.A.L. Visceral leishmaniasis caused by Leishmania (Leishmania) amazonensis associated with Hodgkin’s lymphoma. Rev. Do Inst. De Med. Trop. De São Paulo 2022, 64, e51. [Google Scholar] [CrossRef]
- Lindoso, J.A.L.; Costa, J.M.L.; Queiroz, I.T.; Goto, H. Review of the current treatments for leishmaniases. Res. Rep. Trop. Med. 2012, 3, 69–77. [Google Scholar] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLOS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Roatt, B.M.; Cardoso, J.M.O.; Brito, R.C.F.; Coura-Vital, W.; Aguiar-Soares, R.D.O.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Pradhan, S.; Schwartz, R.A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for leishmaniasis. Clin. Exp. Dermatol. 2022, 47, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.N.; Borja-Cabrera, G.P.; Miyashiro, L.M.; Grechi, J.; Reis, A.B.; Moreira, M.A.; Martins-Filho, O.A.; Luvizotto, M.C.; Menz, I.; Pessôa, L.M.; et al. Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune vaccine. Vaccine 2007, 14, 6176–6190. [Google Scholar] [CrossRef] [PubMed]
- Borja-Cabrera, G.P.; Santos, F.N.; Santos, F.B.; Trivellato, F.A.; Kawasaki, J.K.; Costa, A.C.; Castro, T.; Nogueira, F.S.; Moreira, M.A.; Luvizotto, M.C.; et al. Immunotherapy with the saponin enriched-Leishmune vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine 2010, 3, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; Aguiar-Soares, R.D.; Reis, L.E.; Cardoso, J.M.; Mathias, F.A.; de Brito, R.C.; da Silva, S.M.; Gontijo, N.F.; Ferreira, S.A.; Valenzuela, J.G.; et al. A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden. Front. Immunol. 2017, 8, 217. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Passos, S.; Schriefer, A.; Carvalho, E.M. Protective and pathologic immune responses in human tegumentary leishmaniasis. Front. Immunol. 2012, 3, 301. [Google Scholar] [CrossRef]
- Valentim, D.L.; Paschoale, O.T.; Rodrigues, G.M.; Mathias, F.A.; Cardoso, J.M.; Roatt, B.M.; Aguiar-Soares, R.D.; Conceição, R.J.; de Melo, R.D.; de Brito, R.C.; et al. A specific Leishmania infantum polyepitope vaccine triggers Th1-type immune response and protects against experimental visceral leishmaniasis. Cell Immunol. 2022, 380, 104592. [Google Scholar]
- Iniesta, V.; Gómez-Nieto, L.C.; Corraliza, I. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J. Exp. Med. 2001, 193, 777–784. [Google Scholar] [CrossRef]
- Reis, L.C.; Brito, M.E.; Souza, M.A.; Medeiros, A.C.; Silva, C.J.; Luna, C.F.; Pereira, V.R. Cellular immune response profile in patients with American tegumentary leishmaniasis prior and post chemotherapy treatment. J. Clin. Lab. Anal. 2009, 23, 63–69. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Costa, L.E.; Lage, D.P.; Ramos, F.F.; Santos, T.T.O.; Ribeiro, P.A.F.; Dias, D.S.; Salles, B.C.S.; Lima, M.P.; Carvalho, L.M.; et al. High-through identification of T cell-specific phage-exposed mimotopes using PBMCs from tegumentary leishmaniasis patients and their use as vaccine candidates against Leishmania amazonensis infection. Parasitology 2019, 146, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.A.F.; Tavares, C.A.; Carvalho, F.A.; Chaves, K.F.; Teixeira, K.N.; Rodrigues, R.C.; Charest, H.; Matlashewski, G.; Gazzinelli, R.T.; Fernandes, A.P. Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2003, 71, 3988–3994. [Google Scholar] [CrossRef]
- Brito, R.C.F.; Ruiz, J.C.; Cardoso, J.M.O.; Ostolin, T.L.V.D.P.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.O.; Roatt, B.M.; Corrêa-Oliveira, R.; Resende, D.M.; et al. Chimeric vaccines designed by immunoinformatics-activated poly-functional and memory T cells that trigger protection against experimental visceral leishmaniasis. Vaccines B 2020, 8, 252. [Google Scholar] [CrossRef]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef]
- Lage, D.P.; Ribeiro, P.A.F.; Dias, D.S.; Mendonça, D.V.C.; Ramos, F.F.; Carvalho, L.M.; Steiner, B.T.; Tavares, G.S.V.; Martins, V.T.; Machado, A.S.; et al. Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis. Vaccines B 2020, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Sundar, S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: Current status and future prospects. Front. Immunol. 2014, 5, 296. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. Front. Immunol. 2019, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, D.V.C.; Tavares, G.S.V.; Lage, D.P.; Soyer, T.G.; Carvalho, L.M.; Dias, D.S.; Ribeiro, P.A.F.; Ottoni, F.M.; Antinarelli, L.M.R.; Vale, D.L.; et al. In vivo antileishmanial efficacy of a naphthoquinone derivate incorporated into a Pluronic® F127-based polymeric micelle system against Leishmania amazonensis infection. Biomed. Pharmacother. 2019, 109, 779–787. [Google Scholar] [CrossRef]
- Freitas, C.S.; Oliveira-da-Silva, J.A.; Lage, D.P.; Costa, R.R.; Mendonça, D.V.C.; Martins, V.T.; Reis, T.A.R.; Antinarelli, L.M.R.; Machado, A.S.; Tavares, G.S.V.; et al. Digitoxigenin presents an effective and selective antileishmanial action against Leishmania infantum and is a potential therapeutic agent for visceral leishmaniasis. Parasitol. Res. 2021, 120, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.E.; Chávez-Fumagalli, M.A.; Martins, V.T.; Duarte, M.C.; Lage, D.P.; Lima, M.I.; Pereira, N.C.; Soto, M.; Tavares, C.A.; Goulart, L.R.; et al. Phage-fused epitopes from Leishmania infantum used as immunogenic vaccines confer partial protection against Leishmania amazonensis infection. Parasitology 2015, 42, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.G.; Gamage, L.N.A.; Marciniuk, K.; Hayes, C.; Napper, S.; Hayes, S.; Griebel, P.J. Lambda display phage as a mucosal vaccine delivery vehicle for peptide antigens. Vaccine 2017, 35, 7256–7263. [Google Scholar] [CrossRef]
- Ramos, F.F.; Costa, L.E.; Dias, D.S.; Santos, T.T.O.; Rodrigues, M.R.; Lage, D.P.; Salles, B.C.S.; Martins, V.T.; Ribeiro, P.A.F.; Chávez-Fumagalli, M.A.; et al. Selection strategy of phage-displayed immunogens based on an in vitro evaluation of the Th1 response of PBMCs and their potential use as a vaccine against Leishmania infantum infection. Parasit. Vectors 2017, 10, 617. [Google Scholar] [CrossRef]
- Silva, R.F.; Oliveira, B.C.; Silva, A.A.; Castro, M.C.A.B.; Ferreira, L.F.G.R.; Hernandes, M.Z.; Brito, M.E.F.; Melo-Neto, O.P.; Rezende, A.M.; Pereira, V.R.A. Immunogenicity of Potential CD4+ and CD8+ T Cell Epitopes Derived from the Proteome of Leishmania braziliensis. Front. Immunol. 2020, 10, 3145. [Google Scholar] [CrossRef]
- Coelho, E.A.; Chávez-Fumagalli, M.A.; Costa, L.E.; Tavares, C.A.; Soto, M.; Goulart, L.R. Theranostic applications of phage display to control leishmaniasis: Selection of biomarkers for serodiagnostics, vaccination, and immunotherapy. Rev. da Soc. Bras. de Med. Trop 2015, 48, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, M.; Martin-Orozco, E.; Goodman, J.S.; Nguyen, M.D.; Sato, Y.; Ronaghy, A.; Kornbluth, R.S.; Richman, D.D.; Carson, D.A.; Raz, E. Immunostimulatory DNA sequences function as Th1 promoting adjuvants. Nat. Med. 1997, 3, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Gomes, A.C.; Cabral-Miranda, G.; Krueger, C.C.; Leoratti, F.M.; Stein, J.V.; Bachmann, M.F. Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J. Control. Release 2017, 251, 92–100. [Google Scholar] [CrossRef]
- Chávez-Fumagalli, M.A.; Ribeiro, T.G.; Castilho, R.O.; Fernandes, S.O.; Cardoso, V.N.; Coelho, C.S.; Mendonça, D.V.; Soto, M.; Tavares, C.A.; Faraco, A.A.; et al. New delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Rev. Soc. Bras. Med. Trop. 2015, 48, 235–242. [Google Scholar] [CrossRef]
- Balasegaram, M.; Ritmeijer, K.; Lima, M.A.; Burza, S.; Ortiz-Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin. Emerg. Drugs 2012, 17, 493–510. [Google Scholar] [CrossRef]
- Harper, D.R. Criteria for Selecting Suitable Infectious Diseases for Phage Therapy. Viruses 2018, 10, 177. [Google Scholar] [CrossRef]
- Murray, H.W.; Brooks, E.B.; DeVecchio, J.L.; Heinzel, F.P. Immunoenhancement combined with amphotericin B as treatment for experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 2003, 8, 2513–2517. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Ferreira, F.C.; Gusmão, M.R.; Costa, A.F.P.; de Brito, R.C.F.; Aguiar-Soares, R.D.O.; Reis, A.B.; Cardoso, J.M.O.; Carneiro, C.M.; Roatt, B.M. Heterologous vaccine therapy associated with half course of Miltefosine promote activation of the proinflammatory response with control of splenic parasitism in a hamster model of visceral leishmaniasis. Curr. Res. Immunol. 2021, 2, 194–201. [Google Scholar] [CrossRef]
- Iniesta, V.; Gómez-Nieto, L.C.; Molano, I.; Mohedano, A.; Carcelén, J.; Mirón, C.; Alonso, C.; Corraliza, I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002, 24, 113–118. [Google Scholar] [CrossRef]
- Vacas, A.; Fernández-Rubio, C.; Larrea, E.; Peña-Guerrero, J.; Nguewa, P.A. LmjF.22.0810 from Leishmania major Modulates the Th2-Type Immune Response and Is Involved in Leishmaniasis Outcome. Biomedicines 2020, 8, 452. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Costa, M.A.; Chavez-Fumagalli, M.A.; Valadares, D.G.; Duarte, M.C.; Costa, L.E.; Martins, V.T.; Gomes, R.F.; Melo, M.N.; Soto, M.; et al. Evaluation of parasitological and immunological parameters of Leishmania chagasi infection in BALB/c mice using different doses and routes of inoculation of parasites. Parasitol. Res. 2012, 110, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Carrión, J.; Nieto, A.; Iborra, S.; Iniesta, V.; Soto, M.; Folgueira, C.; Abanades, D.R.; Requena, J.M.; Alonso, C. Immunohistological features of visceral leishmaniasis in BALB/c mice. Parasite Immunol. 2006, 28, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Domínguez-Bernal, G.; Orden, J.A.; De La Fuente, R.; Madrid-Elena, N.; Carrión, J. Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model. Vet. Res. 2011, 42, 39. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Oryan, A.; Hatam, G. Immunotherapy in treatment of leishmaniasis. Immunol. Lett. 2021, 233, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.; Gusmão, M.R.; Costa, A.F.P.; de Brito, R.C.F.; Aguiar-Soares, R.D.O.; Cardoso, J.M.O.; Reis, A.B.; Carneiro, C.M.; Roatt, B.M. Immunochemotherapy for visceral leishmaniasis: Combinatorial action of Miltefosine plus LBSapMPL vaccine improves adaptative Th1 immune response with control of splenic parasitism in experimental hamster model. Parasitology 2022, 149, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.F.; Miranda, D.F.H.; Moura, L.D.; Pinho, F.A.; Werneck, G.L.; Khouri, R.; Reed, S.G.; Duthie, M.S.; Barral, A.; Barral-Netto, M. Allopurinol therapy provides long term clinical improvement, but additional immunotherapy is required for sustained parasite clearance, in L. infantum-infected dogs. Vaccine X 2020, 4, 100048. [Google Scholar]


| Saline Group | |||||||
|---|---|---|---|---|---|---|---|
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 2.133 | 1.545 | 0.877 | 0.418 | 0.217 | 0.112 | 0.065 |
| IgG2a | 0.443 | 0.244 | 0.133 | 0.069 | 0.035 | 0.017 | 0.010 |
| Wild-type phage (WTP) group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 2.012 | 1.322 | 0.722 | 0.355 | 0.175 | 0.092 | 0.047 |
| IgG2a | 0.765 | 0.398 | 0.212 | 0.099 | 0.047 | 0.022 | 0.010 |
| Amphotericin B (AmpB) group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 1.896 | 1.034 | 0.492 | 0.238 | 0.136 | 0.075 | 0.038 |
| IgG2a | 0.766 | 0.398 | 0.215 | 0.109 | 0.052 | 0.024 | 0.014 |
| WTP/AmpB group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 1.744 | 0.903 | 0.454 | 0.226 | 0.126 | 0.072 | 0.035 |
| IgG2a | 0.776 | 0.387 | 0.223 | 0.111 | 0.048 | 0.026 | 0.014 |
| A4 group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 1.022 | 0.577 | 0.298 | 0.164 | 0.087 | 0.044 | 0.019 |
| IgG2a | 2.033 | 1.166 | 0.612 | 0.303 | 0.166 | 0.094 | 0.052 |
| A4/AmpB group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 0.924 | 0.455 | 0.232 | 0.111 | 0.056 | 0.030 | 0.013 |
| IgG2a | 2.015 | 1.133 | 0.622 | 0.325 | 0.155 | 0.082 | 0.050 |
| A8 group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 0.944 | 0.501 | 0.243 | 0.125 | 0.067 | 0.030 | 0.016 |
| IgG2a | 2.233 | 1.355 | 0.706 | 0.358 | 0.193 | 0.095 | 0.052 |
| A8/AmpB group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 0.733 | 0.378 | 0.201 | 0.096 | 0.055 | 0.031 | 0.020 |
| IgG2a | 2.322 | 1.454 | 0.796 | 0.390 | 0.192 | 0.093 | 0.054 |
| Saline Group | |||||||
|---|---|---|---|---|---|---|---|
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 2.677 | 1.897 | 1.044 | 0.558 | 0.277 | 0.144 | 0.082 |
| IgG2a | 0.709 | 0.395 | 0.201 | 0.094 | 0.053 | 0.023 | 0.017 |
| Wild-type phage (WTP) group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 2.455 | 1.504 | 0.833 | 0.423 | 0.233 | 0.143 | 0.083 |
| IgG2a | 0.702 | 0.387 | 0.221 | 0.117 | 0.056 | 0.031 | 0.014 |
| Amphotericin B (AmpB) group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 2.103 | 1.322 | 0.711 | 0.355 | 0.177 | 0.088 | 0.051 |
| IgG2a | 1.335 | 0.784 | 0.422 | 0.211 | 0.122 | 0.071 | 0.033 |
| WTP/AmpB group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 1.877 | 0.993 | 0.576 | 0.281 | 0.153 | 0.088 | 0.049 |
| IgG2a | 1.277 | 0.698 | 0.388 | 0.202 | 0.093 | 0.047 | 0.024 |
| A4 group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 1.165 | 0.677 | 0.311 | 0.158 | 0.084 | 0.047 | 0.023 |
| IgG2a | 2.233 | 1.324 | 0.733 | 0.347 | 0.176 | 0.094 | 0.050 |
| A4/AmpB group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 0.804 | 0.396 | 0.221 | 0.104 | 0.058 | 0.031 | 0.017 |
| IgG2a | 2.033 | 1.299 | 0.722 | 0.363 | 0.192 | 0.102 | 0.048 |
| A8 group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 0.789 | 0.403 | 0.221 | 0.109 | 0.059 | 0.031 | 0.022 |
| IgG2a | 2.677 | 1.533 | 0.811 | 0.397 | 0.198 | 0.096 | 0.047 |
| A8/AmpB group | |||||||
| Isotype | 1:1000 | 1:2500 | 1:5000 | 1:10,000 | 1:20,000 | 1:40,000 | 1:80,000 |
| IgG1 | 0.504 | 0.289 | 0.157 | 0.088 | 0.051 | 0.032 | 0.015 |
| IgG2a | 2.778 | 1.687 | 0.903 | 0.408 | 0.221 | 0.117 | 0.066 |
| Creatinine | |||||
|---|---|---|---|---|---|
| 01 Day | 30 Days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 0.68 | 0.096 | 0.78 | 0.096 | 0.252 |
| Saline | 2.53 | 0.150 | 2.90 | 0.183 | 0.001 |
| WTP | 1.95 | 0.129 | 2.08 | 0.171 | 0.194 |
| AmpB | 2.48 | 0.330 | 2.65 | 0.129 | 0.457 |
| WTP/AmpB | 2.23 | 0.206 | 2.40 | 0.163 | 0.367 |
| A4 | 1.35 | 0.124 | 1.30 | 0.082 | 0.638 |
| A4/AmpB | 1.65 | 0.238 | 1.63 | 0.171 | 0.903 |
| A8 | 0.95 | 0.208 | 1.08 | 0.250 | 0.555 |
| A8/AmpB | 1.28 | 0.171 | 1.40 | 0.189 | 0.504 |
| Urea | |||||
| 01 Day | 30 Days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 15.00 | 0.816 | 16.50 | 1.291 | 0.245 |
| Saline | 28.25 | 1.708 | 30.75 | 1.689 | 0.194 |
| WTP | 22.50 | 1.291 | 24.00 | 1.633 | 0.297 |
| AmpB | 27.50 | 1.286 | 29.75 | 2.630 | 0.299 |
| WTP/AmpB | 25.75 | 1.698 | 26.25 | 1.711 | 0.752 |
| A4 | 20.75 | 2.217 | 19.00 | 1.826 | 0.293 |
| A4/AmpB | 21.25 | 1.708 | 21.50 | 1.291 | 0.861 |
| A8 | 16.25 | 1.258 | 18.00 | 2.160 | 0.367 |
| A8/AmpB | 18.25 | 1.712 | 18.75 | 1.708 | 0.783 |
| Alanine Transaminase | |||||
| 01 Day | 30 Days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 10.50 | 1.291 | 11.50 | 1.291 | 0.182 |
| Saline | 24.75 | 1.708 | 26.00 | 1.414 | 0.239 |
| WTP | 19.50 | 1.288 | 20.50 | 1.291 | 0.495 |
| AmpB | 23.75 | 1.708 | 24.00 | 2.160 | 0.873 |
| WTP/AmpB | 21.50 | 1.279 | 22.50 | 1.288 | 0.391 |
| A4 | 15.00 | 1.826 | 15.00 | 1.414 | 1.000 |
| A4/AmpB | 17.25 | 1.708 | 17.75 | 1.708 | 0.761 |
| A8 | 12.50 | 1.299 | 13.00 | 2.155 | 0.769 |
| A8/AmpB | 14.50 | 1.274 | 14.25 | 2.217 | 0.873 |
| Aspartate Transaminase | |||||
| 01 Day | 30 Days | ||||
| Groups | Mean | Std. Deviation | Mean | Std. Deviation | Paired t test |
| Naive | 12.25 | 1.708 | 13.50 | 1.289 | 0.239 |
| Saline | 23.50 | 1.271 | 25.50 | 1.291 | 0.219 |
| WTP | 18.50 | 1.280 | 20.75 | 1.708 | 0.117 |
| AmpB | 22.00 | 1.826 | 24.00 | 0.816 | 0.182 |
| WTP/AmpB | 20.50 | 1.299 | 22.25 | 2.217 | 0.354 |
| A4 | 15.75 | 1.708 | 15.75 | 1.714 | 1.000 |
| A4/AmpB | 16.75 | 1.258 | 17.75 | 1.698 | 0.252 |
| A8 | 13.50 | 1.287 | 14.00 | 0.816 | 0.638 |
| A8/AmpB | 14.50 | 1.291 | 16.25 | 1.717 | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyer, T.G.; Ramos, F.F.; Pereira, I.A.G.; Lage, D.P.; Bandeira, R.S.; de Jesus, M.M.; Costa, G.P.; Machado, A.S.; Freitas, C.S.; Vale, D.L.; et al. Immunotherapy Using Immunogenic Mimotopes Selected by Phage Display plus Amphotericin B Inducing a Therapeutic Response in Mice Infected with Leishmania amazonensis. Pathogens 2023, 12, 314. https://doi.org/10.3390/pathogens12020314
Soyer TG, Ramos FF, Pereira IAG, Lage DP, Bandeira RS, de Jesus MM, Costa GP, Machado AS, Freitas CS, Vale DL, et al. Immunotherapy Using Immunogenic Mimotopes Selected by Phage Display plus Amphotericin B Inducing a Therapeutic Response in Mice Infected with Leishmania amazonensis. Pathogens. 2023; 12(2):314. https://doi.org/10.3390/pathogens12020314
Chicago/Turabian StyleSoyer, Tauane G., Fernanda F. Ramos, Isabela A. G. Pereira, Daniela P. Lage, Raquel S. Bandeira, Marcelo M. de Jesus, Guilherme P. Costa, Amanda S. Machado, Camila S. Freitas, Danniele L. Vale, and et al. 2023. "Immunotherapy Using Immunogenic Mimotopes Selected by Phage Display plus Amphotericin B Inducing a Therapeutic Response in Mice Infected with Leishmania amazonensis" Pathogens 12, no. 2: 314. https://doi.org/10.3390/pathogens12020314








