Insights into Synonymous Codon Usage Bias in Hepatitis C Virus and Its Adaptation to Hosts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequence Collection
2.2. Base Composition
2.3. Dinucleotide Odds Ratio
2.4. Nucleotide Skew
2.5. Codon Usage
2.6. Relative Synonymous Codon Usage (RSCU)
2.7. Neutrality Plot Analysis
2.8. The Effective Number of Codons (ENc)
2.9. Codon Adaptation Index (CAI)
2.10. Parity Rule 2 (PR2) Bias Plot
2.11. Codon Pair Context and Preferred Codons
2.12. Protein Properties
2.13. Similarity Index Analysis
2.14. The Relative Codon Deoptimization Index (RCDI) Analysis
2.15. Principal Component Analysis
2.16. Phylogenetic Tree Construction
3. Results
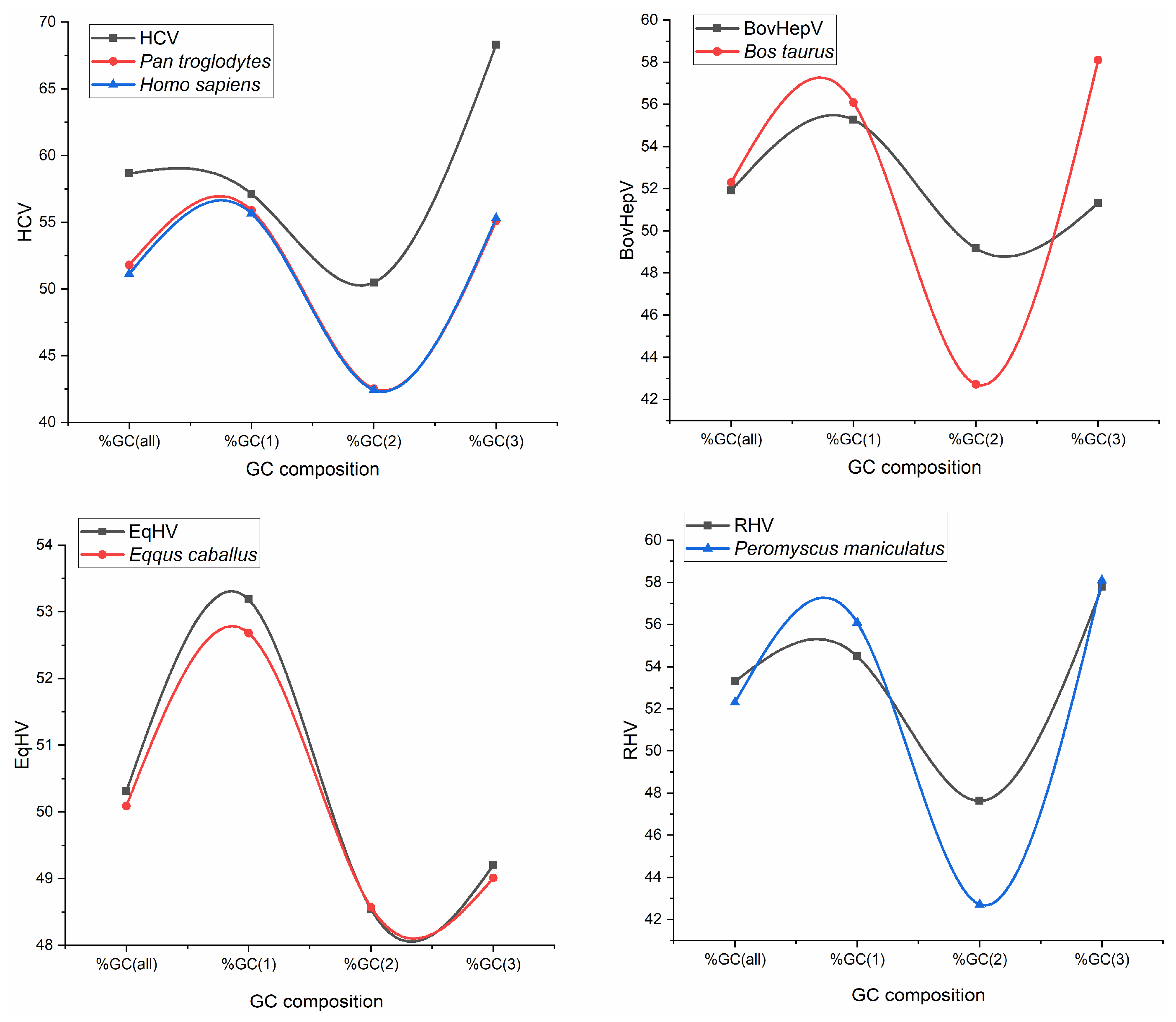
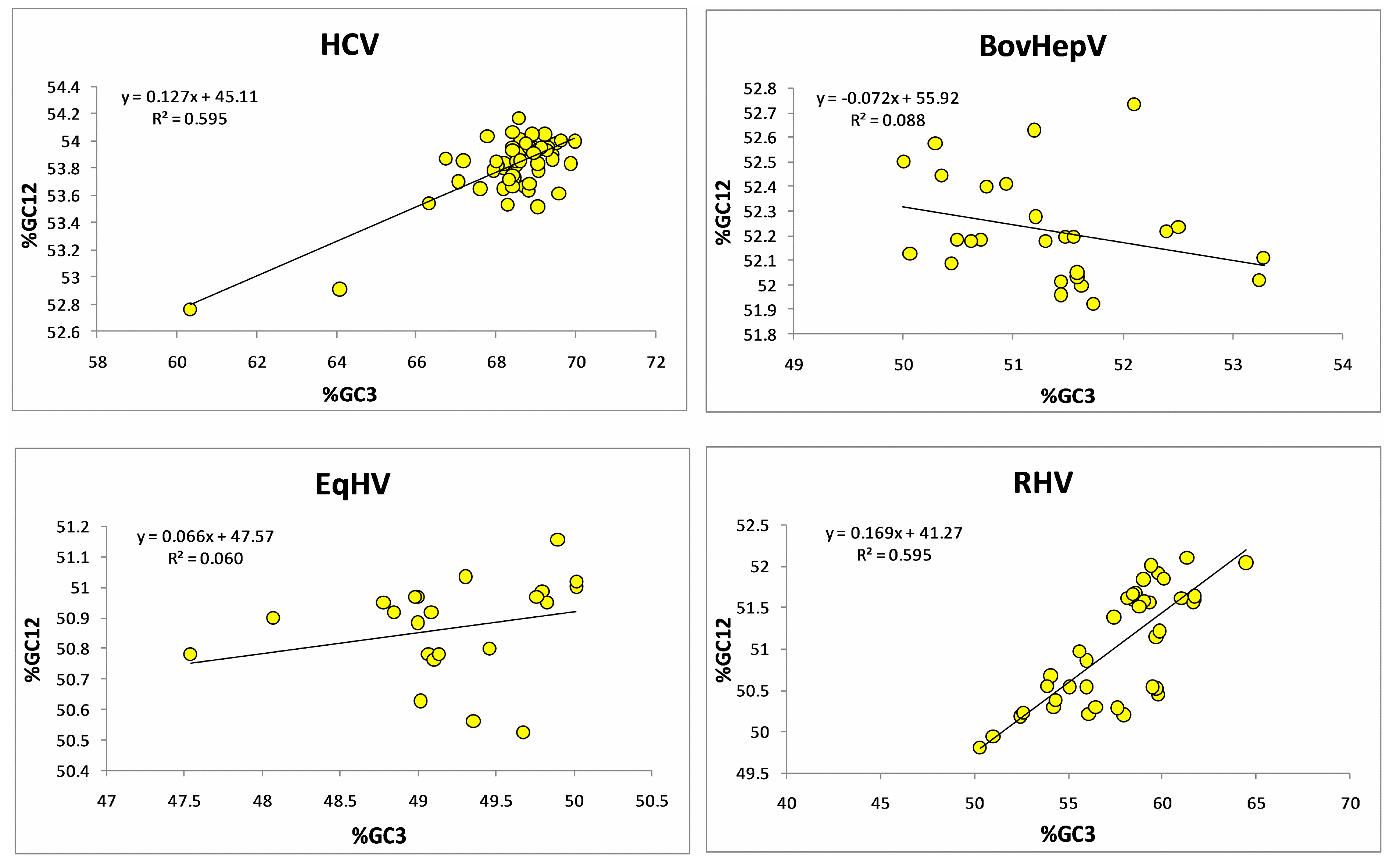
3.1. Compositional Analysis
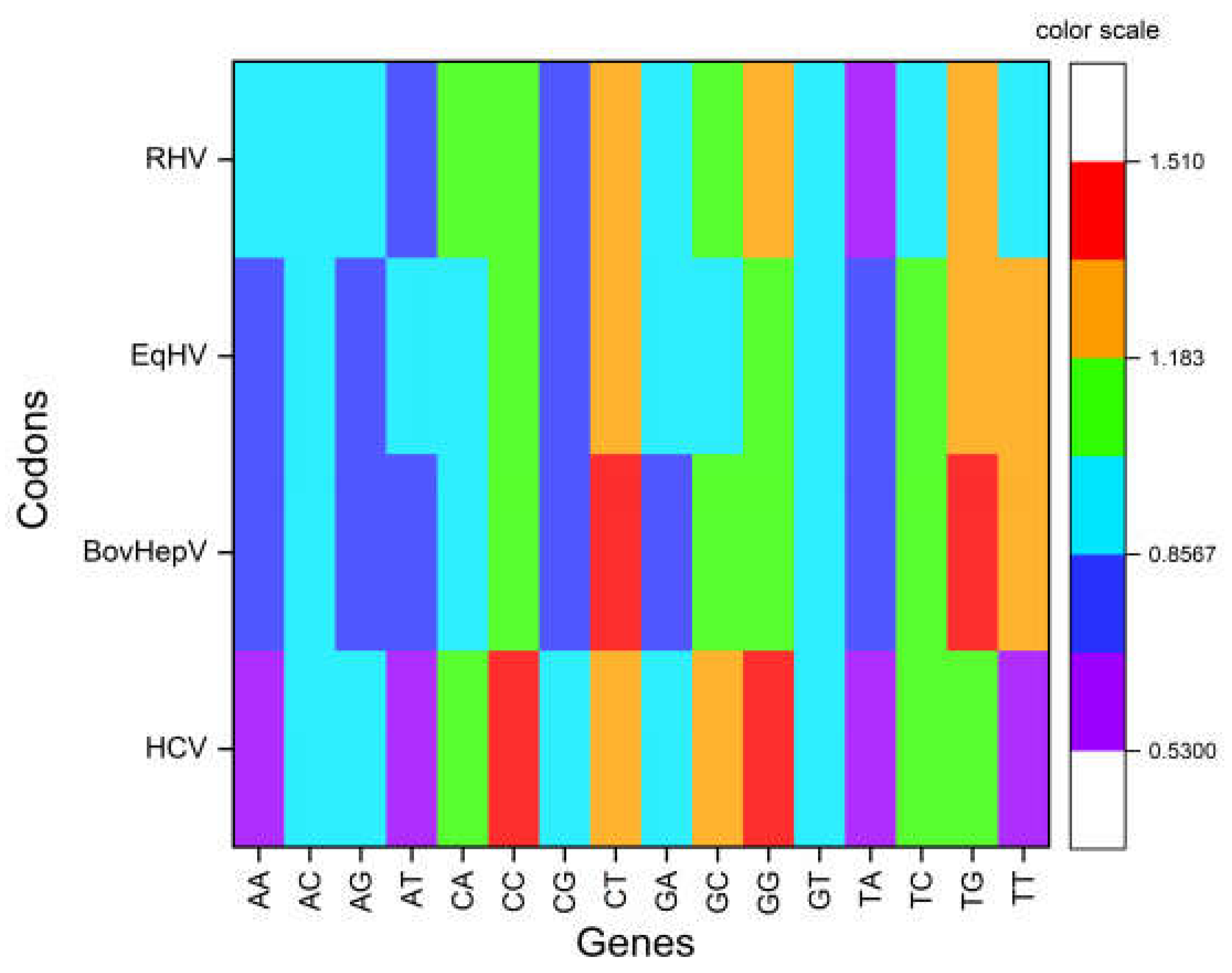
3.2. Odds Ratio Analysis Revealed Overrepresentation of GpG and CpCwhile Underrepresentation of TpA, ApA, TpT, and ApT
3.3. Selection Force Is Dominant Force in the Shaping Codon Usage
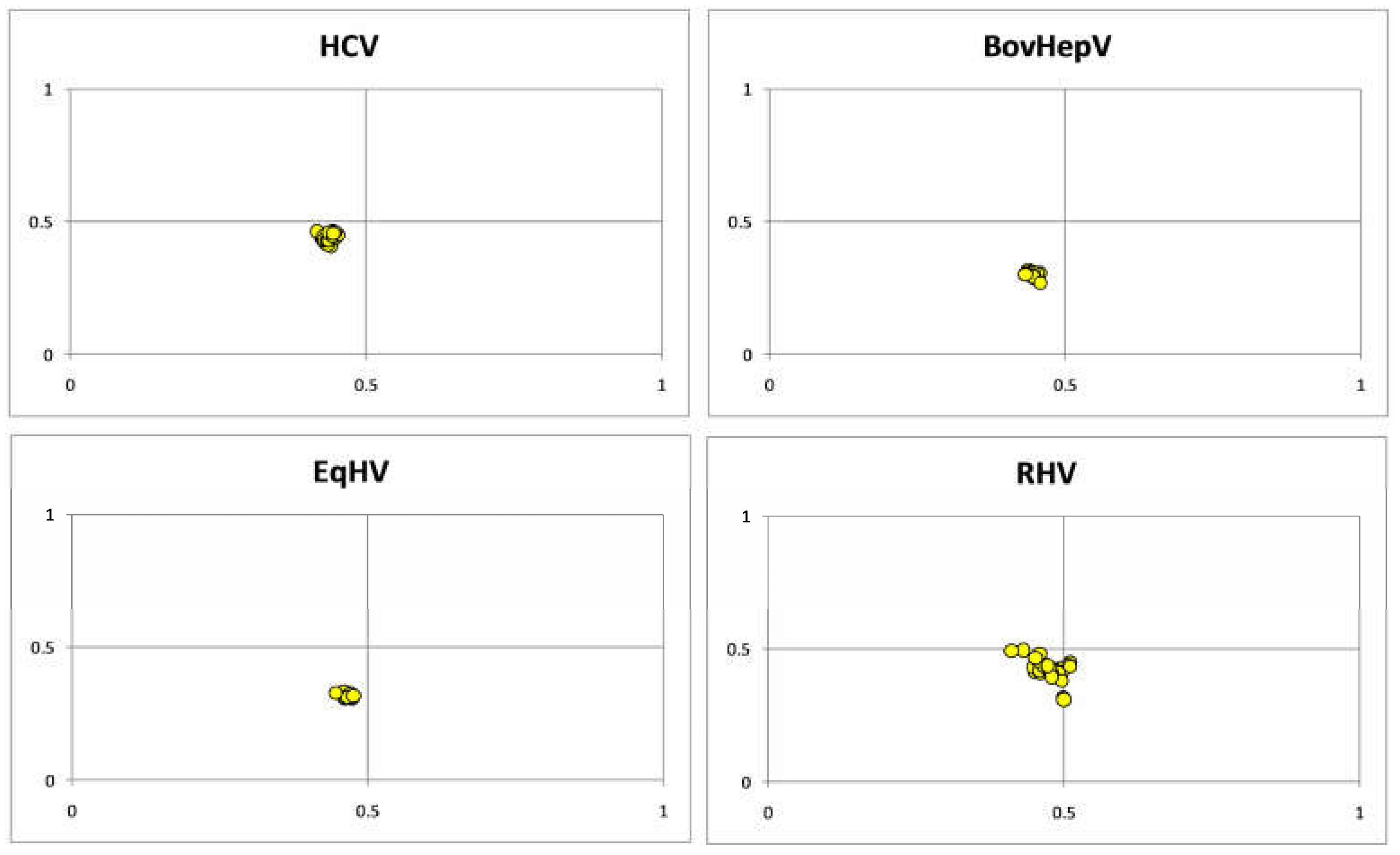
3.4. Parity Plot Analysis Indicated Dominance of Pyrimidine over Purines
3.5. Result of Skew
3.6. RSCU Analysis Revealed the Overrepresentation of G/C Ending Codons
3.7. Protein Properties Are Dependent on the Composition and Codon Bias
3.8. ENc Indicated Low Bias
3.9. Codon Context Analysis Revealed an Abundance of CTC-CTG Codon Pair and Rarity of CGA and TTA
3.10. PCA Analysis
3.11. Phylogeney Analysis
3.12. Adaptability of HCV Genome for its Hosts Human and Chimpanzee
3.12.1. The Codon Adaptation Index Reveals More Adaptability of HCV for Humans Compared to Chimpanzees
3.12.2. Codon Usage Pattern of HCV Is More Similar with That of Chimpanzee Codon Usage Pattern
3.12.3. HCV Displays the Highest Codon Usage Deoptimization for Human
3.12.4. Similarity Index showed Pan Troglodytes Is Primary Host
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Zhang, G.; Li, Q.; Han, L.; Hu, X.; Guo, Y.; Tao, W.; Zhao, X.; Guo, M.; Gan, T.; et al. TRIM26 Is a Critical Host Factor for HCV Replication and Contributes to Host Tropism. Sci. Adv. 2021, 7, eabd9732. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, P.S.; Callahan, K.E.; Jones, P.D.; Morris, C.; Ransdell, J.M.; Kwon, D.; Brown, C.P.; Kobetz, E.N. Liver Cancer: A Leading Cause of Cancer Death in the United States and the Role of the 1945–1965 Birth Cohort by Ethnicity. JHEP Rep. 2019, 1, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, V.; Tefferi, A. Prevention of Liver Cancer with New Curative Hepatitis C Antivirals: Real-World Challenges. Cancer 2018, 124, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Forman, L.M.; Lewis, J.D.; Berlin, J.A.; Feldman, H.I.; Lucey, M.R. The Association between Hepatitis C Infection and Survival after Orthotopic Liver Transplantation. Gastroenterology 2002, 122, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Couto, L.B.; Kolykhalov, A.A. Animal Models for HCV Study. In Hepatitis C Viruses: Genomes and Molecular Biology; Tan, S.-L., Ed.; Horizon Bioscience: Norfolk, UK, 2006; ISBN 978-1-904933-20-5. [Google Scholar]
- Kames, J.; Alexaki, A.; Holcomb, D.D.; Santana-Quintero, L.V.; Athey, J.C.; Hamasaki-Katagiri, N.; Katneni, U.; Golikov, A.; Ibla, J.C.; Bar, H.; et al. TissueCoCoPUTs: Novel Human Tissue-Specific Codon and Codon-Pair Usage Tables Based on Differential Tissue Gene Expression. J. Mol. Biol. 2020, 432, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Camiolo, S.; Farina, L.; Porceddu, A. The Relation of Codon Bias to Tissue-Specific Gene Expression in Arabidopsis Thaliana. Genetics 2012, 192, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Parvathy, S.T.; Udayasuriyan, V.; Bhadana, V. Codon Usage Bias. Mol. Biol. Rep. 2022, 49, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Arella, D.; Dilucca, M.; Giansanti, A. Codon Usage Bias and Environmental Adaptation in Microbial Organisms. Mol. Genet. Genom. 2021, 296, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Gao, Z.-L.; Zhang, J.; Ding, Y.-Z.; Stipkovits, L.; Szathmary, S.; Pejsak, Z.; Liu, Y.-S. The Analysis of Codon Bias of Foot-and-Mouth Disease Virus and the Adaptation of This Virus to the Hosts. Infect. Genet. Evol. 2013, 14, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Quax, T.E.F.; Claassens, N.J.; Söll, D.; van der Oost, J. Codon Bias as a Means to Fine-Tune Gene Expression. Mol. Cell 2015, 59, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandia, R.; Saeed, M.; Alharbi, A.M.; Ashraf, G.M.; Greig, N.H.; Kamal, M.A. Codon Usage Bias Correlates With Gene Length in Neurodegeneration Associated Genes. Front. Neurosci. 2022, 16, 895607. [Google Scholar] [CrossRef]
- Chan, C.T.Y.; Deng, W.; Li, F.; DeMott, M.S.; Babu, I.R.; Begley, T.J.; Dedon, P.C. Highly Predictive Reprogramming of TRNA Modifications Is Linked to Selective Expression of Codon-Biased Genes. Chem. Res. Toxicol. 2015, 28, 978–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hia, F.; Takeuchi, O. The Effects of Codon Bias and Optimality on MRNA and Protein Regulation. Cell. Mol. Life Sci. 2021, 78, 1909–1928. [Google Scholar] [CrossRef]
- Sheikh, A.; Al-Taher, A.; Al-Nazawi, M.; Al-Mubarak, A.I.; Kandeel, M. Analysis of Preferred Codon Usage in the Coronavirus N Genes and Their Implications for Genome Evolution and Vaccine Design. J. Virol. Methods 2020, 277, 113806. [Google Scholar] [CrossRef] [PubMed]
- Sexton, N.R.; Ebel, G.D. Effects of Arbovirus Multi-Host Life Cycles on Dinucleotide and Codon Usage Patterns. Viruses 2019, 11, E643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, A.J.; Kithil, M.; Langhans, M.; Rauh, O.; Cartolano, M.; Van Etten, J.L.; Moroni, A.; Thiel, G. Codon Bias Can Determine Sorting of a Potassium Channel Protein. Cells 2021, 10, 1128. [Google Scholar] [CrossRef]
- Mittal, P.; Brindle, J.; Stephen, J.; Plotkin, J.B.; Kudla, G. Codon Usage Influences Fitness through RNA Toxicity. Proc. Natl. Acad Sci. USA 2018, 115, 8639–8644. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Yu, C.-H.; Zhao, F.; Dang, Y.; Wu, C.; Xie, P.; Sachs, M.S.; Liu, Y. ERF1 Mediates Codon Usage Effects on MRNA Translation Efficiency through Premature Termination at Rare Codons. Nucleic Acids Res. 2019, 47, 9243–9258. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Q.; Zhao, F. Synonymous but Not Silent: The Codon Usage Code for Gene Expression and Protein Folding. Annu. Rev. Biochem. 2021, 90, 375–401. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, Y.; Rao, J.; Zeng, F.; Yang, R.; Tan, H.; Liu, Z.; Du, W.; Liu, L. Host Adaptation of Codon Usage in SARS-CoV-2 from Mammals Indicates Potential Natural Selection and Viral Fitness. Arch. Virol. 2022, 167, 2677–2688. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Legnardi, M.; Cecchinato, M. Effect of Genome Composition and Codon Bias on Infectious Bronchitis Virus Evolution and Adaptation to Target Tissues. BMC Genom. 2021, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Karlin, S.; Mrázek, J. Compositional Differences within and between Eukaryotic Genomes. Proc. Natl. Acad Sci. USA 1997, 94, 10227–10232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, B. SkewDB: A Comprehensive Database of GC and 10 Other Skews for over 28,000 Chromosomes and Plasmids. bioRxiv 2021. [Google Scholar] [CrossRef]
- Necşulea, A.; Lobry, J.R. A New Method for Assessing the Effect of Replication on DNA Base Composition Asymmetry. Mol. Biol. Evol. 2007, 24, 2169–2179. [Google Scholar] [CrossRef] [Green Version]
- Uddin, A.; Chakraborty, S. Codon Usage Trend in Mitochondrial CYB Gene. Gene 2016, 586, 105–114. [Google Scholar] [CrossRef]
- Puigbò, P.; Bravo, I.G.; Garcia-Vallve, S. CAIcal: A Combined Set of Tools to Assess Codon Usage Adaptation. Biol. Direct 2008, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Liu, J.; Li, H.; Liu, B.; Zhao, B.; Ning, Z. Comprehensive Analysis of Synonymous Codon Usage Patterns and Influencing Factors of Porcine Epidemic Diarrhea Virus. Arch. Virol. 2021, 166, 157–165. [Google Scholar] [CrossRef]
- Butt, A.M.; Nasrullah, I.; Qamar, R.; Tong, Y. Evolution of Codon Usage in Zika Virus Genomes Is Host and Vector Specific. Emerg. Microbes Infect. 2016, 5, e107. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Pandey, S.P. Phylogenetic and Evolutionary Analysis of Plant ARGONAUTES. Methods Mol. Biol. 2017, 1640, 267–294. [Google Scholar] [CrossRef]
- Deb, B.; Uddin, A.; Chakraborty, S. Codon Usage Pattern and Its Influencing Factors in Different Genomes of Hepadnaviruses. Arch. Virol. 2020, 165, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Wright, F. The “effective Number of Codons” Used in a Gene. Gene 1990, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bourret, J.; Alizon, S.; Bravo, I.G. COUSIN (COdon Usage Similarity INdex): A Normalized Measure of Codon Usage Preferences. Genome Biol. Evol. 2019, 11, 3523–3528. [Google Scholar] [CrossRef]
- Henry, I.; Sharp, P.M. Predicting Gene Expression Level from Codon Usage Bias. Mol. Biol. Evol. 2007, 24, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Singhal, S.; Kumar, U.; Ansari, A.; Tiwari, R.; Dhama, K.; Das, J.; Munjal, A.; Singh, R.K. Analysis of Nipah Virus Codon Usage and Adaptation to Hosts. Front. Microbiol. 2019, 10, 886. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lyu, X.; Cheng, J.; Fu, Y.; Lin, Y.; Abdoulaye, A.H.; Jiang, D.; Xie, J. Codon Usage Provides Insights into the Adaptive Evolution of Mycoviruses in Their Associated Fungi Host. Int. J. Mol. Sci. 2022, 23, 7441. [Google Scholar] [CrossRef]
- Moura, G.; Pinheiro, M.; Silva, R.; Miranda, I.; Afreixo, V.; Dias, G.; Freitas, A.; Oliveira, J.L.; Santos, M.A. Comparative Context Analysis of Codon Pairs on an ORFeome Scale. Genome Biol. 2005, 6, R28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeza, M.; Alcaíno, J.; Barahona, S.; Sepúlveda, D.; Cifuentes, V. Codon Usage and Codon Context Bias in Xanthophyllomyces Dendrorhous. BMC Genom. 2015, 16, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, X.; Chi, X.; Wang, S.; Ma, Y.; Chen, J. Comprehensive Analysis of the Codon Usage Patterns in the Envelope Glycoprotein E2 Gene of the Classical Swine Fever Virus. PLoS One 2017, 12, e0183646. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A Simple Method for Displaying the Hydropathic Character of a Protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Chandan, J.; Gupta, S.; Babu, V.; Singh, D.; Singh, R. Comprehensive Analysis of Codon Usage Pattern in Withania Somnifera and Its Associated Pathogens: Meloidogyne Incognita and Alternaria Alternata. Genetica 2022, 150, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Sun, D.; Ma, Q.; Chen, H.; Ma, L.; Ding, Y.; Liu, Y. The Distribution of Synonymous Codon Choice in the Translation Initiation Region of Dengue Virus. PLoS One 2013, 8, e77239. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Li, X.-R.; Lan, X.; Han, S.-Y.; Wang, Y.-N.; Hu, Y.; Pan, Q. The Genetic Divergences of Codon Usage Shed New Lights on Transmission of Hepatitis E Virus from Swine to Human. Infect. Genet. Evol. 2019, 68, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Papamichail, D.; Coleman, J.R.; Skiena, S.; Wimmer, E. Reduction of the Rate of Poliovirus Protein Synthesis through Large-Scale Codon Deoptimization Causes Attenuation of Viral Virulence by Lowering Specific Infectivity. J. Virol. 2006, 80, 9687–9696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigbò, P.; Aragonès, L.; Garcia-Vallvé, S. RCDI/ERCDI: A Web-Server to Estimate Codon Usage Deoptimization. BMC Res. Notes 2010, 3, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, G.M.; Holmes, E.C. The Extent of Codon Usage Bias in Human RNA Viruses and Its Evolutionary Origin. Virus Res. 2003, 92, 1–7. [Google Scholar] [CrossRef]
- Munjal, A.; Khandia, R.; Shende, K.K.; Das, J. Mycobacterium Lepromatosis Genome Exhibits Unusually High CpG Dinucleotide Content and Selection Is Key Force in Shaping Codon Usage. Infect. Genet. Evol. 2020, 84, 104399. [Google Scholar] [CrossRef]
- Khandia, R.; Sharma, A.; Alqahtani, T.; Alqahtani, A.M.; Asiri, Y.I.; Alqahtani, S.; Alharbi, A.M.; Kamal, M.A. Strong Selectional Forces Fine-Tune CpG Content in Genes Involved in Neurological Disorders as Revealed by Codon Usage Patterns. Front. Neurosci. 2022, 16, 596. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Song, Y.; Jing, L. Analysis of Codon Usage Bias of WRKY Transcription Factors in Helianthus Annuus. BMC Genom. Data 2022, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.J.; Wolfe, K.H.; Devine, K.M. Base Composition Skews, Replication Orientation, and Gene Orientation in 12 Prokaryote Genomes. J. Mol. Evol. 1998, 47, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Khandia, R.; Puranik, N.; Alqahtani, A.M.; Chidambaram, K.; Kamal, M.A. Codon Usage Is Influenced by Compositional Constraints in Genes Associated with Dementia. Front. Genet. 2022, 13, 884348. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Deb, B.; Barbhuiya, P.A.; Uddin, A. Analysis of Codon Usage Patterns and Influencing Factors in Nipah Virus. Virus Res. 2019, 263, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Khandia, R.; Puranik, N.; Alqahtani, A.M.; Almikhlafi, M.A.; Algahtany, M.A. Leucine Encoding Codon TTG Shows an Inverse Relationship with GC Content in Genes Involved in Neurodegeneration with Iron Accumulation. J. Integr. Neurosci. 2021, 20, 905–918. [Google Scholar] [CrossRef]
- Khandia, R.; Pandey, M.K.; Rzhepakovsky, I.V.; Khan, A.A.; Alexiou, A. Synonymous Codon Variant Analysis for Autophagic Genes Dysregulated in Neurodegeneration. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Deb, B.; Uddin, A.; Chakraborty, S. Genome-Wide Analysis of Codon Usage Pattern in Herpesviruses and Its Relation to Evolution. Virus Res. 2021, 292, 198248. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, N.; Dong, L.; Wang, Y.; Zhang, S.; Young, H.A.; Feng, X.; Gu, Y.Q. Complete Chloroplast Genome Sequences of Mongolia Medicine Artemisia Frigida and Phylogenetic Relationships with Other Plants. PLoS One 2013, 8, e57533. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Khandia, R.; Papadakis, M.; Alexiou, A.; Simonov, A.N.; Khan, A.A. An Investigation of Codon Usage Pattern Analysis in Pancreatitis Associated Genes. BMC Genom. Data 2022, 23, 81. [Google Scholar] [CrossRef]
- Khandia, R.; Pandey, M.K.; Khan, A.A.; Rzhepakovsky, I.V.; Gurjar, P.; Karobari, M.I. Codon Usage and Context Analysis of Genes Modulated during SARS-CoV-2 Infection and Dental Inflammation. Vaccines 2022, 10, 1874. [Google Scholar] [CrossRef]
- Moura, G.; Pinheiro, M.; Arrais, J.; Gomes, A.C.; Carreto, L.; Freitas, A.; Oliveira, J.L.; Santos, M.A.S. Large Scale Comparative Codon-Pair Context Analysis Unveils General Rules That Fine-Tune Evolution of MRNA Primary Structure. PLoS One 2007, 2, e847. [Google Scholar] [CrossRef]
- Silverj, A.; Rota-Stabelli, O. On the Correct Interpretation of Similarity Index in Codon Usage Studies: Comparison with Four Other Metrics and Implications for Zika and West Nile Virus. Virus Res. 2020, 286, 198097. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Gerold, G.; Qaisar, N.; Jain, K.; Henriquez, J.A.; Firth, C.; Hirschberg, D.L.; Rice, C.M.; Shields, S.; et al. Characterization of a Canine Homolog of Hepatitis C Virus. Proc. Natl. Acad Sci. USA 2011, 108, 11608–11613. [Google Scholar] [CrossRef] [Green Version]
- Burbelo, P.D.; Dubovi, E.J.; Simmonds, P.; Medina, J.L.; Henriquez, J.A.; Mishra, N.; Wagner, J.; Tokarz, R.; Cullen, J.M.; Iadarola, M.J.; et al. Serology-Enabled Discovery of Genetically Diverse Hepaciviruses in a New Host. J. Virol. 2012, 86, 6171–6178. [Google Scholar] [CrossRef] [Green Version]
- Quan, P.-L.; Firth, C.; Conte, J.M.; Williams, S.H.; Zambrana-Torrelio, C.M.; Anthony, S.J.; Ellison, J.A.; Gilbert, A.T.; Kuzmin, I.V.; Niezgoda, M.; et al. Bats Are a Major Natural Reservoir for Hepaciviruses and Pegiviruses. Proc. Natl. Acad Sci. USA 2013, 110, 8194–8199. [Google Scholar] [CrossRef] [Green Version]
- Baechlein, C.; Fischer, N.; Grundhoff, A.; Alawi, M.; Indenbirken, D.; Postel, A.; Baron, A.L.; Offinger, J.; Becker, K.; Beineke, A.; et al. Identification of a Novel Hepacivirus in Domestic Cattle from Germany. J. Virol. 2015, 89, 7007–7015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Lukashev, A.N.; Gmyl, A.; Coutard, B.; Adam, A.; Ritz, D.; Leijten, L.M.; van Riel, D.; et al. Evidence for Novel Hepaciviruses in Rodents. PLoS Pathog. 2013, 9, e1003438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauck, M.; Sibley, S.D.; Lara, J.; Purdy, M.A.; Khudyakov, Y.; Hyeroba, D.; Tumukunde, A.; Weny, G.; Switzer, W.M.; Chapman, C.A.; et al. A Novel Hepacivirus with an Unusually Long and Intrinsically Disordered NS5A Protein in a Wild Old World Primate. J. Virol. 2013, 87, 8971–8981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaver, B.; van der Velden, Y.; van Hemert, F.; van der Kuyl, A.C.; Berkhout, B. HIV-1 Tolerates Changes in A-Count in a Small Segment of the Pol Gene. Retrovirology 2017, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Ulveling, D.; Dinger, M.E.; Francastel, C.; Hubé, F. Identification of a Dinucleotide Signature That Discriminates Coding from Non-Coding Long RNAs. Front. Genet. 2014, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Shackelton, L.A.; Parrish, C.R.; Holmes, E.C. Evolutionary Basis of Codon Usage and Nucleotide Composition Bias in Vertebrate DNA Viruses. J. Mol. Evol. 2006, 62, 551–563. [Google Scholar] [CrossRef]
- Karlin, S.; Doerfler, W.; Cardon, L.R. Why Is CpG Suppressed in the Genomes of Virtually All Small Eukaryotic Viruses but Not in Those of Large Eukaryotic Viruses? J. Virol. 1994, 68, 2889–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, M.; Vivekanandan, P. Depletion of CpG Dinucleotides in Papillomaviruses and Polyomaviruses: A Role for Divergent Evolutionary Pressures. PLoS One 2015, 10, e0142368. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.K.; McFerran, N.V. Dinucleotide and Stop Codon Frequencies in Single-Stranded RNA Viruses. J. Gen. Virol. 1997, 78 Pt 11, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.P.; Mota, B.E.F.; Pena, S.D.J.; Azevedo, V.; Macedo, A.M.; Tauch, A.; Machado, C.R.; Franco, G.R. Virus-Host Coevolution: Common Patterns of Nucleotide Motif Usage in Flaviviridae and Their Hosts. PLoS One 2009, 4, e6282. [Google Scholar] [CrossRef] [Green Version]
- Khandia, R.; Ali Khan, A.; Alexiou, A.; Povetkin, S.N.; Verevkina, M.N. Codon Usage Analysis of Pro-Apoptotic Bim Gene Isoforms. J. Alzheimer’s Dis. 2022, 86, 1711–1725. [Google Scholar] [CrossRef]
- Trinh, R.; Gurbaxani, B.; Morrison, S.L.; Seyfzadeh, M. Optimization of Codon Pair Use within the (GGGGS)3 Linker Sequence Results in Enhanced Protein Expression. Mol. Immunol. 2004, 40, 717–722. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, T.; Lu, L.; Cai, F.; Lin, J.; Jiang, Y.E.; Lin, Y. Codon Pair Optimization (CPO): A Software Tool for Synthetic Gene Design Based on Codon Pair Bias to Improve the Expression of Recombinant Proteins in Pichia Pastoris. Microb. Cell Fact. 2021, 20, 209. [Google Scholar] [CrossRef]
- Kunec, D.; Osterrieder, N. Codon Pair Bias Is a Direct Consequence of Dinucleotide Bias. Cell Rep. 2016, 14, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Martrus, G.; Nevot, M.; Andres, C.; Clotet, B.; Martinez, M.A. Changes in Codon-Pair Bias of Human Immunodeficiency Virus Type 1 Have Profound Effects on Virus Replication in Cell Culture. Retrovirology 2013, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Jordan-Paiz, A.; Franco, S.; Martinez, M.A. Synonymous Codon Pair Recoding of the HIV-1 Env Gene Affects Virus Replication Capacity. Cells 2021, 10, 1636. [Google Scholar] [CrossRef]
- RajBhandary, U.L. More Surprises in Translation: Initiation without the Initiator TRNA. Proc. Natl. Acad Sci. USA 2000, 97, 1325–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bletsa, M.; Vrancken, B.; Gryseels, S.; Boonen, I.; Fikatas, A.; Li, Y.; Laudisoit, A.; Lequime, S.; Bryja, J.; Makundi, R.; et al. Molecular Detection and Genomic Characterization of Diverse Hepaciviruses in African Rodents. Virus Evol. 2021, 7, veab036. [Google Scholar] [CrossRef] [PubMed]
- Furió, V.; Garijo, R.; Durán, M.; Moya, A.; Bell, J.C.; Sanjuán, R. Relationship between Within-Host Fitness and Virulence in the Vesicular Stomatitis Virus: Correlation with Partial Decoupling. J. Virol. 2012, 86, 12228–12236. [Google Scholar] [CrossRef] [PubMed]
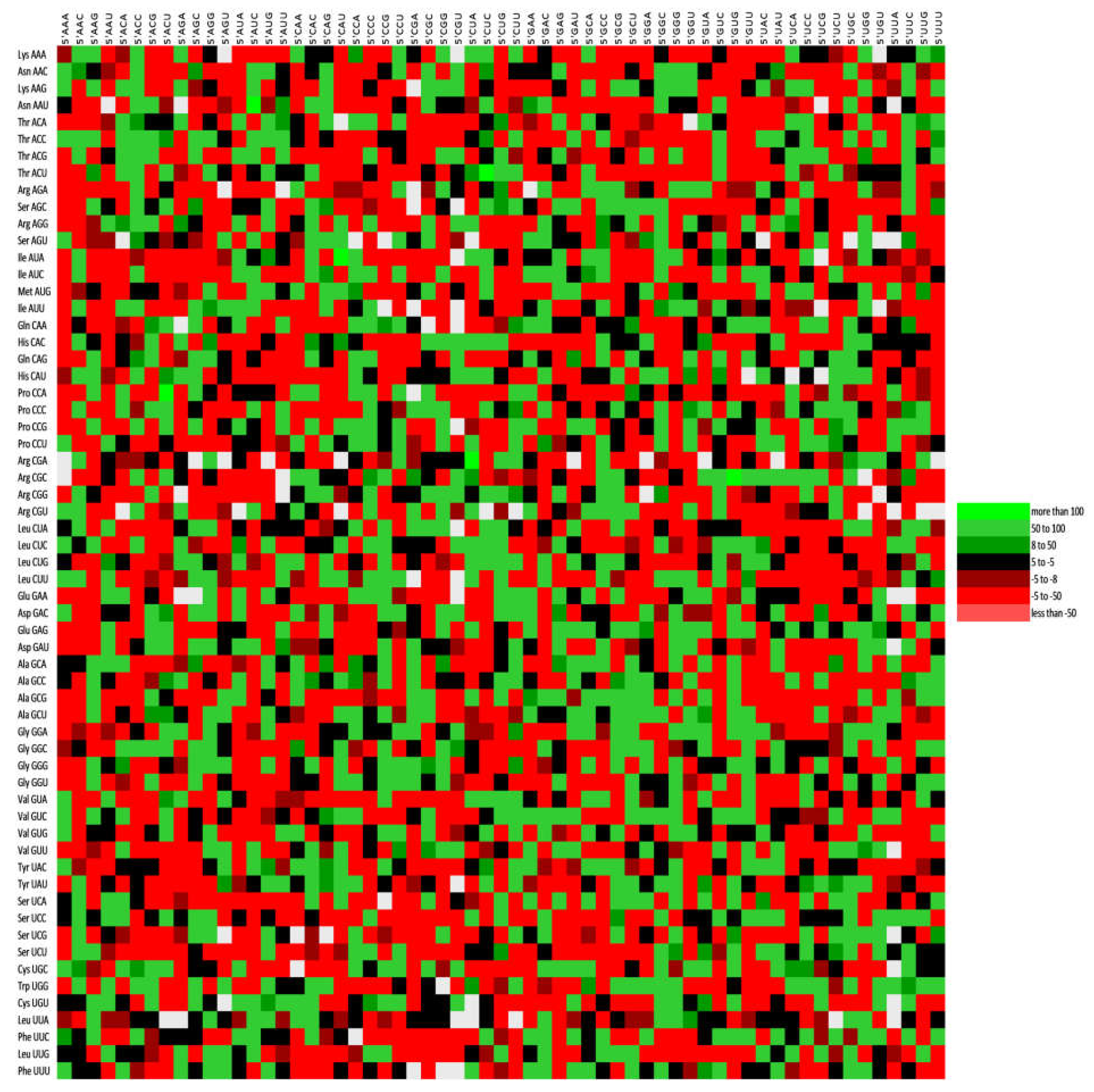
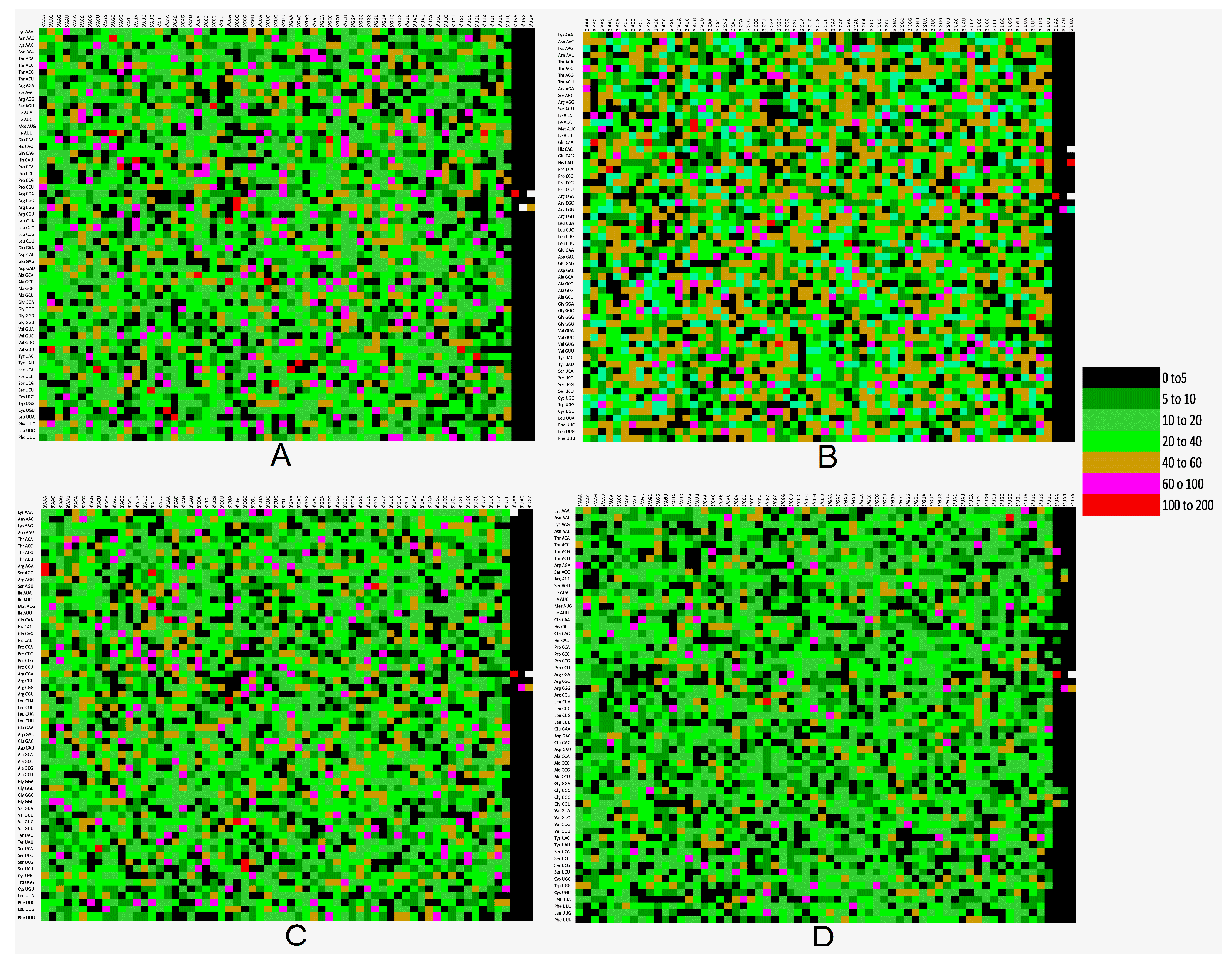
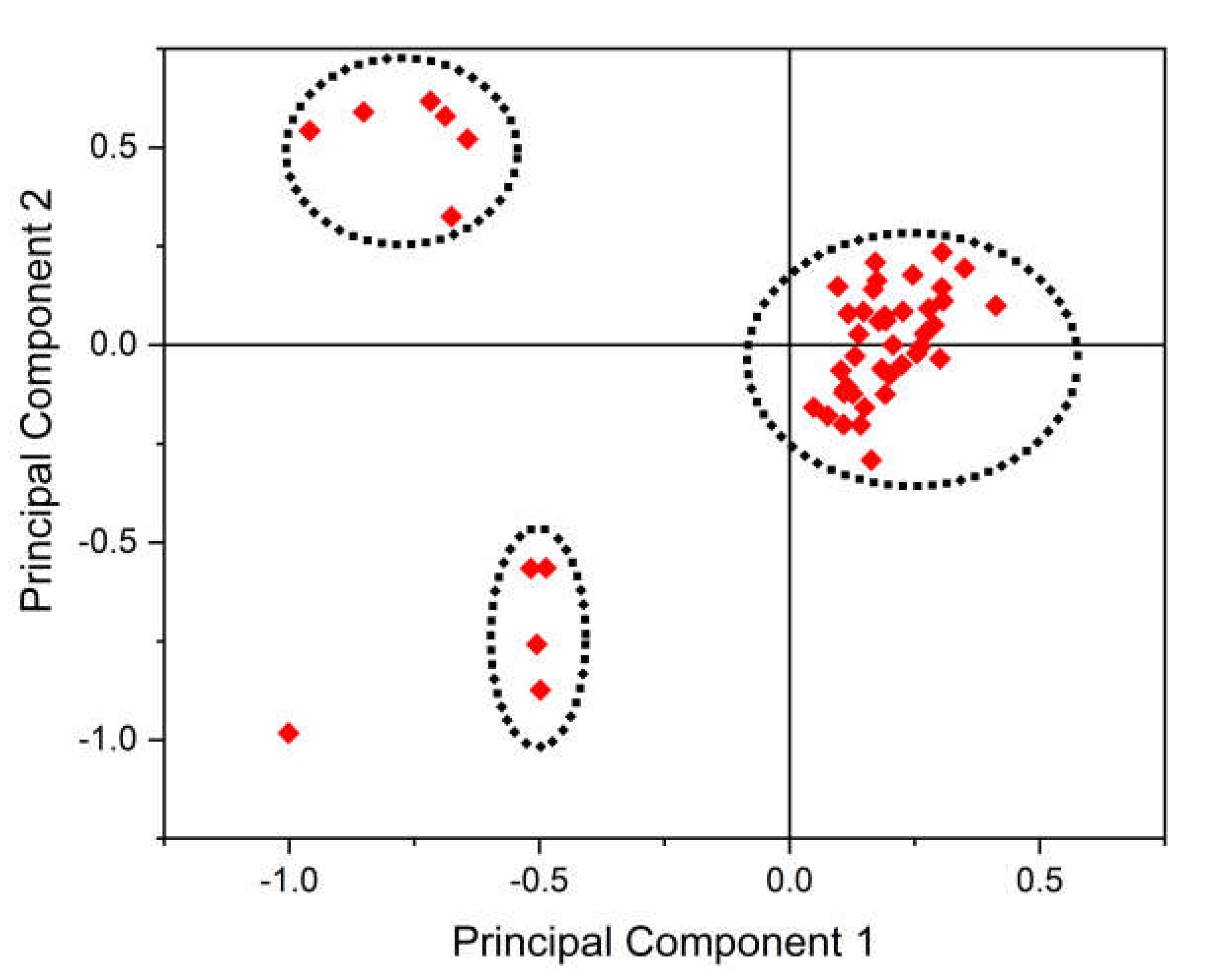
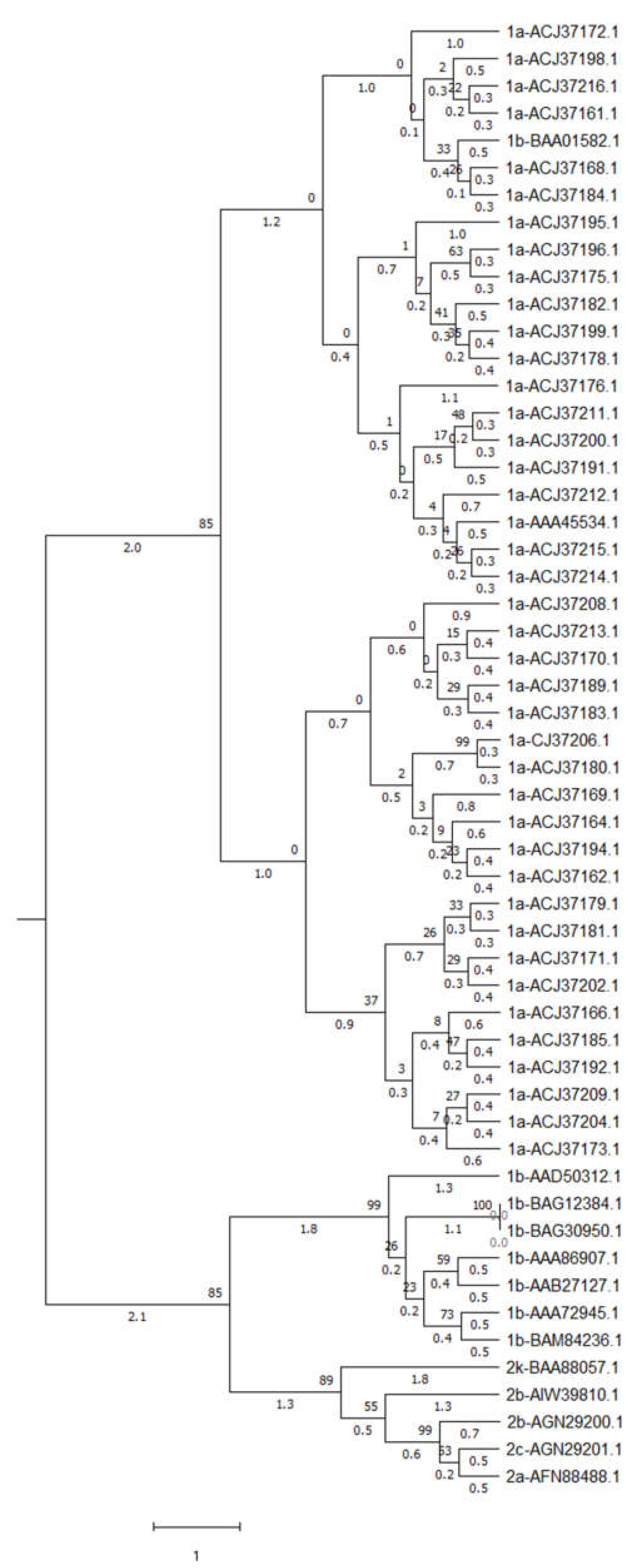
| %A | %A1 | %A2 | %A3 | %T | %T1 | %T2 | %T3 | %C | %C1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| GRAVY (r value) | −0.680 | −0.118 | −0.487 | −0.701 | −0.384 | −0.553 | 0.201 | −0.303 | 0.617 | 0.635 |
| p value | *** | NS | *** | *** | ** | *** | NS | * | *** | *** |
| AROMA | −0.134 | 0.310 | −0.270 | −0.226 | −0.175 | −0.132 | −0.073 | −0.153 | 0.282 | −0.001 |
| p value | NS | * | * | NS | NS | NS | NS | NS | * | NS |
| %C2 | %C3 | %G | %G1 | %G2 | %G3 | %GC(all) | %GC(1) | %GC(2) | %GC(3) | |
| GRAVY | −0.655 | 0.650 | 0.469 | −0.110 | 0.641 | 0.176 | 0.602 | 0.510 | 0.277 | 0.592 |
| p value | *** | *** | *** | NS | *** | NS | *** | *** | *** | *** |
| AROMA | −0.441 | 0.418 | −0.041 | −0.345 | 0.464 | −0.282 | 0.169 | −0.187 | 0.245 | 0.221 |
| p value | *** | ** | NS | * | *** | * | NS | NS | NS | NS |
| HCVs (Recombinants) | HCVs (Non- Recombinants) | Bovine Hepacivirus BovHepV | Equine Hepacivirus (EqHV) | Rodent Hepacivirus (RHV) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. No. | Codon Pair | Frequency | Codon Pair | Frequency | Codon Pair | Frequency | Codon Pair | Frequency | Codon Pair | Frequency |
| 1 | GCC-CTC | 88 | CTC-CTG | 369 | GCT-GCT | 258 | GGC-GCT | 160 | GCT-GCT | 229 |
| 2 | CCC-CCC | 81 | GTG-GCC | 320 | CTT-GAG | 153 | TGG-GCT | 137 | GAG-GAG | 216 |
| 3 | GTC-ATC | 75 | AAC-ACC | 295 | GTC-ACC | 149 | GCT-TGG | 132 | AAG-AAG | 190 |
| 4 | GGC-GCC | 71 | GCC-ATC | 293 | CTT-GCT | 142 | CTT-GCT | 130 | GCT-GGC | 174 |
| 5 | TAT-GAC | 69 | GTC-ACC | 270 | GTC-ACT | 135 | GCT-TCT | 129 | GAG-GAC | 164 |
| 6 | GAG-GTC | 63 | CTC-ACT | 270 | GTT-GCT | 134 | GAC-ACT | 121 | GCT-GCC | 163 |
| 7 | GCG-GCC | 62 | GTG-TGC | 267 | GGT-GCT | 134 | ACT-GGC | 112 | GCT-GAG | 163 |
| 8 | GTG-GAC | 61 | CTG-GAC | 267 | GGC-ACT | 133 | GAT-GTT | 107 | GAG-GCT | 156 |
| 9 | GAC-GCC | 61 | GCT-GCC | 263 | GCT-GTG | 133 | TTT-GAC | 101 | TTT-GAC | 154 |
| 10 | ACC-ATC | 60 | AAC-TGG | 258 | GCT-GTT | 131 | GCT-TTT | 101 | GTG-GTG | 148 |
| 11 | ACC-ACC | 59 | GTG-CGC | 257 | CCT-TAC | 127 | GCT-GTT | 101 | TTG-GCT | 146 |
| 12 | TGC-TCC | 58 | ATC-ACC | 255 | ACT-GCT | 127 | TCT-GTT | 100 | ACT-GGC | 144 |
| 13 | TGC-GGC | 58 | GTG-GGG | 253 | TGG-GCT | 126 | TGT-GGC | 98 | ACC-AAG | 143 |
| 14 | GAG-GAG | 56 | ATC-ATG | 249 | GAT-GTT | 123 | GCT-GTC | 98 | TAC-ACC | 141 |
| 15 | TAC-TCC | 53 | TGG-GCG | 248 | GGT-GCC | 121 | ACT-GTC | 97 | GAC-ACC | 134 |
| 16 | GAC-ATC | 53 | TAC-GTG | 246 | GCT-ACT | 119 | CCT-TAT | 95 | TGT-GAC | 131 |
| 17 | GGG-TAC | 51 | GCC-ACC | 243 | CCT-GCT | 116 | GGG-GAT | 94 | GTG-GCC | 130 |
| 18 | TCC-TGG | 50 | ATC-AAC | 236 | GCT-GGC | 114 | ATG-GGC | 92 | AAG-GAG | 130 |
| 19 | TAC-ATC | 50 | GTC-ATC | 234 | GTT-TGG | 111 | GAG-GAA | 91 | AAG-AAA | 130 |
| 20 | GGT-GTG | 50 | CTG-CTG | 234 | GCT-GTC | 111 | TAT-GAC | 90 | GGG-AAG | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandia, R.; Khan, A.A.; Karuvantevida, N.; Gurjar, P.; Rzhepakovsky, I.V.; Legaz, I. Insights into Synonymous Codon Usage Bias in Hepatitis C Virus and Its Adaptation to Hosts. Pathogens 2023, 12, 325. https://doi.org/10.3390/pathogens12020325
Khandia R, Khan AA, Karuvantevida N, Gurjar P, Rzhepakovsky IV, Legaz I. Insights into Synonymous Codon Usage Bias in Hepatitis C Virus and Its Adaptation to Hosts. Pathogens. 2023; 12(2):325. https://doi.org/10.3390/pathogens12020325
Chicago/Turabian StyleKhandia, Rekha, Azmat Ali Khan, Noushad Karuvantevida, Pankaj Gurjar, Igor Vladimirovich Rzhepakovsky, and Isabel Legaz. 2023. "Insights into Synonymous Codon Usage Bias in Hepatitis C Virus and Its Adaptation to Hosts" Pathogens 12, no. 2: 325. https://doi.org/10.3390/pathogens12020325








