Lipidomics Analysis of Multilamellar Bodies Produced by Amoeba Acanthamoeba castellanii in Co-Culture with Klebsiella aerogenes
Abstract
1. Introduction
2. Results
2.1. MLB Production in A. castellanii
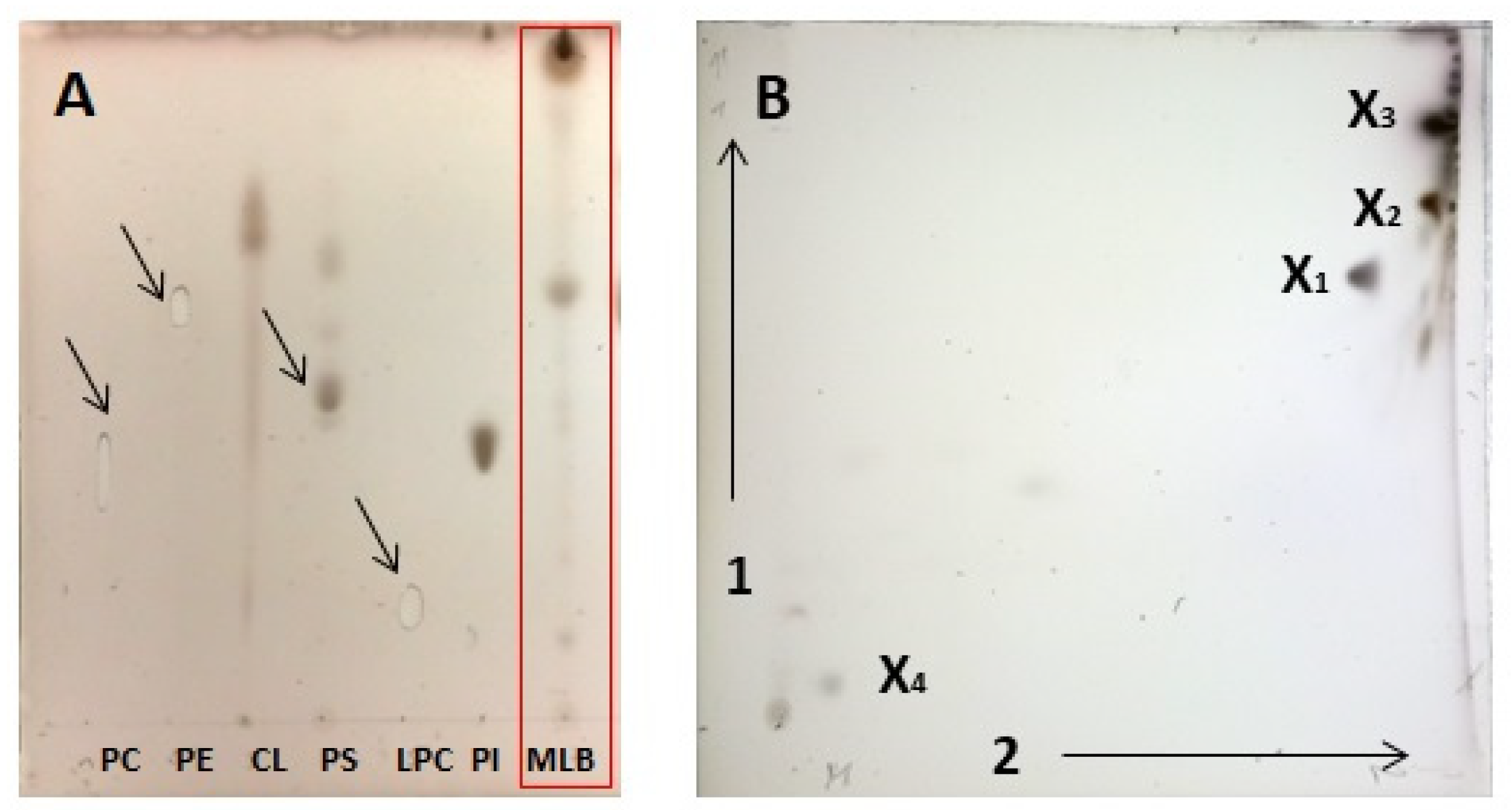
2.2. Chromatographic Analysis of MLB Components
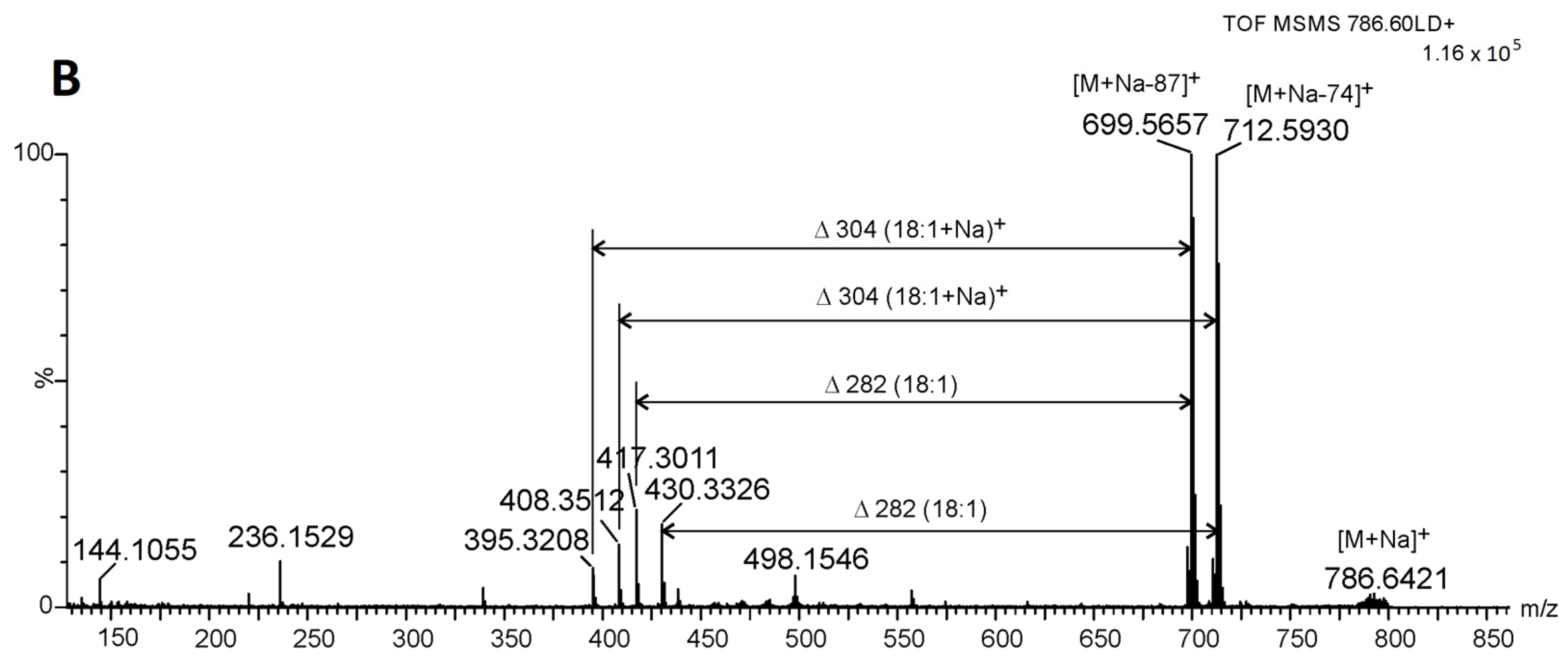
2.3. Identification of Betaine Lipids in MLBs
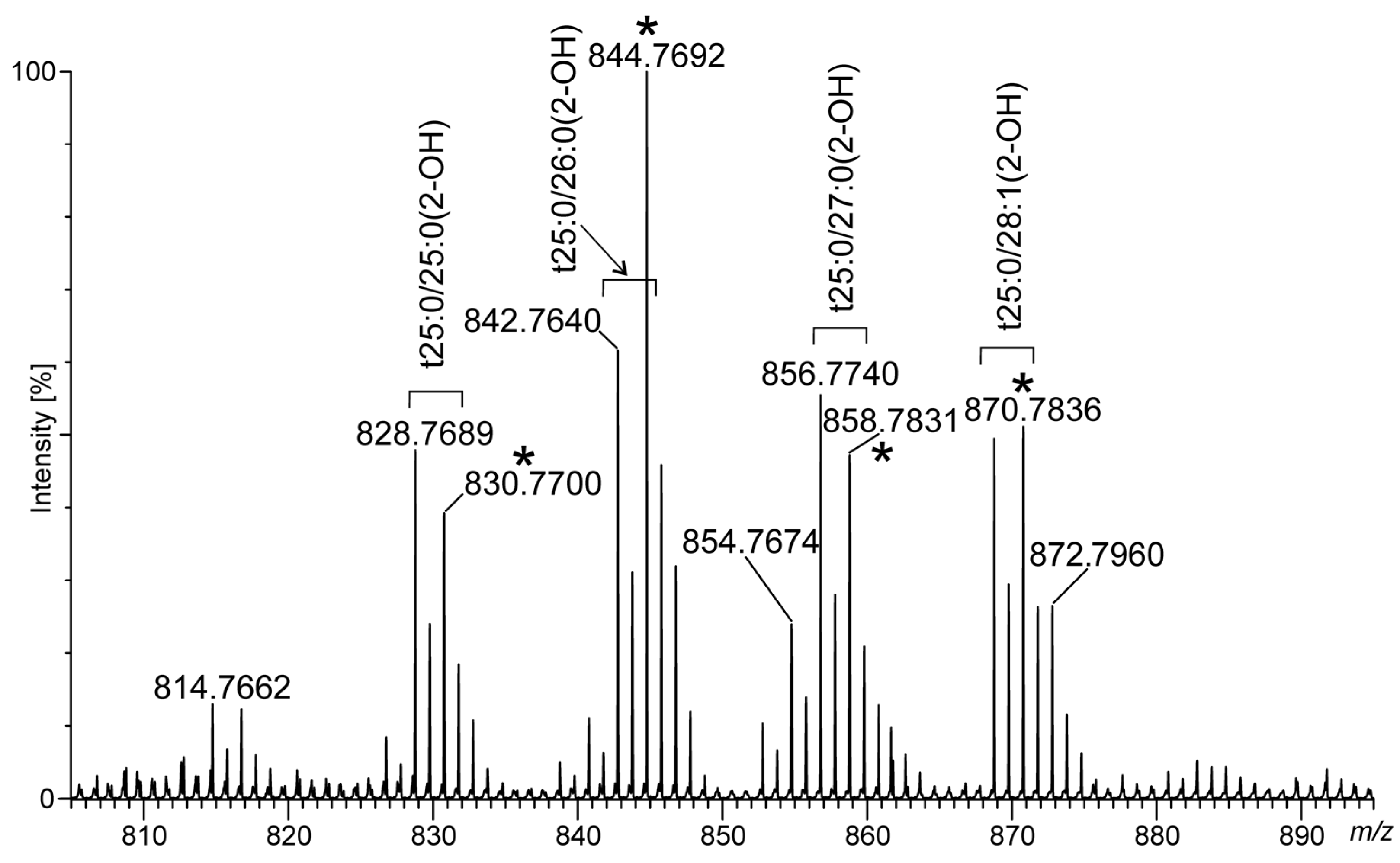
2.4. Ceramide Components in Acanthamoeba Derived MLBs
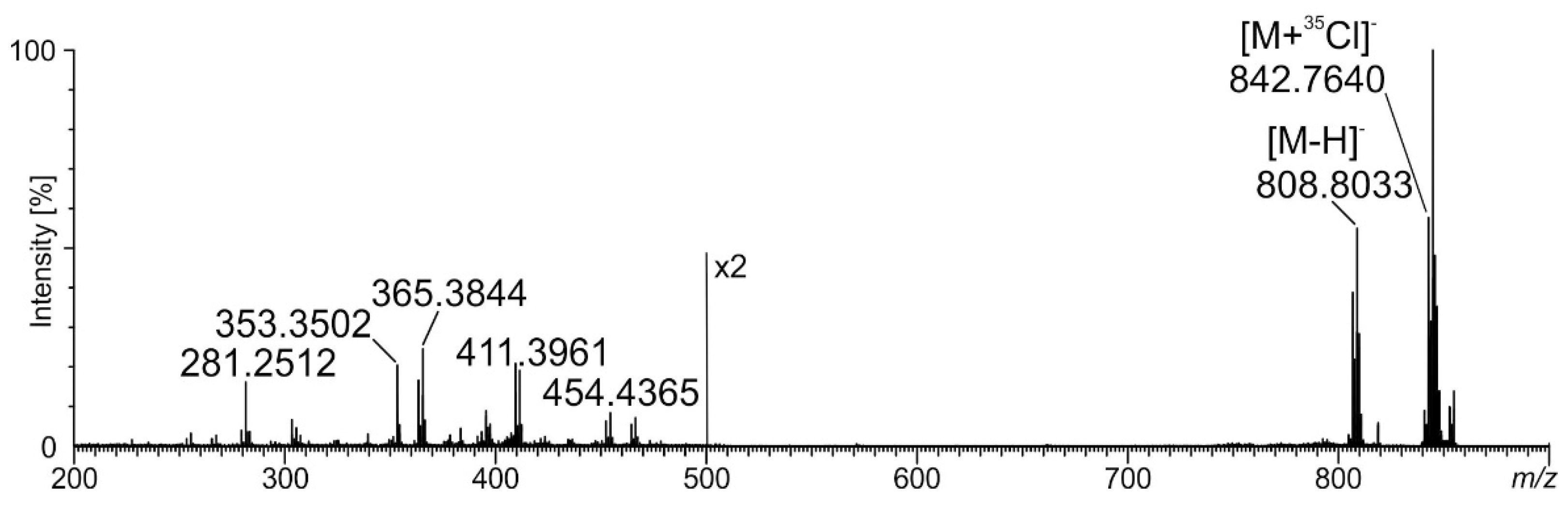
2.5. Phospholipids Identified in MLBs by Spectrometric Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Strains and Culture Conditions
4.3. Purification of MLBs
4.4. LIVE/DEAD Assay
4.5. TEM
4.6. Lipid Extraction
4.7. Lipid Separation by TLC
4.8. Gas Liquid Chromatography with Mass Spectrometry (GC–MS) Analysis of Fatty Acids
4.9. Electrospray Ionization–Mass Spectrometry (ESI–MS) Analysis of Lipids
4.10. MALDI–TOF–MS and MS–MS Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paquet, V.E.; Lessirec, R.; Domerguec, F.; Fouillenc, L.; Filiona, G.; Sedighia, A.; Charette, S.J. Lipid composition of multilamellar bodies secreted by Dictyostelium discoideum reveals their amoebal origin. Eukaryot. Cell 2013, 12, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Steer, D.; Leung, S.S.W.; Meiselman, H.; Topgaard, D.; Leal, C. Structure of lung-mimetic multilamellar bodies with lipid compositions relevant in pneumonia. Langmuir 2018, 34, 7561–7574. [Google Scholar] [CrossRef]
- Denoncourt, A.M.; Paquet, V.E.; Sedighi, A.; Charette, S.J. Identification of proteins associated with multilamellar bodies produced by Dictyostelium discoideum. PLoS ONE 2016, 11, e0158270. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Rupasinghe, T.; Desmarini, D.; Kaufman-Francis, K.; Sorrell, T.C.; Roessner, U.; Djordjevic, J.T. The PHO signaling pathway directs lipid remodeling in Cryptococcus neoformans via DGTS synthase to recycle phosphate during phosphate deficiency. PLoS ONE 2019, 14, e0212651. [Google Scholar] [CrossRef] [PubMed]
- Furlong, S.T.; Leary, J.A.; Costello, C.E.; Dawidowicz, E.A. Isolation and identification of 1,2-diacylglyceryl-(3)-O-4′-(N,N,N-trimethyl)homoserine from the soil amoeba, Acanthamoeba castellanii. J. Lipid Res. 1986, 27, 1182–1189. [Google Scholar] [CrossRef]
- Korn, E.D.; Wright, P.L. Macromolecular composition of an amoeba plasma membrane. J. Biol. Chem. 1973, 248, 439–447. [Google Scholar] [CrossRef]
- Karaś, M.A.; Russa, R. New long chain bases in lipophosphonoglycan of Acanthamoeba castellanii. Lipids 2013, 48, 639–650. [Google Scholar] [CrossRef]
- Murakami, H.; Nobusawa, T.; Hori, K.; Shimojima, M.; Ohta, H. Betaine lipid is crucial for adapting to low temperature and phosphate deficiency in Nannochloropsis. Plant Physiol. 2018, 177, 181–193. [Google Scholar] [CrossRef]
- Popko, J.; Herrfurth, C.; Feussner, K.; Ischebeck, T.; Iven, T.; Haslam, R.; Hamilton, M.; Sayanova, O.; Napier, J.; Khozin-Goldberg, I.; et al. Metabolome analysis reveals betaine lipids as major source for triglyceride formation, and the accumulation of sedoheptulose during nitrogen-starvation of Phaeodactylum tricornutum. PLoS ONE 2016, 11, e0164673. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Jia, L.J.; Yuan, T.L.; Zhang, D.; Guo, Y.; Wang, Y.; Tang, W.H. Cellular tracking and gene profiling of Fusarium graminearum during maize stalk rot disease development elucidates its strategies in confronting phosphorus limitation in the host apoplast. PLoS Pathog. 2016, 12, e1005485. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Gallant, P.; McGinn, P. Mono- and digalactosyldiacylglycerols: Potent nitric oxide inhibitors from the marine microalga Nannochloropsis granulata. J. Appl. Phycol. 2013, 25, 349–357. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Berk, S.G.; Ting, R.S.; Turner, G.W.; Ashburn, R.J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 1998, 64, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.A.; Turska-Szewczuk, A.; Marczak, M.; Jaszek, M.; Janczarek, M.; Dworaczek, K.; Stefaniuk, D.; Wydrych, J. A mutation in the Mesorhizobium loti oatB gene alters the physicochemical properties of the bacterial cell wall and reduces survival inside Acanthamoeba castellanii. Int. J. Mol. Sci. 2018, 19, 3510. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. A liquid chromatography–mass spectrometry method for analysis of intact fatty-acid-based lipids extracted from soil. Eur. J. Soil Sci. 2018, 69, 791–803. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Y.; Mu, T.; Xu, J.; Zhou, C.; Yan, X. Simultaneous structural identification of diacylglyceryl-N-trimethylhomoserine (DGTS) and diacylglycerylhydroxymethyl-N,N,N-trimethyl-β-alanine (DGTA) in microalgae using dual Li+/H+ adduct ion mode by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F. Complete structural characterization of ceramides as [M-H]- ions by multiple-stage linear ion trap mass spectrometry. Biochimie 2016, 130, 63–75. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Schmitz, G.; Müller, G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991, 32, 1539–1570. [Google Scholar] [CrossRef]
- Padzik, M.; Starościak, B.; Szaflik, J.P.; Pietruczuk-Padzik, A.; Siczek, P.; Chomicz, L. Assessment of in vitro dynamics of pathogenic Acanthamoeba strains originating from contact lens wearers with infectious keratitis. Ann. Parasitol. 2016, 62, 331–336. [Google Scholar] [CrossRef]
- Henriquez, F.L.; Mooney, R.; Bandel, T.; Giammarini, E.; Zeroual, M.; Fiori, P.L.; Margarita, V.; Rappelli, P.; Dessì, D. Paradigms of protist/bacteria symbioses affecting human health: Acanthamoeba species and Trichomonas vaginalis. Front. Microbiol. 2021, 11, 616213. [Google Scholar] [CrossRef] [PubMed]
- Denoncourt, A.M.; Paquet, V.E.; Charette, S.J. Potential role of bacteria packaging by protozoa in the persistence and transmission of pathogenic bacteria. Front. Microbiol. 2014, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Otaki, R.; Iijima, Y.; Kumagai, E.; Aoki, M.; Tsuzuki, M.; Fujiwara, S.; Sato, N. Diacylglyceryl-N,N,N-trimethylhomoserine-dependent lipid remodeling in a green alga, Chlorella kessleri. Commun. Biol. 2022, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.S.; Du, Z.; Chen, Z. Membrane lipid biosynthesis in Chlamydomonas reinhardtii. In vitro biosynthesis of diacylglyceryltrimethylhomoserine. Plant Physiol. 2001, 125, 423–429. [Google Scholar] [CrossRef]
- Palusinska-Szysz, M.; Kania, M.; Turska-Szewczuk, A.; Danikiewicz, W.; Russa, R.; Fuchs, B. Identification of unusual phospholipid fatty acyl compositions of Acanthamoeba castellanii. PLoS ONE 2014, 9, e101243. [Google Scholar] [CrossRef]
- Cañavate, J.P.; Armada, I.; Ríos, J.L.; Hachero-Cruzado, I. Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 2016, 124, 68–78. [Google Scholar] [CrossRef]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar lipidomic profile shows Chlorococcum amblystomatis as a promising source of value-added lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef]
- Vogel, G.; Eichenberger, W. Betaine lipids in lower plants. Biosynthesis of DGTS and DGTA in Ochromonas danica (Chrysophyceae) and the possible role of DGTS in lipid metabolism. Plant Cell Physiol. 1992, 33, 427–436. [Google Scholar] [CrossRef]
- Denoncourt, A.M.; Durocher, A.F.; Paquet, V.E.; Charette, S.J. The fate of multilamellar bodies produced and secreted by Dictyostelium discoideum amoebae. Eur. J. Cell Biol. 2017, 96, 767–773. [Google Scholar] [CrossRef]
- Alizadeh, H.; Neelam, S.; Niederkorn, J.Y. Role of activated macrophages in Acanthamoeba keratitis. J. Parasitol. 2007, 93, 1114–1120. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New diacylglyceryltrimethylhomoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Heung, L.J.; Luberto, C.; Del Poeta, M. Role of sphingolipids in microbial pathogenesis. Infect. Immun. 2006, 74, 28–39. [Google Scholar] [CrossRef]
- Custódio, L.; Mendes, L.A.; Alvares, D.S.; Moreto, J.A.; Slade, N.B.L. Charge and rigidity effects on the encapsulation of quercetin by multilamellar vesicles. Bull. Mater. Sci. 2022, 45, 159. [Google Scholar] [CrossRef]
- Nele, V.; Holme, M.N.; Kauscher, U.; Thomas, M.R.; Doutch, J.J.; Stevens, M. Effect of formulation method, lipid composition, and pegylation on vesicle lamellarity: A small-angle neutron scattering study. Langmuir 2019, 35, 6064–6074. [Google Scholar] [CrossRef] [PubMed]
- Czolkoss, S.; Fritz, C.; Hölzl, G.; Aktas, M. Two distinct cardiolipin synthases operate in agrobacterium tumefaciens. PLoS ONE 2016, 11, e0160373. [Google Scholar] [CrossRef]

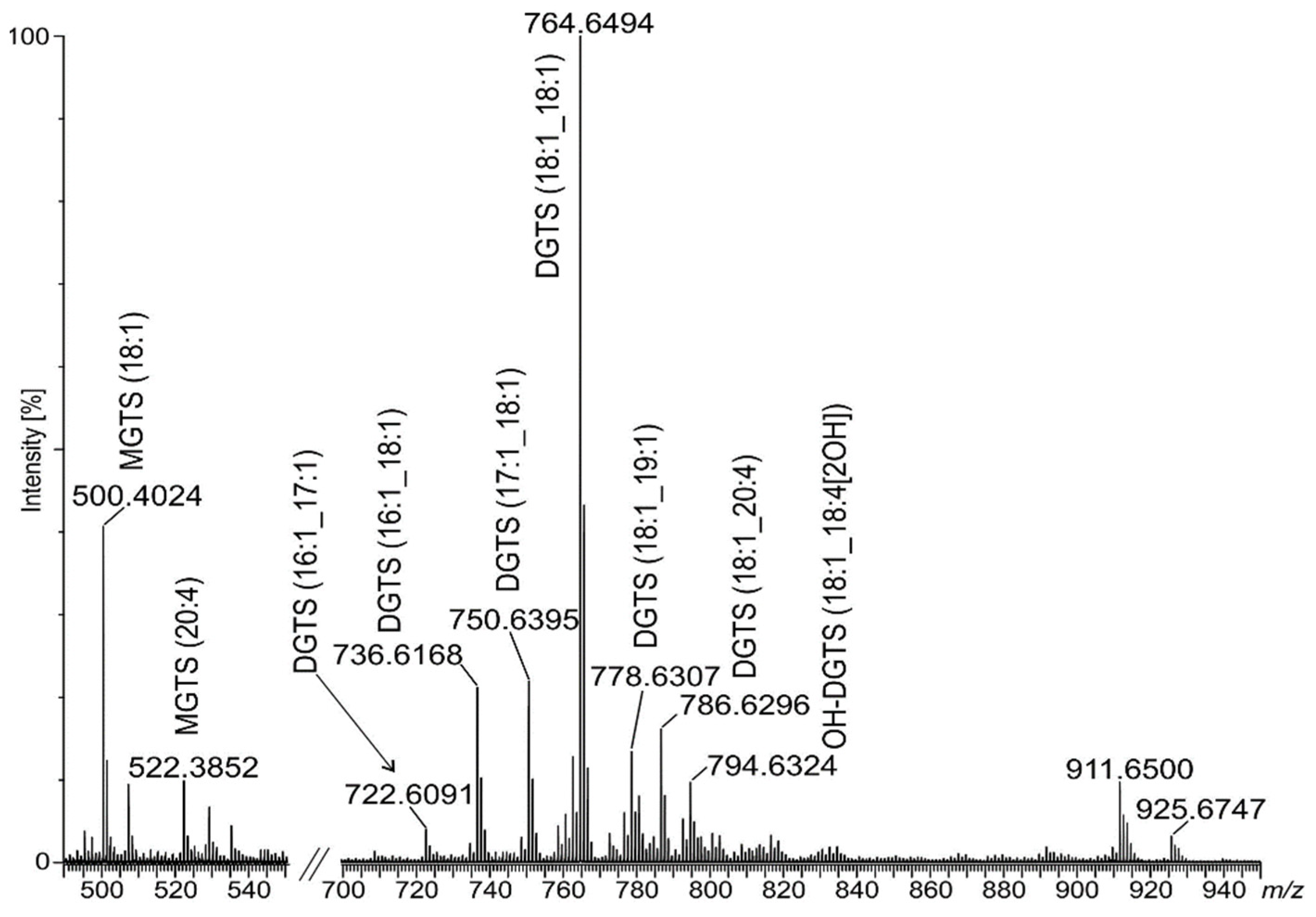

| Lipid Ion/Formula | Obs m/z | Calc m/z | Product Ion |
|---|---|---|---|
| MGTS (18:1_0) + H C28H54O6N | 500.4024 | 500.3951 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2 |
| MGTS (20:4_0) + H C30H52O6N | 522.3852 | 522.3795 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL(C2H4N(CH3)3) 435.3200:MS2 |
| a DGTS/DGTA (32:0) + H C42H82O7N | 712.6046 | 712.6091 | - |
| DGTS (16:1_17:1) + H C43H80O7N | 722.6091 | 722.5935 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL[FA(17:1)] 454.3679:MS2, NL[FA(17:1)] + H2O (472.3710):MS2, NL[FA(16:1)] 468.3758:MS2, NL[FA(16:1)] + H2O (486.3916):MS2 |
| DGTS (16:1_18:1) + H C44H82O7N | 736.6168 | 736.6091 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL[FA(18:1)] 454.3679:MS2, NL[FA(18:1)] + H2O (472.3710):MS2, NL[FA(16:1)] 482.3949:MS2, NL[FA(16:1)] + H2O (500.4024):MS2 |
| DGTS (17:1_18:4) + H C45H78O7N | 744.5764 | 744.5778 | (C7H14O2N) 144.1020:MS3, (C7H16O3N) 162.1134:MS3, (C10H22O5N) 236.1521: MS2, NL[FA(18:4)] 468.3679:MS2, NL[FA(18:4)] + H2O (486.3679):MS2,NL(C2H4N(CH3)3) 657.5064:MS2 |
| DGTS (17:1_18:1) + H C45H84O7N | 750.6395 | 750.6248 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N)+ 236.1568: MS2,NL[FA(18:1)] 468.3758:MS2, NL[FA(17:1)] 482.3949:MS2, NL[FA(18:1)] + H2O (486.3916):MS2, NL[FA(17:1)] + H2O (500.4024):MS2 |
| b DGTS (18:1_18:4) + H C46H80O7N | 758.6021 | 758.5935 | (C7H14O2N) 144.1020:MS3, (C7H16O3N) 162.1134:MS3, (C10H22O5N) 236.1521: MS2, NL[FA(18:4)] 482.3869:MS2, NL[FA(18:4)] + H2O (500.3942):MS2, NL(C2H4N(CH3)3) 671.5272:MS2 |
| b DGTS (18:1/18:1) + H C46H86O7N | 764.6494 | 764.6404 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL[FA(18:1)] 482.3949:MS2, NL[FA(18:1)] + H2O (500.4024):MS2 |
| DGTS (18:1_19:1) + H C47H88O7N | 778.6428 | 778.6561 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL[FA(19:1)] 482.3949:MS2, NL[FA(18:1)] 496.3694:MS2, NL[FA(19:1)] + H2O (500.4024):MS2, NL[FA(18:1)] + H2O (514.3845):MS2 |
| DGTS (18:2_20:4) + H C48H82O7N | 784.6108 | 784.6091 | (C7H14O2N) 144.1020:MS2, (C10H22O5N) 236.1521: MS2, NL[FA(18:2)] 504.3665:MS2, NL[FA(18:2)] + H2O (522.3866):MS2, NL[C2H4N(CH3)3] 697.5432:MS2 |
| b DGTS (18:1_20:4) + H C48H84O7N | 786.6296 | 786.6248 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL[FA(20:4)] 482.3949:MS2, NL[FA(20:4)] + H2O (500.4122):MS2, NL[C2H4N(CH3)3] 699.5732:MS2 |
| OH-DGTS (18:1_18:4[2OH]) + H C46H84O9N | 794.6324 | 794.6146 | (C7H14O2N) 144.1049:MS3, (C7H16O3N) 162.1168:MS3, (C10H22O5N) 236.1568:MS2, NL[FA(18:4)[2OH]] 482.3949:MS2, NL[FA(18:4)[2OH]] + H2O (500.4122):MS2, NL[FA(18:1)] 512.3668:MS2, NL[FA(18:1)] + H2O (528.3685):MS2, NL(2H2O) 758.6138, NL(H2O) 776.6221 |
| b DGTA (19:1_18:4) + H C47H82O7N | 772.6129 | 772.6091 | (C7H14O2N) 144.1020:MS3, (C7H16O3N) 162.1134:MS3, (C10H22O5N) 236.1521:MS2, NL[FA(18:4)] 496.3694:MS2, NL[FA(18:4)] + H2O (514.3760):MS2, NL[C2H4N(CH3)3] 685.5473:MS2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaś, M.A.; Turska-Szewczuk, A.; Komaniecka, I.; Łotocka, B. Lipidomics Analysis of Multilamellar Bodies Produced by Amoeba Acanthamoeba castellanii in Co-Culture with Klebsiella aerogenes. Pathogens 2023, 12, 411. https://doi.org/10.3390/pathogens12030411
Karaś MA, Turska-Szewczuk A, Komaniecka I, Łotocka B. Lipidomics Analysis of Multilamellar Bodies Produced by Amoeba Acanthamoeba castellanii in Co-Culture with Klebsiella aerogenes. Pathogens. 2023; 12(3):411. https://doi.org/10.3390/pathogens12030411
Chicago/Turabian StyleKaraś, Magdalena Anna, Anna Turska-Szewczuk, Iwona Komaniecka, and Barbara Łotocka. 2023. "Lipidomics Analysis of Multilamellar Bodies Produced by Amoeba Acanthamoeba castellanii in Co-Culture with Klebsiella aerogenes" Pathogens 12, no. 3: 411. https://doi.org/10.3390/pathogens12030411
APA StyleKaraś, M. A., Turska-Szewczuk, A., Komaniecka, I., & Łotocka, B. (2023). Lipidomics Analysis of Multilamellar Bodies Produced by Amoeba Acanthamoeba castellanii in Co-Culture with Klebsiella aerogenes. Pathogens, 12(3), 411. https://doi.org/10.3390/pathogens12030411






