Major Emerging Fungal Diseases of Reptiles and Amphibians
Abstract
1. Introduction
2. Overview of Fungal Pathogens
2.1. Reptiles
2.1.1. Nannizziopsis spp.
2.1.2. Ophidiomyces ophidiicola
2.2. Amphibians
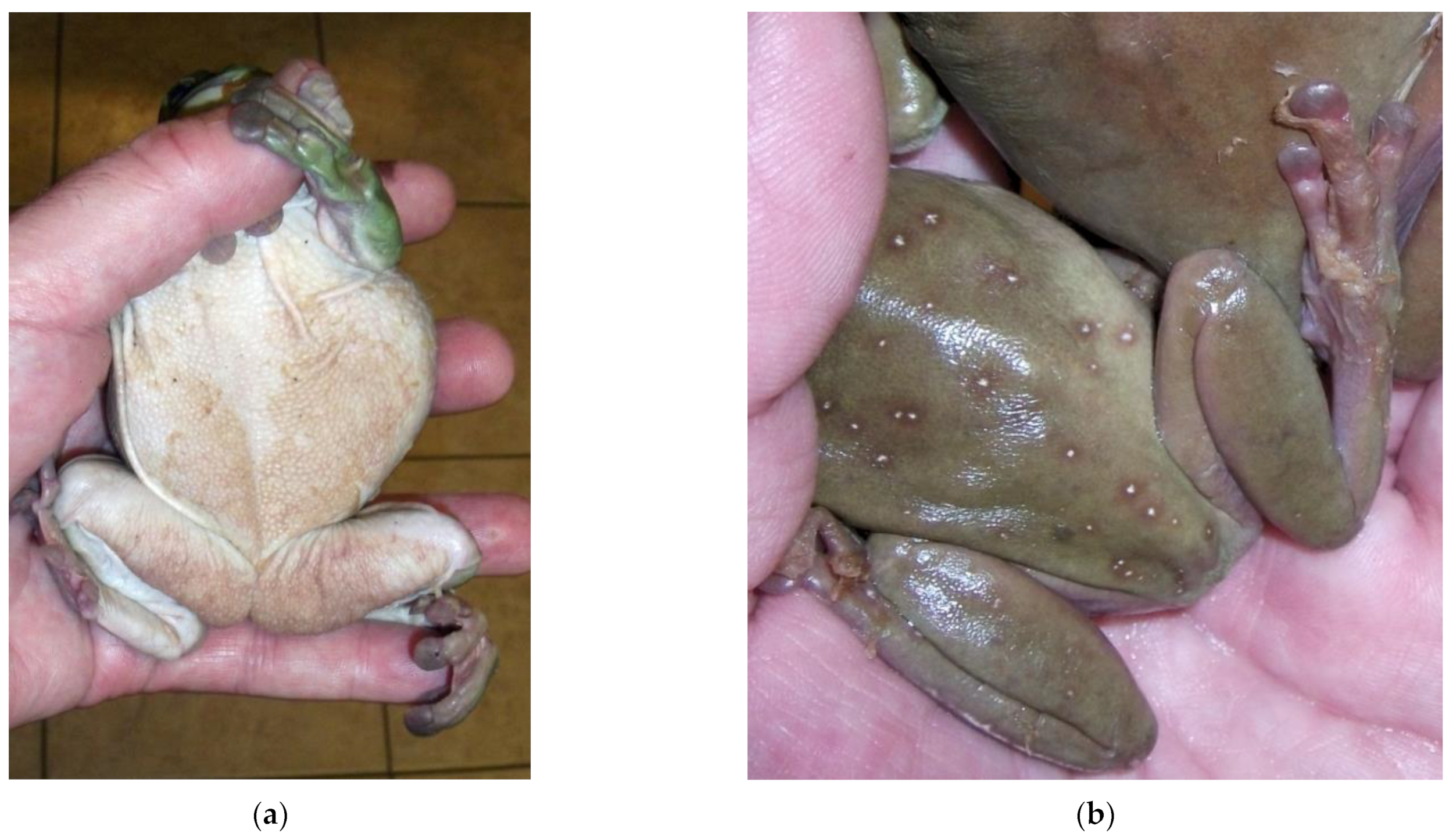
2.2.1. Chytridiomycosis
Batrachochytrium dendrobatidis
Batrachochytrium salamandrivorans
3. Host–Pathogen Relationship
3.1. Host-Related Factors
3.2. Pathogen-Related Factors
3.3. Environment-Related Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McArthur, D.B. Emerging Infectious Diseases. Nurs. Clin. North Am. 2019, 54, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Weldon, C. Chytridiomycosis, an Emerging Infectious Disease of Amphibians in South Africa. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2005. [Google Scholar]
- Gibbons, P.; Steffes, Z. Emerging Infectious Diseases of Chelonians. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.; Knowles, S.; Lankton, J.; Michell, K.; Edwards, J.; Kapfer, J.; Staffen, R.; Wild, E.; Schmidt, K.; Ballmann, A.; et al. Snake Fungal Disease: An Emerging Threat to Wild Snakes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150457. [Google Scholar] [CrossRef] [PubMed]
- Latney, L.V.; Wellehan, J. Selected Emerging Infectious Diseases of Squamata. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.; Beckmann, K.; Perkins, M.; Fitzpatrick, L.; Cromie, R.; Redbond, J.; O’Brien, M.; Ghosh, P.; Shelton, J.; Fisher, M. Emerging Disease in UK Amphibians. Vet. Rec. 2015, 176, 468. [Google Scholar] [CrossRef]
- Xie, G.Y.; Olson, D.H.; Blaustein, A.R. Projecting the Global Distribution of the Emerging Amphibian Fungal Pathogen, Batrachochytrium Dendrobatidis, Based on IPCC Climate Futures. PLoS ONE 2016, 11, e0160746. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Urbina, J.; Snyder, P.W.; Reynolds, E.; Dang, T.; Hoverman, J.T.; Han, B.; Olson, D.H.; Searle, C.; Hambalek, N.M. Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies. Diversity 2018, 10, 81. [Google Scholar] [CrossRef]
- Latney, L.V.; Klaphake, E. Selected Emerging Infectious Diseases of Amphibians. Vet. Clin. North Am. Exot. Anim. Pract. 2020, 23, 397–412. [Google Scholar] [CrossRef]
- Adamovicz, L.; Allender, M.C.; Gibbons, P.M. Emerging Infectious Diseases of Chelonians: An Update. Vet. Clin. North Am.-Exot. Anim. Pract. 2020, 23, 263–283. [Google Scholar] [CrossRef]
- Ladner, J.T.; Palmer, J.M.; Ettinger, C.L.; Stajich, J.E.; Farrell, T.M.; Glorioso, B.M.; Lawson, B.; Price, S.J.; Stengle, A.G.; Grear, D.A.; et al. The Population Genetics of the Causative Agent of Snake Fungal Disease Indicate Recent Introductions to the USA. PLoS Biol. 2022, 20, e3001676. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Martel, A.; Asselberghs, J.; Bales, E.; Beukema, W.; Bletz, M.; Dalbeck, L.; Goverse, E.; Kerres, A.; Kinet, T.; et al. Expanding Distribution of Lethal Amphibian Fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 2016, 22, 1286–1288. [Google Scholar] [CrossRef]
- Woodburn, D.; Miller, A.; Allender, M.; Maddox, C.; Terio, K. Emydomyces testavorans, a New Genus and Species of Onygenalean Fungus Isolated from Shell Lesions of Freshwater Aquatic Turtles. J. Clin. Microbiol. 2019, 57, e00628-18. [Google Scholar] [CrossRef]
- Parrish, K.; Kirkland, P.D.; Skerratt, L.F.; Ariel, E. Nidoviruses in Reptiles: A Review. Front. Vet. Sci. 2021, 8, 733404. [Google Scholar] [CrossRef]
- Baker, S.; Kessler, E.; Darville-Bowleg, L.; Merchant, M. Different Mechanisms of Serum Complement Activation in the Plasma of Common (Chelydra serpentina) and Alligator (Macrochelys temminckii) Snapping Turtles. PLoS ONE 2019, 14, e0217626. [Google Scholar]
- Haynes, E.; Allender, M. History, Epidemiology, and Pathogenesis of Ophidiomycosis: A Review. Herpetol. Rev. 2021, 52, 521–536. [Google Scholar]
- Cabañes, F. Chytridiomycosis in Amphibians. Rev. Iberoam. Micol. 2019, 36, 171–172. [Google Scholar] [CrossRef]
- Latney, L.V.; Wellehan, J.F. Selected Emerging Infectious Diseases of Squamata: An Update. Vet. Clin. North Am.-Exot. Anim. Pract. 2020, 23, 353–371. [Google Scholar] [CrossRef]
- Stchigel, A.M.; Sutton, D.; Cano, J.; Cabañes, F.; Abarca, L.; Tintelnot, K.; Wickes, B.; García, D.; Guarro, J. Phylogeny of Chrysosporia Infecting Reptiles: Proposal of the New Family Nannizziopsiaceae and Five New Species. Persoonia 2013, 31, 86–100. [Google Scholar] [CrossRef]
- Paré, J.; Sigler, L. An Overview of Reptile Fungal Pathogens in the Genera nannizziopsis, paranannizziopsis, and ophidiomyces. J. Herpetol. Med. Surg. 2016, 26, 46–53. [Google Scholar] [CrossRef]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Ostell, J.; Pruitt, K.D.; Karsch-Mizrachi, I. GenBank. Nucleic Acids Res. 2020, 48, D84–D86. [Google Scholar] [CrossRef]
- Paré, J.; Wellehan, J.; Perry, S.; Scheelings, T.; Keller, K.; Boyer, T. Onygenalean Dermatomycoses (Formerly Yellow Fungus Disease, Snake Fungal Disease) in Reptiles. J. Herpetol. Med. Surg. 2021, 30, 198–209. [Google Scholar] [CrossRef]
- Allain, S.; Duffus, A.; Marschang, R. Editorial: Emerging Infections and Diseases of Herpetofauna. Front. Vet. Sci 2022, 9, 9616. [Google Scholar] [CrossRef] [PubMed]
- Paré, J.A.; Sigler, L.; Hunter, D.; Summerbell, R.; Smith, D.; Machin, K. Cutaneous Mycoses in Chameleons Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii (Apinis) Currah. J. Zoo Wildl. Med. 1997, 28, 443–453. [Google Scholar] [PubMed]
- Bowman, M.; Paré, J.; Sigler, L.; Naeser, J.; Sladky, K.; Hanley, C.; Helmer, P.; Phillips, L.; Brower, A.; Porter, R. Deep Fungal Dermatitis in Three Inland Bearded Dragons (Pogona vitticeps) Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii. Med. Mycol. 2007, 45, 371–376. [Google Scholar] [CrossRef]
- Mitchell, M.A.; Walden, M.R. Chrysosporium Anamorph Nannizziopsis vriesii: An Emerging Fungal Pathogen of Captive and Wild Reptiles. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 659–668. [Google Scholar] [CrossRef]
- Sigler, L.; Hambleton, S.; Paré, J. Molecular Characterization of Reptile Pathogens Currently Known as Members of the Chrysosporium Anamorph of Nannizziopsis vriesii Complex and Relationship with Some Human-Associated Isolates. J. Clin. Microbiol. 2013, 51, 3338–3357. [Google Scholar] [CrossRef]
- Peterson, N.; Rose, K.; Shaw, S.; Hyndman, T.; Sigler, L.; Kurtböke, D.; Llinas, J.; Littleford Colquhoun, B.; Cristescu, R.; Frere, C. Cross-Continental Emergence of Nannizziopsis Barbatae Disease May Threaten Wild Australian Lizards. Sci. Rep. 2020, 10, 20976. [Google Scholar] [CrossRef]
- Johnson, R.; Sangster, C.; Sigler, L.; Hambleton, S.; Paré, J.A. Deep Fungal Dermatitis Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii in Captive Coastal Bearded Dragons (Pogona barbata). Aust. Vet. J. 2011, 89, 515–519. [Google Scholar] [CrossRef]
- Gentry, S.L.; Lorch, J.M.; Lankton, J.S.; Pringle, A. Koch’s Postulates: Confirming Nannizziopsis guarroi as the Cause of Yellow Fungal Disease in Pogona Vitticeps. Mycologia 2021, 113, 1253–1263. [Google Scholar] [CrossRef]
- Wellehan, J.; Divers, S. Mycology. In Mader’s Reptile and Amphibian Medicine and Surgery; Divers, S., Stahl, S., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 270–280. [Google Scholar]
- Hill, A.; Sandy, J.; Begg, A. Mycotic Dermatitis in Juvenile Freshwater Crocodiles (Crocodylus johnstoni) Caused by Nannizziopsis crocodili. J. Zoo Wildl. Med. 2019, 50, 225–230. [Google Scholar] [CrossRef]
- Paré, J.A.; Coyle, K.A.; Sigler, L.; Maas, A.K., III; Mitchell, R.L. Pathogenicity of the Chrysosporium Anamorph of Nannizziopsis vriesii for Veiled Chameleons (Chamaeleo calyptratus). Med. Mycol. 2006, 44, 25–31. [Google Scholar] [CrossRef]
- Hellebuyck, T.; Pasmans, F.; Haesebrouck, F.; Martel, A. Dermatological Diseases in Lizards. Vet. J. 2012, 193, 38–45. [Google Scholar] [CrossRef]
- Hellebuyck, T.; Scheelings, T. Dermatology—Skin. In Mader’s Reptile and Amphibian Medicine and Surgery; Divers, S., Stahl, S., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 699–711. [Google Scholar]
- Schneider, J.; Heydel, T.; Klasen, L.; Pees, M.; Schrödl, W.; Schmidt, V. Characterization of Nannizziopsis guarroi with Genomic and Proteomic Analysis in Three Lizard Species. Med. Mycol. 2018, 56, 610–620. [Google Scholar] [CrossRef]
- Foltin, E.T.; Keller, K.A. Successful Treatment of Nannizziopsis guarroi Infection Using Systemic Terbinafine in a Central Bearded Dragon (Pogona vitticeps). J. Herpetol. Med. Surg. 2022, 32, 20–25. [Google Scholar] [CrossRef]
- Van Waeyenberghe, L.; Baert, K.; Pasmans, F.; Van Rooij, P.; Hellebuyck, T.; Beernaert, L.; Backer, P.; Haesebrouck, F.; Martel, A. Voriconazole, a Safe Alternative for Treating Infections Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii in Bearded Dragons (Pogona vitticeps). Med. Mycol. 2010, 48, 880–885. [Google Scholar] [CrossRef]
- McEntire, M.S.; Reinhart, J.M.; Cox, S.K.; Keller, K.A. Single-Dose Pharmacokinetics of Orally Administered Terbinafine in Bearded Dragons (Pogona vitticeps) and the Antifungal Susceptibility Patterns of Nannizziopsis guarroi. Am. J. Vet. Res. 2022, 83, 256–263. [Google Scholar] [CrossRef]
- Jourdan, B.; Hemby, C.; Allender, M.C.; Levy, I.; Foltin, E.; Keller, K.A. Effectiveness of Common Disinfecting Agents Against Isolates of Nannizziopsis guarroi. J. Herpetol. Med. Surg. 2022. [Google Scholar] [CrossRef]
- Gray, M.; Duffus, A.; Haman, K.; Harris, R.; Allender, M.; Thompson, T.; Christman, M.; Sacredote-Velat, A.; Sprague, L.; Williams, J.; et al. Pathogen Surveillance in Herpetofaunal Populations: Guidance on Study Design, Sample Collection, Biosecurity, and Intervention Strategies. Herpetol. Rev. 2017, 48, 334. [Google Scholar]
- Rajeev, S.; Sutton, D.; Wickes, B.; Miller, D.; Giri, D.; Meter, M.; Thompson, E.; Rinaldi, M.; Romanelli, A.; Cano, J.; et al. Isolation and Characterization of a New Fungal Species, Chrysosporium ophiodiicola, from a Mycotic Granuloma of a Black Rat Snake (Elaphe obsoleta obsoleta). J. Clin. Microbiol. 2009, 47, 1264–1268. [Google Scholar] [CrossRef]
- Nichols, D.; Weyant, R.; Lamirande, E.; Sigler, L.; Mason, R. Fatal Mycotic Dermatitis in Captive Brown Tree Snakes (Boiga irregularis). J. Zoo Wildl. Med. 1999, 30, 111–118. [Google Scholar]
- Cheatwood, J.; Jacobson, E.; May, P.; Farrell, T.; Homer, B.; Samuelson, D.; Kimbrough, J. An Outbreak of Fungal Dermatitis and Stomatitis in a Free-Ranging Population of Pigmy Rattlesnakes (Sistrurus miliarius barbouri) in Florida. J. Wildl. Dis. 2003, 39, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Steeil, J.; Neiffer, D.; Evans, M.; Peters, A.; Allender, M.; Cartoceti, A. Retrospective Review of Ophidiomycosis (Ophidiomyces ophiodiicola) at the Smithsonian’s National Zoological Park (1983–2017). J. Zoo Wildl. Med. 2021, 52, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.; Price, S.; Lankton, J.; Drayer, A. Confirmed Cases of Ophidiomycosis in Museum Specimens from as Early as 1945, United States. Emerg. Infect. Dis. J. 2021, 27, 1986. [Google Scholar] [CrossRef] [PubMed]
- Origgi, F.; Pisano, S.; Glaizot, O.; Hertwig, S.; Schmitz, A.; Ursenbacher, S. Ophiodimyces Ophiodiicola, Etiologic Agent of Snake Fungal Disease, in Europe since Late 1950s. Emerg. Infect. Dis. 2022, 28, 2064–2068. [Google Scholar] [CrossRef]
- Di Nicola, M.R.; Coppari, L.; Notomista, T.; Marini, D. Ophidiomyces ophidiicola Detection and Infection: A Global Review on a Potential Threat to the World’s Snake Populations. Eur. J. Wildl. Res. 2022, 68, 64. [Google Scholar] [CrossRef]
- McBride, M.P.; Wojick, K.B.; Georoff, T.A.; Kimbro, J.; Garner, M.M.; Wang, X.; Childress, A.L.; Wellehan, J.F.X. Ophidiomyces ophiodiicola Dermatitis in Eight Free-Ranging Timber Rattlesnakes (Crotalus horridus) from Massachusetts. J. Zoo Wildl. Med. 2015, 46, 86–94. [Google Scholar] [CrossRef]
- Lindemann, D.M.; Allender, M.C.; Rzadkowska, M.; Archer, G.; Kane, L.; Baitchman, E.; Driskell, E.A.; Chu, C.T.; Singh, K.; Hsiao, S.-H.; et al. Pharmacokinetics, Efficacy, and Safety of Voriconazole and Itraconazole in Healthy Cottonmouths (Agkistrodon piscivorus) and Massasauga Rattlesnakes (Sistrurus catenatus) with Snake Fungal Disease. J. Zoo Wildl. Med. 2017, 48, 757–766. [Google Scholar] [CrossRef]
- Meier, G.; Notomista, T.; Marini, D.; Ferri, V. First Case of Snake Fungal Disease Affecting a Free-Ranging Natrix natrix (Linnaeus, 1758) in Ticino Canton, Switzerland. Herpetol. Notes 2018, 11, 885–891. [Google Scholar]
- Allender, M.C.; Raudabaugh, D.B.; Gleason, F.H.; Miller, A.N. The Natural History, Ecology, and Epidemiology of Ophidiomyces ophiodiicola and Its Potential Impact on Free-Ranging Snake Populations. Fungal Ecol. 2015, 17, 187–196. [Google Scholar] [CrossRef]
- McKenzie, C.; Oesterle, P.; Stevens, B.; Shirose, L.; Lillie, B.; Davy, C.; Jardine, C.; Nemeth, N. Pathology Associated with Ophidiomycosis in Wild Snakes in Ontario, Canada. Can. Vet. J. 2020, 2020, 957–962. [Google Scholar]
- Sun, P.; Yang, C.K.; Li, W.-T.; Lai, W.-Y.; Chen, F.; Huang, H.; Yu, P. Infection with Nannizziopsis guarroi and Ophidiomyces ophiodiicola in Reptiles in Taiwan. Transbound. Emerg. Dis. 2021, 69, 764–775. [Google Scholar] [CrossRef]
- Grioni, A.; To, K.; Crow, P.; Rose-Jeffreys, L.; Ching, K.; Chu, L.; Hill, F.; Chan, K.H.-K.; Cheung, K. Detection of Ophidiomyces Ophidiicola in a Wild Burmese Python (Python bivittatus) in Hong Kong SAR, China. J. Herpetol. Med. Surg. 2021, 31, 283–291. [Google Scholar] [CrossRef]
- Takami, Y.; Une, Y.; Mitsui, I.; Hemmi, C.; Takaki, Y.; Hosoya, T.; Nam, K.-O. First Report of Emerging Snake Fungal Disease Caused by Ophidiomyces ophiodiicola from Asia in Imported Captive Snakes in Japan. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lorch, J.; Lankton, J.; Werner, K.; Falendysz, E.; McCurley, K.; Blehert, D. Experimental Infection of Snakes with Ophidiomyces ophiodiicola Causes Pathological Changes That Typify Snake Fungal Disease. mBio 2015, 6, e01534-15. [Google Scholar] [CrossRef]
- Bohuski, E.; Lorch, J.M.; Griffin, K.M.; Blehert, D.S. TaqMan Real-Time Polymerase Chain Reaction for Detection of Ophidiomyces ophiodiicola, the Fungus Associated with Snake Fungal Disease. BMC Vet. Res. 2015, 11, 95. [Google Scholar] [CrossRef]
- Paré, J.; Sigler, L.; Rypien, K.; Gibas, C. Cutaneous Mycobiota of Captive Squamate Reptiles with Notes on the Scarcity of Chrysosporium Anamorph of Nannizziopsis vriesii. J. Herpetol. Med. Surg. 2003, 13, 10–15. [Google Scholar] [CrossRef]
- Stengle, A.G.; Farrell, T.M.; Freitas, K.S.; Lind, C.M.; Price, S.J.; Butler, B.O.; Tadevosyan, T.; Isidoro-Ayza, M.; Taylor, D.R.; Winzeler, M.; et al. Evidence of Vertical Transmission of the Snake Fungal Pathogen Ophidiomyces ophiodiicola. J. Wildl. Dis. 2019, 55, 961–964. [Google Scholar] [CrossRef]
- Kane, L.P.; Allender, M.C.; Archer, G.; Leister, K.; Rzadkowska, M.; Boers, K.; Souza, M.; Cox, S. Pharmacokinetics of Nebulized and Subcutaneously Implanted Terbinafine in Cottonmouths (Agkistrodon piscivorus). J. Vet. Pharmacol. Ther. 2017, 40, 575–579. [Google Scholar] [CrossRef]
- Rzadkowska, M.; Allender, M.C.; O’Dell, M.; Maddox, C. Evaluation of Common Disinfectants Effective against Ophidiomyces ophiodiicola, the Causative Agent of Snake Fungal Disease. J. Wildl. Dis. 2016, 52, 759–762. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans Sp. Nov. Causes Lethal Chytridiomycosis in Amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef]
- Van Rooij, P.; Martel, A.; Haesebrouck, F.; Pasmans, F. Amphibian Chytridiomycosis: A Review with Focus on Fungus-Host Interactions. Vet. Res. 2015, 46, 137. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Whitaker, B. Amphibian Chytridiomycosis. In Mader’s Reptile and Amphibian Medicine and Surgery; Divers, S., Stahl, S., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 1292–1293. [Google Scholar]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis Gen. et Sp. Nov., a Chytrid Pathogenic to Amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Sonn, J.; Berman, S.; Richards Zawacki, C. The Influence of Temperature on Chytridiomycosis In Vivo. Ecohealth 2017, 14, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Hagman, M.; Alford, R. Patterns of Batrachochytrium dendrobatidis Transmission between Tadpoles in a High-Elevation Rainforest Stream in Tropical Australia. Dis. Aquat. Organ. 2015, 115, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a Chytrid Pathogen of Amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef]
- Stegen, G.; Pasmans, F.; Schmidt, B.; Rouffaer, L.; Praet, S.; Schaub, M.; Canessa, S.; Laudelout, A.; Kinet, T.; Adriaensen, C.; et al. Drivers of Salamander Extirpation Mediated by Batrachochytrium salamandrivorans. Nature 2017, 544, 353. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Spikmans, F.; Bosman, W.; Zeeuw, M.; Meij, T.; Goverse, E.; Kik, M.; Pasmans, F.; Martel, A. Rapid Enigmatic Decline Drives the Fire Salamander (Salamandra salamandra) to the Edge of Extinction in the Netherlands. Amphib.-Reptil. 2013, 34, 233–239. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Skerratt, L. Distribution of Batrachochytrium dendrobatidis and Pathology in the Skin of Green Tree Frogs Litoria Caerulea with Severe Chytridiomycosis. Dis. Aquat. Organ. 2006, 68, 65–70. [Google Scholar] [CrossRef]
- Kelly, M.; Pasmans, F.; Muñoz, J.F.; Shea, T.; Carranza, S.; Cuomo, C.; Martel, A. Diversity, Multifaceted Evolution, and Facultative Saprotrophism in the European Batrachochytrium salamandrivorans Epidemic. Nat. Commun. 2021, 12, 6688. [Google Scholar] [CrossRef]
- Hanlon, S.; Lynch, K.; Kerby, J. Batrachochytrium dendrobatidis Exposure Effects on Foraging Efficiencies and Body Size in Anuran Tadpoles. Dis. Aquat. Organ. 2015, 112, 237–242. [Google Scholar] [CrossRef]
- Borteiro, C.; Kolenc, F.; Verdes, J.; Martinez Debat, C.; Ubilla, M. Sensitivity of Histology for the Detection of the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis. J. Vet. Diagn. Investig. 2019, 31, 246–249. [Google Scholar] [CrossRef]
- Kamoroff, C.; Goldberg, C.; Grasso, R. Rapid Detection of the Amphibian Chytrid Fungus (Batrachochytrium dendrobatidis) Using In-Situ DNA Extraction and a Handheld Mobile Thermocycler. Authorea 2020. [Google Scholar] [CrossRef]
- Kamoroff, C.; Goldberg, C. Using Environmental DNA for Early Detection of Amphibian Chytrid Fungus Batrachochytrium dendrobatidis Prior to a Ranid Die Off. Dis. Aquat. Organ. 2017, 127, 75–79. [Google Scholar] [CrossRef]
- Lastra González, D.; Baláž, V.; Vojar, J.; Chajma, P. Dual Detection of the Chytrid Fungi Batrachochytrium Spp. with an Enhanced Environmental DNA Approach. J. Fungi 2021, 7, 258. [Google Scholar] [CrossRef]
- Osman, O.A.; Andersson, J.; Martin-Sanchez, P.M.; Eiler, A. National EDNA-Based Monitoring of Batrachochytrium dendrobatidis and Amphibian Species in Norway. Metabarcoding Metagenom. 2022, 6, e85199. [Google Scholar] [CrossRef]
- Congram, M.; Torres Vilaça, S.; Wilson, C.C.; Kyle, C.J.; Lesbarrères, D.; Wikston, M.J.H.; Beaty, L.; Murray, D.L. Tracking the Prevalence of a Fungal Pathogen, Batrachochytrium dendrobatidis (Chytrid Fungus), Using Environmental DNA. Environ. DNA 2022, 4, 687–699. [Google Scholar] [CrossRef]
- Mutschmann, F. Chytridiomycosis in Amphibians. J. Exot. Pet Med. 2015, 24, 276–282. [Google Scholar] [CrossRef]
- Kueneman, J.G.; Woodhams, D.C.; Harris, R.; Archer, H.M.; Knight, R.; McKenzie, V.J. Probiotic Treatment Restores Protection against Lethal Fungal Infection Lost during Amphibian Captivity. Proc. Biol. Sci. 2016, 283, 20161553. [Google Scholar] [CrossRef]
- Bletz, M.; Loudon, A.; Becker, M.; Bell, S.; Woodhams, D.; Minbiole, K.; Harris, R. Mitigating Amphibian Chytridiomycosis with Bioaugmentation: Characteristics of Effective Probiotics and Strategies for Their Selection and Use. Ecol. Lett. 2013, 16, 807–820. [Google Scholar] [CrossRef]
- Harrison, X.A.; Sewell, T.; Fisher, M.; Antwis, R.E. Designing Probiotic Therapies With Broad-Spectrum Activity Against a Wildlife Pathogen. Front. Microbiol. 2020, 10, 3134. [Google Scholar] [CrossRef]
- Blooi, M.; Martel, A.; Haesebrouck, F.; Vercammen, F.; Bonte, D.; Pasmans, F. Treatment of Urodelans Based on Temperature Dependent Infection Dynamics of Batrachochytrium salamandrivorans. Sci. Rep. 2015, 5, 8037. [Google Scholar] [CrossRef] [PubMed]
- Voyles, J.; Johnson, L.; Briggs, C.; Cashins, S.; Alford, R.; Berger, L.; Skerratt, L.; Speare, R.; Rosenblum, E. Temperature Alters Reproductive Life History Patterns in Batrachochytrium dendrobatidis, a Lethal Pathogen Associated with the Global Loss of Amphibians. Ecol. Evol. 2012, 2, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, M.; Richards-Zawacki, C. Elevated Temperature as a Treatment for Batrachochytrium dendrobatidis Infection in Captive Frogs. Dis. Aquat. Organ. 2011, 94, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Andre, S.; Parker, J.; Briggs, C. Effect of Temperature on Host Response to Batrachochytrium dendrobatidis Infection in the Mountain Yellow-Legged Frog (Rana muscosa). J. Wildl. Dis. 2008, 44, 716–720. [Google Scholar] [CrossRef]
- Geiger, C.; Küpfer, E.; Schär, S.; Wolf, S.; Schmidt, B. Elevated Temperature Clears Chytrid Fungus Infections from Tadpoles of the Midwife Toad, Alytes Obstetricans. Amphib.-Reptil. 2011, 32, 276–280. [Google Scholar] [CrossRef]
- Johnson, M.; Berger, L.; Philips, L.; Speare, R. Fungicidal Effects of Chemical Disinfectants, UV Light, Desiccation and Heat on the Amphibian Chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2004, 57, 255–260. [Google Scholar] [CrossRef]
- Poole, V.; Grow, S. Amphibian Husbandry Resource Guide, 2nd ed.; Poole, V., Grow, S., Eds.; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2012. [Google Scholar]
- Speare, R. Developing Management Strategies to Control Amphibian Diseases; School of Public Health and Tropical Medicine, James Cook University: Douglas, Australia, 2001; pp. 171–183. [Google Scholar]
- Garmyn, A.; Van Rooij, P.; Pasmans, F.; Hellebuyck, T.; Van Den Broeck, W.; Haesebrouck, F.; Martel, A. Waterfowl: Potential Environmental Reservoirs of the Chytrid Fungus Batrachochytrium dendrobatidis. PLoS ONE 2012, 7, e35038. [Google Scholar] [CrossRef]
- Hanlon, S.; Henson, J.; Kerby, J. Detection of Amphibian Chytrid Fungus on Waterfowl Integument in Natural Settings. Dis. Aquat. Organ. 2017, 126, 71–74. [Google Scholar] [CrossRef][Green Version]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Infectious Disease and Amphibian Population Declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef]
- Johnson, M.L.; Speare, R. Possible Modes of Dissemination of the Amphibian Chytrid Batrachochytrium dendrobatidis in the Environment. Dis. Aquat. Organ. 2005, 65, 181–186. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis Causes Amphibian Mortality Associated with Population Declines in the Rain Forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef]
- O’Hanlon, S.; Rieux, A.; Farrer, R.; Rosa, G.; Waldman, B.; Bataille, A.; Kosch, T.; Murray, K.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian Origin of Chytrid Fungi Causing Global Amphibian Declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef]
- Talley, B.; Muletz Wolz, C.; Vredenburg, V.; Fleischer, R.; Lips, K. A Century of Batrachochytrium dendrobatidis in Illinois Amphibians (1888–1989). Biol. Conserv. 2015, 182, 254–261. [Google Scholar] [CrossRef]
- Rodriguez, D.; Becker, C.G.; Pupin, N.C.; Haddad, C.F.B.; Zamudio, K.R. Long-term Endemism of Two Highly Divergent Lineages of the Amphibian-Killing Fungus in the Atlantic Forest of Brazil. Mol. Ecol. 2014, 23, 774–787. [Google Scholar] [CrossRef]
- Goka, K.; Yokoyama, J.; Une, Y.; Kuroki, T.; Suzuki, K.; Nakahara, M.; Kobayashi, A.; Inaba, S.; Mizutani, T.; Hyatt, A. Amphibian Chytridiomycosis in Japan: Distribution, Haplotypes and Possible Route of Entry into Japan. Mol. Ecol. 2009, 18, 4757–4774. [Google Scholar] [CrossRef]
- Soto-Azat, C.; Clarke, B.; Poynton, J.; Cunningham, A. Widespread Historical Presence of Batrachochytrium dendrobatidis in African Pipid Frogs. Divers. Distrib. 2010, 16, 126–131. [Google Scholar] [CrossRef]
- Ouellet, M.; Mikaelian, I.; Pauli, B.; Rodrigue, J.; Green, D. Historical Evidence of Widespread Chytrid Infection in North American Amphibian Populations. Conserv. Biol. 2005, 19, 1431–1440. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Walker, S.; Bosch, J.; Hyatt, A.D.; Cunningham, A.A.; Fisher, M.C. Chytrid Fungus in Europe. Emerg. Infect. Dis. 2005, 11, 1639–1641. [Google Scholar] [CrossRef]
- Davidson, E.; Parris, M.; Collins, J.; Longcore, J.; Pessier, A.; Brunner, J.; Beaupre, S. Pathogenicity and Transmission of Chytridiomycosis in Tiger Salamanders (Ambystoma tigrinum). Copeia 2003, 2003, 601–607. [Google Scholar] [CrossRef]
- Olson, D.; Ronnenberg, K.; Glidden, C.; Christiansen, K.; Blaustein, A. Global Patterns of the Fungal Pathogen Batrachochytrium dendrobatidis Support Conservation Urgency. Front. Vet. Sci. 2021, 8, 685877. [Google Scholar] [CrossRef]
- Gower, D.; Doherty-Bone, T.; Loader, S.; Wilkinson, M.; Kouete, M.; Tapley, B.; Orton, F.; Daniel, O.; Wynne, F.; Flach, E.; et al. Batrachochytrium dendrobatidis Infection and Lethal Chytridiomycosis in Caecilian Amphibians (Gymnophiona). Ecohealth 2013, 10, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Vredenburg, V.; Martel, A.; Pasmans, F.; Bell, R.; Blackburn, D.; Bletz, M.; Bosch, J.; Briggs, C.; Brown, R.; et al. Cryptic Diversity of a Widespread Global Pathogen Reveals Expanded Threats to Amphibian Conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, R.; Cunningham, A.; Daszak, P.; Apolo, A.; Perdomo, E.; Speranza, G. Emerging Pathogen of Wild Amphibians in Frogs (Rana catesbeiana) Farmed for International Trade. Emerg. Infect. Dis. 2003, 9, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Schloegel, L.; Picco, A.; Kilpatrick, A.; Davies, A.; Hyatt, A.; Daszak, P. Magnitude of the US Trade in Amphibians and Presence of Batrachochytrium dendrobatidis and Ranavirus Infection in Imported North American Bullfrogs (Rana catesbeiana). Biol. Conserv. 2009, 142, 1420–1426. [Google Scholar] [CrossRef]
- Bai, C.-M.; Garner, T.; Yiming, L. First Evidence of Batrachochytrium dendrobatidis in China: Discovery of Chytridiomycosis in Introduced American Bullfrogs and Native Amphibians in the Yunnan Province, China. EcoHealth 2010, 7, 127–134. [Google Scholar] [CrossRef]
- Weldon, C.; Preez, L.; Hyatt, A.; Muller, R.; Spears, R. Origin of the Amphibian Chytrid Fungus. Emerg. Infect. Dis. 2005, 10, 2100–2105. [Google Scholar] [CrossRef]
- Scheele, B.; Pasmans, F.; Skerratt, L.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.; Burrowes, P.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- González-Maya, J.; Belant, J.; Wyatt, S.; Schipper, J.; Cardenal, J.; Corrales, D.; Cruz-Lizano, I.; Hoepker, A.; Escobedo-Galván, A.; Castañeda, F.; et al. Renewing Hope: The Rediscovery of Atelopus Varius in Costa Rica. Amphib.-Reptil. 2013, 34, 573–578. [Google Scholar] [CrossRef]
- Belasen, A.M.; Russell, I.D.; Zamudio, K.R.; Bletz, M.C. Endemic Lineages of Batrachochytrium dendrobatidis Are Associated With Reduced Chytridiomycosis-Induced Mortality in Amphibians: Evidence From a Meta-Analysis of Experimental Infection Studies. Front. Vet. Sci. 2022, 9, 756686. [Google Scholar] [CrossRef]
- Schloegel, L.; Toledo, L.F.; Longcore, J.; Greenspan, S.; Vieira, C.; Lee, M.; Zhao, S.; Wangen, C.; Mosterio, C.; Hipolito, M.; et al. Novel, Panzootic and Hybrid Genotypes of Amphibian Chytridiomycosis Associated with the Bullfrog Trade. Mol. Ecol. 2012, 21, 5162–5177. [Google Scholar] [CrossRef]
- Jenkinson, T.S.; Betancourt Román, C.M.; Lambertini, C.; Valencia-Aguilar, A.; Rodriguez, D.; Nunes-de-Almeida, C.H.L.; Ruggeri, J.; Belasen, A.M.; da Silva Leite, D.; Zamudio, K.R.; et al. Amphibian-Killing Chytrid in Brazil Comprises Both Locally Endemic and Globally Expanding Populations. Mol. Ecol. 2016, 25, 2978–2996. [Google Scholar] [CrossRef]
- Martel, A.; Blooi, M.; Adriaensen, C.; Van Rooij, P.; Beukema, W.; Fisher, M.; Farrer, R.; Schmidt, B.; Tobler, U.; Goka, K.; et al. Recent Introduction of a Chytrid Fungus Endangers Western Palearctic Salamanders. Science 2014, 346, 630–631. [Google Scholar] [CrossRef]
- Fitzpatrick, L.; Pasmans, F.; Martel, A.; Cunningham, A. Epidemiological Tracing of Batrachochytrium salamandrivorans Identifies Widespread Infection and Associated Mortalities in Private Amphibian Collections. Sci. Rep. 2018, 8, 13845. [Google Scholar] [CrossRef]
- Sabino Pinto, J.; Bletz, M.; Hendrix, R.; Perl, R.; Martel, A.; Pasmans, F.; Lötters, S.; Mutschmann, F.; Schmeller, D.; Schmidt, B.; et al. First Detection of the Emerging Fungal Pathogen Batrachochytrium salamandrivorans in Germany. Amphib.-Reptil. 2015, 36, 411–416. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Van Nguyen, T.; Ziegler, T.; Pasmans, F.; Martel, A. Trade in Wild Anurans Vectors the Urodelan Pathogen Batrachochytrium salamandrivorans into Europe. Amphib.-Reptil. 2017, 38, 554–556. [Google Scholar] [CrossRef]
- Yuan, Z.; Martel, A.; Wu, J.; Praet, S.; Canessa, S.; Pasmans, F. Widespread Occurrence of an Emerging Fungal Pathogen in Heavily Traded Chinese Urodelan Species. Conserv. Lett. 2018, 11, e12436. [Google Scholar] [CrossRef]
- Laking, A.; Ngo, H.; Pasmans, F.; Martel, A.; Nguyen, T. Batrachochytrium salamandrivorans Is the Predominant Chytrid Fungus in Vietnamese Salamanders. Sci. Rep. 2017, 7, 44443. [Google Scholar] [CrossRef]
- North American Bsal Task Force. A North American Strategic Plan to Prevent and Control Invasions of the Lethal Salamander Pathogen Batrachochytrium salamandrivorans; North American Bsal Task Force: Fort Collins, CO, USA, 2022. [Google Scholar]
- Yap, T.; Nguyen, N.; Serr, M.; Shepack, A.; Vredenburg, V. Batrachochytrium salamandrivorans and the Risk of a Second Amphibian Pandemic. EcoHealth 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Towe, A.; Gray, M.; Carter, E.; Wilber, M.; Ossiboff, R.; Ash, K.; Bohanon, M.; Bajo, B.; Miller, D. Batrachochytrium salamandrivorans Can Devour More than Salamanders. J. Wildl. Dis. 2021, 57, 942–948. [Google Scholar] [CrossRef]
- Bosch, J.; Martel, A.; Sopniewski, J.; Thumsová, B.; Ayres, C.; Scheele, B.; Velo-Antón, G.; Pasmans, F. Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot. J. Fungi 2021, 7, 644. [Google Scholar] [CrossRef]
- Can, Ö.E.; D’Cruze, N.; Macdonald, D.W. Dealing in Deadly Pathogens: Taking Stock of the Legal Trade in Live Wildlife and Potential Risks to Human Health. Glob. Ecol. Conserv. 2019, 17, e00515. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.; Barnhart, K.; Bletz, M.; Campos, A.; Ganem, S.; Hertz, A.; Labumbard, B.; Nanjappa, P.; Tokash-Peters, A. Batrachochytrium: Biology and Management of Amphibian Chytridiomycosis. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2018; pp. 1–18. ISBN 978-0-47001-617-6. [Google Scholar]
- Beukema, W.; Martel, A.; Nguyen, T.; Goka, K.; Schmeller, D.; Yuan, Z.; Laking, A.; Nguyen, T.; Lin, C.-F.; Shelton, J.; et al. Environmental Context and Differences between Native and Invasive Observed Niches of Batrachochytrium salamandrivorans Affect Invasion Risk Assessments in the Western Palaearctic. Divers. Distrib. 2018, 24, 1788–1801. [Google Scholar] [CrossRef]
- Brucker, R.M.; Harris, R.N.; Schwantes, C.; Gallaher, T.N.; Flaherty, D.C.; Lam, B.A.; Minbiole, K.P.C. Amphibian Chemical Defense: Antifungal Metabolites of the Microsymbiont Janthinobacterium lividum on the Salamander Plethodon cinereus. J. Chem. Ecol. 2008, 34, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.K.; Pasmans, F.; Dhaenens, M.; Deforce, D.; Bonte, D.; Verheyen, K.; Lens, L.; Martel, A. Skin Mucosome Activity as an Indicator of Batrachochytrium salamandrivorans Susceptibility in Salamanders. PLoS ONE 2018, 13, e0199295. [Google Scholar] [CrossRef]
- Jiménez, R.R.; Carfagno, A.; Linhoff, L.; Gratwicke, B.; Woodhams, D.C.; Chafran, L.S.; Bletz, M.C.; Bishop, B.; Muletz-Wolz, C.R. Inhibitory Bacterial Diversity and Mucosome Function Differentiate Susceptibility of Appalachian Salamanders to Chytrid Fungal Infection. Appl. Environ. Microbiol. 2022, 88, e01818-21. [Google Scholar] [CrossRef]
- Myers, J.M.; Ramsey, J.P.; Blackman, A.L.; Nichols, A.E.; Minbiole, K.P.C.; Harris, R.N. Synergistic Inhibition of the Lethal Fungal Pathogen Batrachochytrium dendrobatidis: The Combined Effect of Symbiotic Bacterial Metabolites and Antimicrobial Peptides of the Frog Rana Muscosa. J. Chem. Ecol. 2012, 38, 958–965. [Google Scholar] [CrossRef]
- Lam, B.; Walke, J.; Vredenburg, V.; Harris, R. Proportion of Individuals with Anti-Batrachochytrium dendrobatidis Skin Bacteria Is Associated with Population Persistence in the Frog Rana Muscosa. Biol. Conserv. 2010, 143, 529–531. [Google Scholar] [CrossRef]
- Lam, B.A.; Walton, D.B.; Harris, R.N. Motile Zoospores of Batrachochytrium dendrobatidis Move Away from Antifungal Metabolites Produced by Amphibian Skin Bacteria. EcoHealth 2011, 8, 36–45. [Google Scholar] [CrossRef]
- Ramsey, J.; Reinert, L.; Harper, L.; Woodhams, D.; Rollins-Smith, L. Immune Defenses against Batrachochytrium dendrobatidis, a Fungus Linked to Global Amphibian Declines, in the South African Clawed Frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Gutiérrez-Preciado, A.; Noecker, C.; Eng, A.; Hughey, M.C.; Medina, D.; Walke, J.B.; Borenstein, E.; Jensen, R.V.; Belden, L.K.; et al. The Skin Microbiome of the Neotropical Frog Craugastor fitzingeri: Inferring Potential Bacterial-Host-Pathogen Interactions from Metagenomic Data. Front. Microbiol. 2018, 9, 466. [Google Scholar] [CrossRef]
- Bosch, J.; Thumsová, B.; López-Rojo, N.; Pérez, J.; Alonso, A.; Fisher, M.; Boyero, L. Microplastics Increase Susceptibility of Amphibian Larvae to the Chytrid Fungus Batrachochytrium dendrobatidis. Sci. Rep. 2021, 11, 22438. [Google Scholar] [CrossRef]
- McKenzie, C.; Oesterle, P.; Stevens, B.; Shirose, L.; Mastromonaco, G.; Lillie, B.; Davy, C.; Jardine, C.; Nemeth, N. Ophidiomycosis in Red Cornsnakes (Pantherophis guttatus): Potential Roles of Brumation and Temperature on Pathogenesis and Transmission. Vet. Pathol. 2020, 57, 825–837. [Google Scholar] [CrossRef]
- Romer, A.; Grinath, J.; Moe, K.; Walker, D. Host Microbiome Responses to the Snake Fungal Disease Pathogen (Ophidiomyces ophidiicola) Are Driven by Changes in Microbial Richness. Sci. Rep. 2022, 12, 3078. [Google Scholar] [CrossRef]
- Origgi, F.; Tecilla, M. Immunology of Reptiles. In Infectious Diseases and Pathology of Reptiles Color Atlas and Text; Jacobson, E., Garner, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 215–266. [Google Scholar]
- Zapata, A.G.; Varas, A.; Torroba, M. Seasonal Variations in the Immune System of Lower Vertebrates. Immunol. Today 1992, 13, 142–147. [Google Scholar] [CrossRef]
- Wright, R.K.; Cooper, E. Temperature Effects on Ectotherm Immune Responses. Dev. Comp. Immunol. 1981, 5, 117–122. [Google Scholar] [CrossRef]
- Campbell, T. Peripheral Blood of Reptiles. In Exotic Animal Hematology and Cytology; John Wiley & Sons, I.: Hoboken, NJ, USA, 2015; pp. 67–87. [Google Scholar]
- Rakus, K.; Ronsmans, M.; Vanderplasschen, A. Behavioral Fever in Ectothermic Vertebrates. Dev. Comp. Immunol. 2016, 66, 84–91. [Google Scholar] [CrossRef]
- Merchant, M.; Williams, S.; Trosclair, P.L.; Elsey, R.M.; Mills, K. Febrile Response to Infection in the American Alligator (Alligator mississippiensis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 921–925. [Google Scholar] [CrossRef]
- Ramos, A.B.; Don, M.T.; Muchlinski, A.E. The Effect of Bacteria Infection on Mean Selected Body Temperature in the Common Agama, Agama Agama: A Dose-Response Study. Comp. Biochem. Physiol. Part A Physiol. 1993, 105, 479–484. [Google Scholar] [CrossRef]
- Muchlinski, A.E.; Estany, A.; Don, M.T. The Response of Anolis Equestris and Oplurus cyclurus (Reptilia: Iguanidae) to Bacterial Endotoxin. J. Therm. Biol. 1995, 20, 315–320. [Google Scholar] [CrossRef]
- Richards-Zawacki, C.L. Thermoregulatory Behaviour Affects Prevalence of Chytrid Fungal Infection in a Wild Population of Panamanian Golden Frogs. Proc. Biol. Sci. 2010, 277, 519–528. [Google Scholar] [CrossRef]
- Agha, M.; Price, S.; Nowakowski, A.; Augustine, B.; Todd, B. Mass Mortality of Eastern Box Turtles with Upper Respiratory Disease Following Atypical Cold Weather. Dis. Aquat. Organ. 2017, 124, 91–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohen, J.M.; Venesky, M.D.; Sauer, E.L.; Civitello, D.J.; McMahon, T.A.; Roznik, E.A.; Rohr, J.R. The Thermal Mismatch Hypothesis Explains Host Susceptibility to an Emerging Infectious Disease. Ecol. Lett. 2017, 20, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Venesky, M.D.; DeMarchi, J.; Hickerson, C.; Anthony, C.D. Does the Thermal Mismatch Hypothesis Predict Disease Outcomes in Different Morphs of a Terrestrial Salamander? J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Civitello, D.J.; Venesky, M.D.; McMahon, T.A.; Rohr, J.R. Thermal Mismatches Explain How Climate Change and Infectious Disease Drove Widespread Amphibian Extinctions. bioRxiv 2017. [Google Scholar] [CrossRef]
- Chandler, H.; Allender, M.; Stegenga, B.; Haynes, E.; Ospina, E.; Stevenson, D. Ophidiomycosis Prevalence in Georgia’s Eastern Indigo Snake (Drymarchon couperi) Populations. PLoS ONE 2019, 14, e0218351. [Google Scholar] [CrossRef]
- Lind, C.; McCoy, C.; Farrell, T. Tracking Outcomes of Snake Fungal Disease in Free-Ranging Pigmy Rattlesnakes (Sistrurus miliarius). J. Wildl. Dis. 2018, 54, 352–356. [Google Scholar] [CrossRef]
- McKenzie, J.; Price, S.; Fleckenstein, L.; Drayer, A.; Connette, G.; Bohuski, E.; Lorch, J. Field Diagnostics and Seasonality of Ophidiomyces ophiodiicola in Wild Snake Populations. EcoHealth 2018, 16, 141–150. [Google Scholar] [CrossRef]
- Narayan, E. Non-Invasive Reproductive and Stress Endocrinology in Amphibian Conservation Physiology. Conserv. Physiol. 2013, 1, cot011. [Google Scholar] [CrossRef]
- Gabor, C.; Fisher, M.; Bosch, J. A Non-Invasive Stress Assay Shows That Tadpole Populations Infected with Batrachochytrium dendrobatidis Have Elevated Corticosterone Levels. PLoS ONE 2013, 8, e56054. [Google Scholar] [CrossRef]
- Searle, C.; Belden, L.; Du, P.; Blaustein, A. Stress and Chytridiomycosis: Exogenous Exposure to Corticosterone Does Not Alter Amphibian Susceptibility to a Fungal Pathogen. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 243–253. [Google Scholar] [CrossRef]
- Fonner, C.; Patel, S.; Boord, S.; Venesky, M.; Woodley, S. Effects of Corticosterone on Infection and Disease in Salamanders Exposed to the Amphibian Fungal Pathogen, Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2016, 123, 159–171. [Google Scholar] [CrossRef]
- Barnhart, K.; Bletz, M.; Labumbard, B.; Tokash-Peters, A.; Gabor, C.; Woodhams, D. Batrachochytrium salamandrivorans Elicits Acute Stress Response in Spotted Salamanders but Not Infection or Mortality. Anim. Conserv. 2020, 23, 533–546. [Google Scholar] [CrossRef]
- McCoy, C.; Lind, C.; Farrell, T. Environmental and Physiological Correlates of the Severity of Clinical Signs of Snake Fungal Disease in a Population of Pigmy Rattlesnakes, Sistrurus miliarius. Conserv. Physiol. 2017, 5, cow077. [Google Scholar] [CrossRef]
- Agugliaro, J.; Lind, C.M.; Lorch, J.M.; Farrell, T.M. An Emerging Fungal Pathogen Is Associated with Increased Resting Metabolic Rate and Total Evaporative Water Loss Rate in a Winter-Active Snake. Funct. Ecol. 2020, 34, 486–496. [Google Scholar] [CrossRef]
- Rosenblum, E.; Poorten, T.; Settles, M.; Murdoch, G. Only Skin Deep: Shared Genetic Response to the Deadly Chytrid Fungus in Susceptible Frog Species. Mol. Ecol. 2012, 21, 3110–3120. [Google Scholar] [CrossRef]
- Farrer, R.A.; Weinert, L.A.; Bielby, J.; Garner, T.W.J.; Balloux, F.; Clare, F.; Bosch, J.; Cunningham, A.A.; Weldon, C.; du Preez, L.H.; et al. Multiple Emergences of Genetically Diverse Amphibian-Infecting Chytrids Include a Globalized Hypervirulent Recombinant Lineage. Proc. Natl. Acad. Sci. USA 2011, 108, 18732–18736. [Google Scholar] [CrossRef]
- Rosenblum, E.; James, T.; Zamudio, K.; Poorten, T.; Ilut, D.; Rodriguez, D.; Eastman, J.; Richards-Hrdlicka, K.; Joneson, S.; Jenkinson, T.; et al. Complex History of the Amphibian-Killing Chytrid Fungus Revealed with Genome Resequencing Data. Proc. Natl. Acad. Sci. USA 2013, 110, 9385–9390. [Google Scholar] [CrossRef]
- Dang, T.; Searle, C.; Blaustein, A. Virulence Variation among Strains of the Emerging Infectious Fungus Batrachochytrium dendrobatidis in Multiple Amphibian Host Species. Dis. Aquat. Organ. 2017, 124, 233–239. [Google Scholar] [CrossRef]
- Franklinos, L.H.V.; Lorch, J.M.; Bohuski, E.; Rodriguez-Ramos Fernandez, J.; Wright, O.N.; Fitzpatrick, L.; Petrovan, S.; Durrant, C.; Linton, C.; Baláž, V.; et al. Emerging Fungal Pathogen Ophidiomyces ophiodiicola in Wild European Snakes. Sci. Rep. 2017, 7, 3844. [Google Scholar] [CrossRef]
- Clark, R.; Marchand, M.; Clifford, B.; Stechert, R.; Stephens, S. Decline of an Isolated Timber Rattlesnake (Crotalus horridus) Population: Interactions between Climate Change, Disease, and Loss of Genetic Diversity. Biol. Conserv. 2011, 144, 886–891. [Google Scholar] [CrossRef]
- Campbell, L.; Burger, J.; Zappalorti, R.; Bunnell, J.; Winzeler, M.; Taylor, D.; Lorch, J. Soil Reservoir Dynamics of Ophidiomyces ophidiicola, the Causative Agent of Snake Fungal Disease. J. Fungi 2021, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Le Donne, V.; Crossland, N.; Brandão, J.; Sokolova, Y.; Fowlkes, N.; Nevarez, J.; Langohr, I.; Gaunt, S. Nannizziopsis guarroi Infection in 2 Inland Bearded Dragons (Pogona vitticeps): Clinical, Cytologic, Histologic, and Ultrastructural Aspects. Vet. Clin. Pathol. 2016, 45, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Sigler, L.; Peucker, S.K.J.; Norton, J.H.; Nielan, A. Chrysosporium Anamorph of Nannizziopsis vriesii Associated with Fatal Cutaneous Mycoses in the Salt-Water Crocodile (Crocodylus porosus). Med. Mycol. 2002, 40, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Sánchez, C.; Becker, D.; Byers, K.; Worsley-Tonks, K.; Craft, M. City Sicker? A Meta-analysis of Wildlife Health and Urbanization. Front. Ecol. Environ. 2019, 17, 575–583. [Google Scholar] [CrossRef]
- Woodhams, D.; Alford, R. Ecology of Chytridiomycosis in Rainforest Stream Frog Assemblages of Tropical Queensland. Conserv. Biol. 2005, 19, 1449–1459. [Google Scholar] [CrossRef]
- Cramp, R.; Ohmer, M.; Franklin, C. UV Exposure Causes Energy Trade-Offs Leading to Increased Chytrid Fungus Susceptibility in Green Tree Frog Larvae. Conserv. Physiol. 2022, 10, coac038. [Google Scholar] [CrossRef]
- Ortiz-Santaliestra, M.; Fisher, M.; Fernández-Beaskoetxea, S.; Fernández-Benéitez, M.; Bosch, J. Ambient Ultraviolet B Radiation and Prevalence of Infection by Batrachochytrium dendrobatidis in Two Amphibian Species. Conserv. Biol. 2011, 25, 975–982. [Google Scholar] [CrossRef]
- Doddington, B.J.; Bosch, J.; Oliver, J.A.; Grassly, N.C.; Garcia, G.; Schmidt, B.; Garner, T.; Fisher, M.C. Context-Dependent Amphibian Host Population Response to an Invading Pathogen. Ecology 2013, 98, 1795–1804. [Google Scholar] [CrossRef]
- Schmeller, D.; Blooi, M.; Martel, A.; Garner, T.; Fisher, M.; Azémar, F.; Clare, F.; Leclerc, C.; Jäger, L.; Guevara-Nieto, H.M.; et al. Microscopic Aquatic Predators Strongly Affect Infection Dynamics of a Globally Emerged Pathogen. Curr. Biol. 2013, 24, 176–180. [Google Scholar] [CrossRef]
- Searle, C.L.; Mendelson, J.R.; Green, L.E.; Duffy, M.A. Daphnia Predation on the Amphibian Chytrid Fungus and Its Impacts on Disease Risk in Tadpoles. Ecol. Evol. 2013, 3, 4129–4138. [Google Scholar] [CrossRef]
- Battaglin, W.; Smalling, K.; Anderson, C.; Calhoun, D.; Chestnut, T.; Muths, E. Potential Interactions among Disease, Pesticides, Water Quality and Adjacent Land Cover in Amphibian Habitats in the United States. Sci. Total Environ. 2016, 566–567, 320–332. [Google Scholar] [CrossRef]
- Tompros, A.; Wilber, M.Q.; Fenton, A.; Carter, E.D.; Gray, M.J. Efficacy of Plant-Derived Fungicides at Inhibiting Batrachochytrium salamandrivorans Growth. J. Fungi 2022, 8, 1025. [Google Scholar] [CrossRef]
- Barbi, A.; Goessens, T.; Strubbe, D.; Deknock, A.; Van Leeuwenberg, R.; De Troyer, N.; Verbrugghe, E.; Greener, M.; De Baere, S.; Lens, L.; et al. Widespread Triazole Pesticide Use Affects Infection Dynamics of a Global Amphibian Pathogen. Ecol. Lett. 2023, 26, 313–322. [Google Scholar] [CrossRef]
- Romansic, J.; Johnson, J.; Wagner, R.; Hill, R.; Gaulke, C.; Vredenburg, V.; Blaustein, A. Complex Interactive Effects of Water Mold, Herbicide, and the Fungus Batrachochytrium dendrobatidis on Pacific Treefrog Hyliola Regilla Hosts. Dis. Aquat. Organ. 2016, 123, 227–238. [Google Scholar] [CrossRef]
- Stockwell, M.P.; Clulow, J.; Mahony, M.J. Evidence of a Salt Refuge: Chytrid Infection Loads Are Suppressed in Hosts Exposed to Salt. Oecologia 2015, 177, 901–910. [Google Scholar] [CrossRef]
- Thomas, V.; Wang, Y.; Van Rooij, P.; Verbrugghe, E.; Baláž, V.; Bosch, J.; Cunningham, A.; Fisher, M.; Garner, T.; Gilbert, M.; et al. Mitigating Batrachochytrium salamandrivorans in Europe. Amphib.-Reptil. 2019, 40, 265–290. [Google Scholar] [CrossRef]
- Woodburn, D.; Kinsel, M.; Poll, C.; Langan, J.; Haman, K.; Gamble, K.; Maddox, C.; Jeon, A.; Wellehan, J.; Ossiboff, R.; et al. Shell Lesions Associated With Emydomyces testavorans Infection in Freshwater Aquatic Turtles. Vet. Pathol. 2021, 58, 578–586. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilliger, L.; Paillusseau, C.; François, C.; Bonwitt, J. Major Emerging Fungal Diseases of Reptiles and Amphibians. Pathogens 2023, 12, 429. https://doi.org/10.3390/pathogens12030429
Schilliger L, Paillusseau C, François C, Bonwitt J. Major Emerging Fungal Diseases of Reptiles and Amphibians. Pathogens. 2023; 12(3):429. https://doi.org/10.3390/pathogens12030429
Chicago/Turabian StyleSchilliger, Lionel, Clément Paillusseau, Camille François, and Jesse Bonwitt. 2023. "Major Emerging Fungal Diseases of Reptiles and Amphibians" Pathogens 12, no. 3: 429. https://doi.org/10.3390/pathogens12030429
APA StyleSchilliger, L., Paillusseau, C., François, C., & Bonwitt, J. (2023). Major Emerging Fungal Diseases of Reptiles and Amphibians. Pathogens, 12(3), 429. https://doi.org/10.3390/pathogens12030429






