Co-Immunization Efficacy of Recombinant Antigens against Rhipicephalus microplus and Hyalomma anatolicumTick Infestations
Abstract
1. Introduction
2. Materials and Methods
2.1. Ticks and Experimental Animals
2.2. In Vitro Amplification, Cloning, and Sequencing of the Genes
2.3. Sequence Homology of Targeted Genes of R. microplus and H. anatolicum
2.4. Relative Gene Expression Profile
2.5. Expression of Proteins in the Eukaryotic Expression System
2.6. Raising of Hyper-Immune Sera in Rabbits
2.7. SDS-PAGE and Western Blot
2.8. Monitoring of Immune Response
2.9. Immunization and Challenge Study
- 10th day of the second booster with ten to twelve-day-old larvae hatched from 50 mg eggs of H. anatolicum and R. microplus;
- 20th day of the second booster with larvae hatched from 50 mg eggs of H. anatolicum;
- 30th day of the second booster with larvae hatched from 50 mg eggs of R. microplus and unfed adults of H. anatolicum (15 female + 30 male on each calf).
2.10. Statistical Analysis
3. Results
3.1. Genetic Homology of the BM86, SUB, and TPM Coding Genes
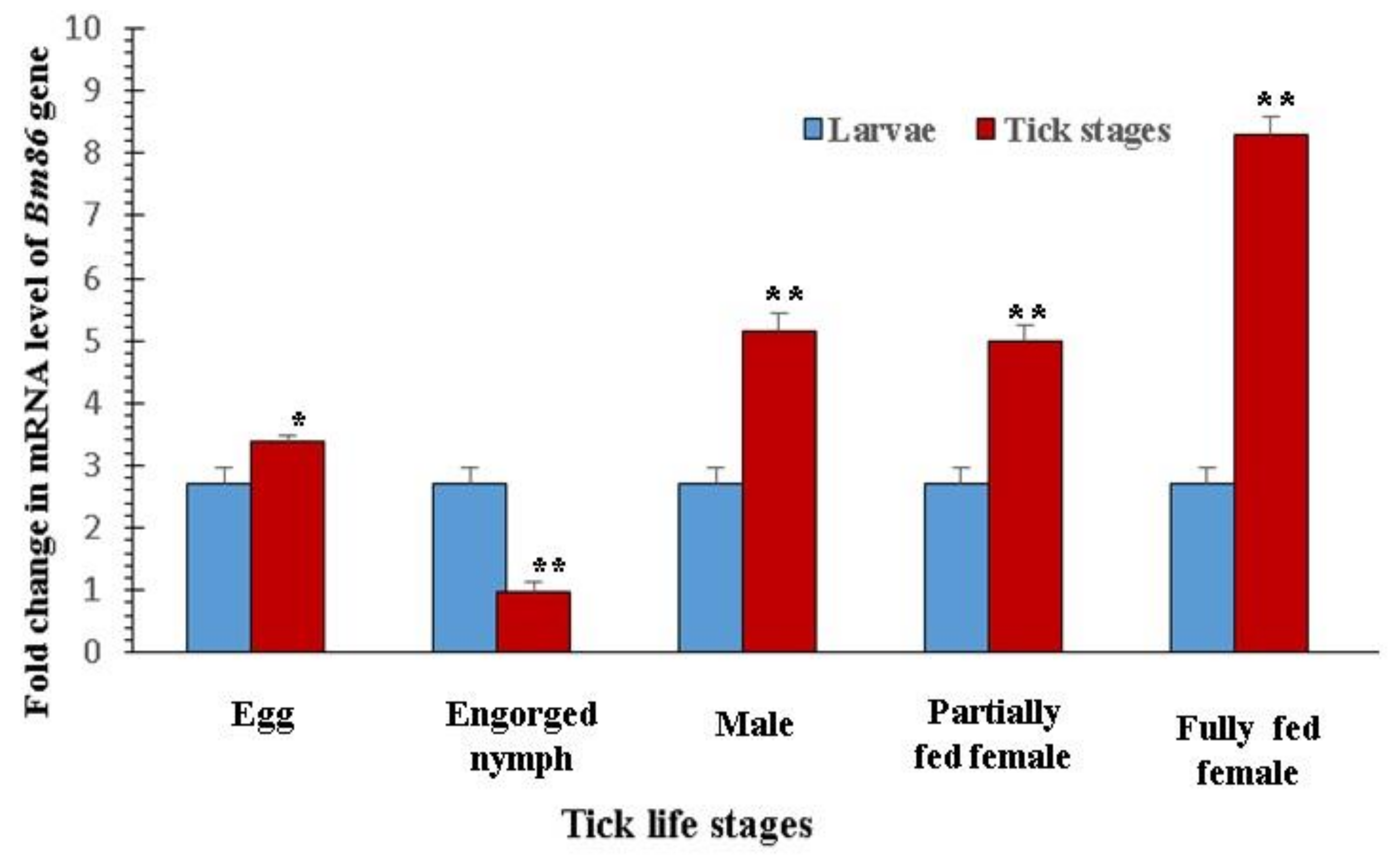
3.2. Expression Profile of the Bm86 Gene in Different Stages of R. microplus
3.3. Expression of Recombinant Proteins
3.4. Antibody Responses
3.5. Protective Efficacy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Accounts Statistics Report. Government of India. 2022. Available online: http://mospi.nic.in/publication/national-accounts-statistics-2020 (accessed on 7 December 2022).
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bansal, G.C.; Gupta, S.C.; Ray, D.; Khan, M.Q.; Irshad, H.; Shahiduzzaman, M.; Seitzer, U.; Ahmed, J.S. Status of tick distribution in Bangladesh, India and Pakistan. Parasitol. Res. 2007, 101, S207–S216. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. S3), S3–S14. [Google Scholar] [CrossRef]
- Brianti, E.; Otranto, D.; Dantas-Torres, F.; Weigl, S.; Latrofa, M.; Gaglio, G.; Napoli, E.; Brucato, G.; Cauquil, L.; Giannetto, S.; et al. Rhipicephalus sanguineus (Ixodida, Ixodidae) as intermediate host of a canine neglected filarial species with dermal microfilariae. Vet. Parasitol. 2012, 183, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Brianti, E.; Latrofa, M.S.; Annoscia, G.; Weigl, S.; Lia, R.P.; Gaglio, G.; Napoli, E.; Giannetto, S.; Papadopoulos, E.; et al. On a Cercopithifilaria sp. transmitted by Rhipicephalus sanguineus: A neglected, but widespread filarioid of dogs. Parasit Vectors 2012, 5, 1. [Google Scholar] [CrossRef]
- Patil, M.M.; Walikar, B.N.; Kalyanshettar, S.S.; Patil, S.V. Tick induced facial palsy. Indian J Pediatr. 2012, 49, 57–67. [Google Scholar] [CrossRef]
- Padula, A.M. Tick paralysis of animals in Australia. Clinical Toxicology. Clin. Toxicol. 2016, 1–20. [Google Scholar]
- Ghosh, S.; Azhahianambi, P.; de la Fuente, J. Control of ticks of ruminants, with special emphasis on livestock farming systems in India: Present and future possibilities for integrated control—a review. Exp. Appl. Acarol. 2006, 40, 49–66. [Google Scholar] [CrossRef]
- Ghosh, S.; Nagar, G. Problem of ticks and tick-borne diseases in India with special emphasis on progress in tick control research: A review. J. Vector Borne Dis. 2014, 51, 259–270. [Google Scholar]
- Mourya, D.T.; Yadav, P.D.; Gurav, Y.K.; Pardeshi, P.G.; Shete, A.M.; Jain, R.; Raval, D.D.; Upadhyay, K.J.; Patil, D.Y. Crimean Congo hemorrhagic fever serosurvey in humans for identifying high-risk populations and high-risk areas in the endemic state of Gujarat, India. BMC Infect. Dis. 2019, 19, 104. [Google Scholar] [CrossRef]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Wong, G.; Babuadze, G.; Camp, J.V.; Ergonul, O.; Kobinger, G.P.; Chinikar, S.; Nowotny, N. Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe. Microorganisms 2021, 9, 1907. [Google Scholar] [CrossRef]
- Sakamoto, J.M. Progress, challenges, and the role of public engagement to improve tick-borne disease literacy. Curr. Opin. Insect. Sci. 2018, 28, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Control of ixodid ticks and prevention of tick-borne diseases in the United States: The prospect of a new Lyme disease vaccine and the continuing problem with tick exposure on residential properties. Ticks Tick-Borne Dis. 2022, 12, 101649. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A. Recent developments in ectoparasiticides. Vet. J. 2001, 161, 253–268. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Price, A.; Hornbostel, V.L.; Benjamin, M.A.; Keesing, F. Controlling Ticks and Tick-borne Zoonoses with Biological and Chemical Agents. BioScience 2006, 56, 383–394. [Google Scholar] [CrossRef]
- FAO. Acaricide Resistance: Test kit Instructions for Use; World Acaricide Resistance Reference Centre: Berlin, Germany, 1984. [Google Scholar]
- WHO. Vector resistance to insecticides. 15th report of WHO expert committee on vector biology and control. Tech. Rep. Serv. 1992, 818, 1–62. [Google Scholar]
- Willadsen, P. Anti-tick vaccines. Parasitol. 2004, 129, S367–S387. [Google Scholar] [CrossRef]
- Chema, S. Summing-up of strategies for the control of ticks in Africa. Parasitologia 1990, 32, 201–202. [Google Scholar]
- Pegram, R.G.; James, A.D.; Oosterwijk, G.P.; Killorn, K.J.; Lemche, J.; Ghirotti, M.; Tekle, Z.; Chizyuka, H.G.; Mwase, E.T.; Chizyuka, F. Studies on the economics of ticks in Zambia. Exp. Appl. Acarol. 1991, 12, 9–26. [Google Scholar] [CrossRef]
- Pegram, R.G.; Tatchell, R.J.; De Castro, J.J.; Chizyuka, H.G.B.; Creek, M.J.; McCosker, P.J.; Moran, M.C.; Nigarura, G. Tick control: New concepts. World Anim. Rev. 1993, 74, 2–11. Available online: https://www.fao.org/AG/aga/AGAP/FRG/FEEDback/War/u9550b/u9550b04.htm (accessed on 7 December 2022).
- Kumar, R.; Sharma, A.K.; Ghosh, S. Menace of acaricide resistance in cattle tick, Rhipicephalus microplus in India: Status and possible mitigation strategies. Vet. Parasitol. 2020, 278, 108993. [Google Scholar] [CrossRef]
- Dzemo, W.D.; Thekisoe, O.; Vudriko, P. Development of acaricide resistance in tick populations of cattle: A systematic review and meta-analysis. Heylion 2022, 8, e08718. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.-F.; Gogolewski, R.; Leach-Bing, N.; Sabatini, G.A.; Molento, M.B.; Bordin, E.L.; Arantes, G.J. Tick control: An industry point of view. Parasitology 2004, 129, S427–S442. [Google Scholar] [CrossRef] [PubMed]
- Duval, H.; Hüe, T. Field efficacy assessment of a vaccine against Rhipicephalus (Boophilus) australis in New-Caledonia. Vet. Parasitol. Reg. Stud. Rep. 2022, 29, 100702. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Kocan, K.M. Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol. 2006, 28, 275–283. [Google Scholar] [CrossRef]
- de la Fuente, J.; Almazán, C.; Canales, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef]
- Merino, O.; Alberdi, P.; Pérez de la Lastra, J.M.; de la Fuente, J. Tick vaccines and the control of tick-borne pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 30. [Google Scholar] [CrossRef]
- Rego, R.; Trentelman, J.; Anguita, J.; Nijhof, A.M.; Sprong, H.; Klempa, B.; Hajdusek, O.; Tomás-Cortázar, J.; Azagi, T.; Strnad, M.; et al. Counter attacking the tick bite: Towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit Vectors 2019, 12, 229. [Google Scholar] [CrossRef]
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328. [Google Scholar] [CrossRef]
- Vaughan, J.A.; Sonenshine, D.E.; Azad, A.F. Kinetics of ingested host immunoglobulin G in hemolymph and whole body homogenates during nymphal development of Dermacentor variabilis and Ixodes scapularis ticks (Acari: Ixodidae). Exp. Appl. Acarol. 2002, 27, 329–340. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Moreno-Cid, J.A.; Canales, M.; Villar, M.; de la Lastra, J.M.; Kocan, K.M.; Galindo, R.C.; Almazán, C.; Blouin, E.F. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet. Parasitol. 2011, 181, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Murugan, K.; Ray, D.D.; Ghosh, S. Efficacy of rBm86 against Rhipicephalus (Boophilus) microplus (IVRI-I line) and Hyalomma anatolicum anatolicum (IVRI-II line) infestations on bovine calves. Parasitol. Res. 2012, 111, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Manjunathachar, H.; Nagar, G.; Ravikumar, G.; de la Fuente, J.; Saravanan, B.; Ghosh, S. Functional characterization of candidate antigens of Hyalomma anatolicum and evaluation of its cross-protective efficacy against Rhipicephalus microplus. Vaccine 2017, 35, 5682–5692. [Google Scholar] [CrossRef] [PubMed]
- Manjunathachar, H.V.; Kumar, B.; Saravanan, B.C.; Choudhary, S.; Mohanty, A.K.; Nagar, G.; Chigure, G.; Ravi Kumar, G.; de la Fuente, J.; Ghosh, S. Identification and characterization of vaccine candidates against Hyalommaanatolicum-Vector of Crimean-Congo haemorrhagic fever virus. Transbound. Emerg. Dis. 2019, 66, 422–434. [Google Scholar] [CrossRef]
- Almazán, C.; Lagunes, R.; Villar, M.; Canales, M.; Rosario-Cruz, R.; Jongejan, F.; de la Fuente, J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010, 106, 471–479. [Google Scholar] [CrossRef]
- Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Villar, M.; Jiménez, M.; Pinal, R.; Estrada-Peña, A.; Molina, R.; Lucientes, J.; Gortázar, C.; de la Fuente, J.; et al. Control of multiple arthropod vector infestations with subolesin/akirin vaccines. Vaccine 2013, 31, 1187–1196. [Google Scholar] [CrossRef]
- Parthasarathi, B.C.; Kumar, B.; Ghosh, S. Current status and future prospects of multi-antigen tick vaccine. J. Vector Borne Dis. 2021, 58, 183–192. [Google Scholar] [CrossRef]
- Shakya, M.; Kumar, B.; Nagar, G.; de la Fuente, J.; Ghosh, S. Subolesin: A candidate vaccine antigen for the control of cattle tick infestations in Indian situation. Vaccine 2014, 32, 3488–3494. [Google Scholar] [CrossRef]
- Parthasarathi, B.C.; Kumar, B.; Nagar, G.; Manjunathachar, H.V.; de la Fuente, J.; Ghosh, S. Analysis of Genetic Diversity in Indian Isolates of Rhipicephalus microplus Based on Bm86 Gene Sequence. Vaccines 2021, 9, 194. [Google Scholar] [CrossRef]
- McKenna, R.V.; Riding, G.A.; Jarmey, J.M.; Pearson, R.D.; Willadsen, P. Vaccination of cattle against the Boophilus microplus using a mucin-like membrane glycoprotein. Parasit Immunol. 1998, 20, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Schetters, T.P.M.; Jansen, T.; Vaccine against Rhipicephalus Ticks. International Application Number: PCT/EP2014/056248. 2014, International Publication Number: WO2014/154847Al. 2015. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014154847 (accessed on 7 December 2022).
- de la Fuente, J.; Rodríguez, M.; Redondo, M.; Montero, C.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enriquez, A.; Canales, M.; et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine 1998, 16, 366–373. [Google Scholar] [CrossRef]
- de Vos, S.; Zeinstra, L.; Taoufik, O.; Willadsen, P.; Jongejan, F. Evidence for the utility of the Bm86 antigen from Boophilus microplus in vaccination against other tick species. Exp. Appl. Acarol. 2001, 25, 245–261. [Google Scholar] [CrossRef]
- Ghosh, S.; Azhahianambi, P. Laboratory rearing of Theileria annulata-free Hyalomma anatolicum anatolicum ticks. Exp. Appl. Acarol. 2007, 43, 137–146. [Google Scholar] [CrossRef]
- Fragoso, H.; Rad, P.H.; Ortiz, M.; Rodriguez, M.; Redondo, M.; Herrera, L.; De la Fuente, J. Protection against Boophilus annulatus infestations in cattle vaccinated with the B. microplus Bm86-containing vaccine Gavac. Vaccine 1998, 16, 1990–1992. [Google Scholar] [CrossRef]
- Kumar, B.; Ray, D.D.; Ghosh, S. Immune responses against rHaa86 in cross-bred cattle. J. Parasit. Dis. 2015, 39, 292–297. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Laemmli, U.K. SDS-page Laemmli method. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Manjunathachar, H.V.; Saravanan, B.C.; Kumar, B.; Ghosh, S. Expression and determination of immunization dose of recombinant tropomyosin protein of Hyalommaanatolicum for the development of anti-tick vaccine. Indian J. Anim. Res. 2017, 87, 275–279. [Google Scholar]
- García-García, J.C.; Gonzalez, I.L.; González, D.M.; Valdés, M.; Méndez, L.; Lamberti, J.; D’Agostino, B.; Citroni, D.; Fragoso, H.; Ortiz, M.; et al. Sequence variations in the Boophilusmicroplus Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Exp. Appl. Acarol. 1999, 23, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Kaewmongkol, S.; Kaewmongkol, N.G.; Inthong, N.; Lakkitjaroen, T.; Sirinarumitr, C.M.; Berry, N.N.; Jonsson, R.W.; Stich, S.; Jittapalapong, S. Variation among Bm86 sequences in Rhipicephalus (Boophilus) microplus ticks collected from cattle across Thailand. Exp. Appl. Acarol. 2015, 66, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, C.; Chongkasikit, N.; Jittapalapong, S.; Gauly, M. Immune response of Bos indicus cattle against the anti-tick antigen Bm91 derived from local Rhipicephalus (Boophilus) microplus ticks and its effect on tick reproduction under natural infestation. Parasitol. Res. 2012, 66, 247–256. [Google Scholar]
- Muhanguzi, D.; Ndekezi, C.; Nkamwesiga, J.; Kalayou, S.; Ochwo, S.; Vuyani, M.; Kimuda, M.P. Anti-Tick Vaccines: Current Advances and Future Prospects. Methods 2022, 2, 253–267. [Google Scholar]
- García-García, J.C.; Montero, C.; Redondo, M.; Vargas, M.; Canales, M.; Boue, O.; Rodríguez, M.; Joglar, M.; Machado, H.; González, I.L.; et al. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilusmicroplus. Vaccine 2000, 18, 2275–2287. [Google Scholar] [CrossRef]
- Parizi, L.F.; Reck, J., Jr.; Oldiges, D.P.; Guizzo, M.G.; Seixas, A.; Logullo, C.; de Oliveira, P.L.; Termignoni, C.; Martins, J.R.; Vaz Ida, S., Jr. Multi-antigenic vaccine against the cattle tick Rhipicephalus (Boophilus) microplus: A field evaluation. Vaccine 2012, 30, 6912–6917. [Google Scholar] [CrossRef]
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccine 2015, 14, 1367–1376. [Google Scholar] [CrossRef]
- Willadsen, P. Antigen cocktails: Valid hypothesis or unsubstantiated hope? Trends Parasitol. 2008, 24, 164–167. [Google Scholar] [CrossRef]
- Canales, M.; Almazán, C.; Naranjo, V.; Jongejan, F.; de la Fuente, J. Vaccination with recombinant Boophilusannulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. 2009, 9, 29. [Google Scholar] [CrossRef]
- Shannon, C.E. The mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Bastos, R.G.; Ueti, M.W.; Knowles, D.P.; Scoles, G.A. The Rhipicephalus (Boophilus) microplus Bm86 gene plays a critical role in the fitness of ticks fed on cattle during acute Babesia bovis infection. Parasit Vectors 2010, 3, 111. [Google Scholar] [CrossRef]
- Ndawula, C., Jr.; Tabor, A.E. Cocktail anti-tick vaccines: The unforeseen constraints and approaches toward enhanced efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Ndawula, C., Jr. From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness. Vaccines 2021, 9, 1185. [Google Scholar] [CrossRef]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Estrada-Peña, A.; Kocan, K.M.; de la Fuente, J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of subolesin vaccination and tick autocidal control after subolesin gene knockdown in ticks fed on cattle. Vaccine 2011, 29, 2248–2254. [Google Scholar] [CrossRef]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Galindo, R.C.; de la Fuente, J. Targeting the tick protective antigen subolesin reduces vector infestations and pathogen infection by Anaplasmamarginale and Babesia bigemina. Vaccine 2011, 29, 8575–8579. [Google Scholar] [CrossRef]
- Carreón, D.; de la Lastra, J.M.; Almazán, C.; Canales, M.; Ruiz-Fons, F.; Boadella, M.; Moreno-Cid, J.A.; Villar, M.; Gortázar, C.; Reglero, M.; et al. Vaccination with BM86, subolesin and akirin protective antigens for the control of tick infestations in white tailed deer and red deer. Vaccine 2012, 30, 273–279. [Google Scholar] [CrossRef]
- Allen, J.R.; Humphreys, S.J. Immunisation of guinea pigs and cattle against ticks. Nature 1979, 280, 491–493. [Google Scholar] [CrossRef]
- Kemp, D.H.; Pearson, R.D.; Gough, J.M.; Willadsen, P. Vaccination against Boophilusmicroplus: Localization of antigens on tick gut cells and their interaction with the host immune system. Exp. Appl. Acarol. 1989, 7, 43–58. [Google Scholar] [CrossRef]
- Penichet, M.; Rodriguez, M.; Castellano, O.; Mandado, S.; Rojas, Y.; Rubiera, R.; Sanchez, P.; Lleonart, R.; De La Fuente, J. Detection of Bm86 antigen in different strains of Boophilusmicroplus and effectiveness of immunization with recombinant Bm86. Parasit Immunol. 1994, 16, 493–500. [Google Scholar] [CrossRef]
- de la Fuente, J.; Rodríguez, M.; Montero, C.; Redondo, M.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enríquez, A.; Canales, M.; et al. Vaccination against ticks (Boophilus spp.): The experience with the Bm86-based vaccine Gavac. Genet. Anal. Biomol. Eng. 1999, 15, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Azhahianambi, P.; de La Fuente, J.; Suryanarayana, V.V.; Ghosh, S. Cloning, expression and immunoprotective efficacy of rHaa86, the homologue of the Bm86 tick vaccine antigen, from Hyalommaanatolicumanatolicum. Parasite Immunol. 2009, 31, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Valle, M.; Montero, C.; Machado, H.; Joglar, M. The evaluation of yeast derivatives as adjuvants for the immune response to the Bm86 antigen in cattle. BMC Biotechnol. 2001, 1, 2. [Google Scholar] [CrossRef]
- Kumar, B.; Azhahianambi, P.; Ray, D.D.; Chaudhuri, P.; De La Fuente, J.; Kumar, R.; Ghosh, S. Comparative efficacy of rHaa86 and rBm86 against Hyalomma anatolicum anatolicum and Rhipicephalus (Boophilus) microplus. Parasite Immunol. 2012, 34, 297–301. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rubiera, R.; Penichet, M.; Montesinos, R.; Cremata, J.; Falcón, V.; Sánchez, G.; Bringas, R.; Cordovés, C.; Valdés, M. High level expression of the B. microplus Bm86 antigen in the yeast Pichia pastoris forming highly immunogenic particles for cattle. J. Biotechnol. 1994, 33, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 2016, 34, 3010–3013. [Google Scholar] [CrossRef]
- Lee, S.H.; Li, J.; Moumouni, P.F.A.; Okado, K.; Zheng, W.; Liu, M.; Ji, S.; Kim, S.; Umemiya-Shirafuji, R.; Xuan, X. Subolesin vaccination inhibits blood feeding and reproduction of Haemaphysalis longicornis in rabbits. Parasit Vectors 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E.; Je, B.D. Primer to the Immune Response, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hatfield, P.R. Detection and localization of antibody ingested with a mosquito blood meal. Med. Vet. Entomol. 1988, 2, 339–345. [Google Scholar] [CrossRef]
- Artigas-Jerónimo, S.; Villar, M.; Cabezas-Cruz, A.; Caignard, G.; Vitour, D.; Richardson, J.; Lacour, S.; Attoui, H.; Bell-Sakyi, L.; Allain, E.; et al. Tick Importin-α is implicated in the interactome and regulome of the cofactor Subolesin. Pathogens 2021, 10, 457. [Google Scholar] [CrossRef]
- Kasaija, P.D.; Contreras, M.; Kabi, F.; Mugerwa, S.; Fuente, J.D. Vaccination with recombinant subolesin antigens provides cross-tick species protection in Bos indicus and crossbred cattle in Uganda. Vaccine 2020, 2020, 319. [Google Scholar] [CrossRef]
- Tellam, R.L.; Smith, D.; Kemp, D.H.; Willadsen, P. Animal Parasite Control Utilizing Biotechnology; Yong, W.K., Ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 303–331. [Google Scholar]
- Valle, M.R.; Mèndez, L.; Valdez, M.; Redondo, M.; Espinosa, C.M.; Vargas, M.; Cruz, R.L.; Barrios, H.P.; Seoane, G.; Ramirez, E.S.; et al. Integrated control of Boophilusmicroplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Exp. Appl. Acarol. 2004, 34, 375–382. [Google Scholar] [CrossRef]
- Maruyama, S.R.; Garcia, G.R.; Teixeira, F.R.; Brandão, L.G.; Anderson, J.M.; Ribeiro, J.; Valenzuela, J.G.; Horackova, J.; Veríssimo, C.J.; Katiki, L.M.; et al. Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations. Parasit Vectors. 2017, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Tafur-Gómez, G.A.; Salcedo, J.H.P.; Vargas, M.I.; Araújo, L.; Fidelis, C.F.; Prates-Patarroyo, P.A.; Cortes-Vecino, J.A.; Portela, R.W. Intestinal changes and performance parameters in ticks feeding on calves immunized with subunits of immunogens against Rhipicephalus microplus. Exp. Appl. Acarol. 2020, 80, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.A.; Kemp, D.H.; Pearson, R.D. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Effects of induced immunity on tick populations. Parasitol. Int. 1986, 16, 27–34. [Google Scholar] [CrossRef]
- Opdebeeck, J.P.; Wong, J.Y.; Jackson, L.A.; Dobson, C. Hereford cattle immunized and protected against Boophilusmicroplus with soluble and membrane-associated antigens from the midgut of ticks. Parasit Immunol. 1988, 10, 405–410. [Google Scholar] [CrossRef]
- Willadsen, P.; Kemp, D.H. Vaccination with ‘concealed’ antigens for tick control. Parasitol. Today 1988, 4, 196–198. [Google Scholar] [CrossRef]





| (A) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Challenge Infestations | Group | No. of Engorged Larvae Dropped | No. of Larvae Moulted to Nymphs | DT% | MO% | E% | ||||||||
| First larval Challenge | Immunized | 198.5 ± 11.11 * | 99.5 ± 10.75 * | 49.05 | 73.74 | 86.62 | ||||||||
| Control | 389.66 ± 64.67 | 379.0 ± 66.68 | ||||||||||||
| Reduction% | 49.05 | 73.4 | ||||||||||||
| Second larval Challenge | Immunization | 247 ± 13.42 ** | 96.5 ± 3.96 ** | 45.47 | 78.44 | 88.24 | ||||||||
| Control | 453 ± 20.30 | 447.66 ± 20.11 | ||||||||||||
| Reduction% | 45.5 | 78.5 | ||||||||||||
| (B) | ||||||||||||||
| Group | Tick Dropped/Animal (Mean ± SE) | Tick wt. (g) (Mean ± SE) | Egg wt.(g) (Mean ± SE) | RI(Mean ± SE) | DT% | DR% | DO% | RF% | E% | |||||
| Immunization | 13.83 ± 0.3073 | 0.2763 ± 0.007 ** | 0.105 ± 0.006 ** | 0.3511 ± 0.01 ** | 5.4 | 29.9 | 66.81 | 56.2 | 86.2 | |||||
| Control | 14.67 ± 0.3333 | 0.3943 ± 0.007 | 0.3164 ± 0.007 | 0.8019 ± 0.030 | ||||||||||
| Reduction% | 5.7 | 29.9 | 66.8 | 56.2 | ||||||||||
| Group | Tick Dropped/Animal (Mean ± SE) | Tick wt. (g) (Mean ± SE) | Egg wt.(g) (Mean ± SE) | RI (Mean ± SE) | DT% | DR% | DO% | RF% | E% |
|---|---|---|---|---|---|---|---|---|---|
| First Larval Challenge | |||||||||
| Immunization | 27.33 ± 1.054 * | 0.1065 ± 0.004 ** | 0.0498 ± 0.002 * | 0.4761 ± 0.013 * | 63 | 26 | 53 | 35 | 88.9 |
| Control | 74 ± 3.606 | 0.1444 ± 0.007284 | 0.1071 ± 0.006013 | 0.7417 ± 0.00515 | |||||
| Reduction% | 63.06 | 26.2 | 53.50 | 35.8 | |||||
| Second Larval Challenge | |||||||||
| Immunization | 46.17 ± 1.108 * | 0.1172 ± 0.0020 * | 0.05506 ± 0.001639 * | 0.476 ± 0.01615 * | 52.8 | 20.4 | 49.8 | 34 | 84.6 |
| Control | 98 ± 1 | 0.1473 ± 0.001866 | 0.1097 ± 0.002133 | 0.734 ± 0.006956 | |||||
| Reduction% | 52.8 | 20.4 | 49.8 | 35.1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parthasarathi, B.C.; Kumar, B.; Bhure, S.K.; Sharma, A.K.; Manisha; Nagar, G.; Kumar, S.; Nandi, A.; Manjunathachar, H.V.; Chigure, G.M.; et al. Co-Immunization Efficacy of Recombinant Antigens against Rhipicephalus microplus and Hyalomma anatolicumTick Infestations. Pathogens 2023, 12, 433. https://doi.org/10.3390/pathogens12030433
Parthasarathi BC, Kumar B, Bhure SK, Sharma AK, Manisha, Nagar G, Kumar S, Nandi A, Manjunathachar HV, Chigure GM, et al. Co-Immunization Efficacy of Recombinant Antigens against Rhipicephalus microplus and Hyalomma anatolicumTick Infestations. Pathogens. 2023; 12(3):433. https://doi.org/10.3390/pathogens12030433
Chicago/Turabian StyleParthasarathi, Balasamudram Chandrasekhar, Binod Kumar, S. K. Bhure, Anil Kumar Sharma, Manisha, Gaurav Nagar, Sachin Kumar, Abhijit Nandi, Haranahally Vasanthachar Manjunathachar, Gajanan M. Chigure, and et al. 2023. "Co-Immunization Efficacy of Recombinant Antigens against Rhipicephalus microplus and Hyalomma anatolicumTick Infestations" Pathogens 12, no. 3: 433. https://doi.org/10.3390/pathogens12030433
APA StyleParthasarathi, B. C., Kumar, B., Bhure, S. K., Sharma, A. K., Manisha, Nagar, G., Kumar, S., Nandi, A., Manjunathachar, H. V., Chigure, G. M., Shakya, M., Sankar, M., Fuente, J. d. l., & Ghosh, S. (2023). Co-Immunization Efficacy of Recombinant Antigens against Rhipicephalus microplus and Hyalomma anatolicumTick Infestations. Pathogens, 12(3), 433. https://doi.org/10.3390/pathogens12030433












