Dual RNA-Seq of Flavobacterium psychrophilum and Its Outer Membrane Vesicles Distinguishes Genes Associated with Susceptibility to Bacterial Cold-Water Disease in Rainbow Trout (Oncorhynchus mykiss)
Abstract
:1. Introduction
2. Results and Discussion
2.1. OMV Isolation and TEM
2.2. RNA-Seq of Fp Whole Cells and OMVs Identify Protein-Coding Genes Associated with Bacterial Virulence and Pathogenesis
2.3. Ribosome
2.4. Two-Component System
2.5. Pantothenate and CoA Biosynthesis
2.6. Glycine, Serine, and Threonine Metabolism
2.7. Genes with Enriched GO Terms
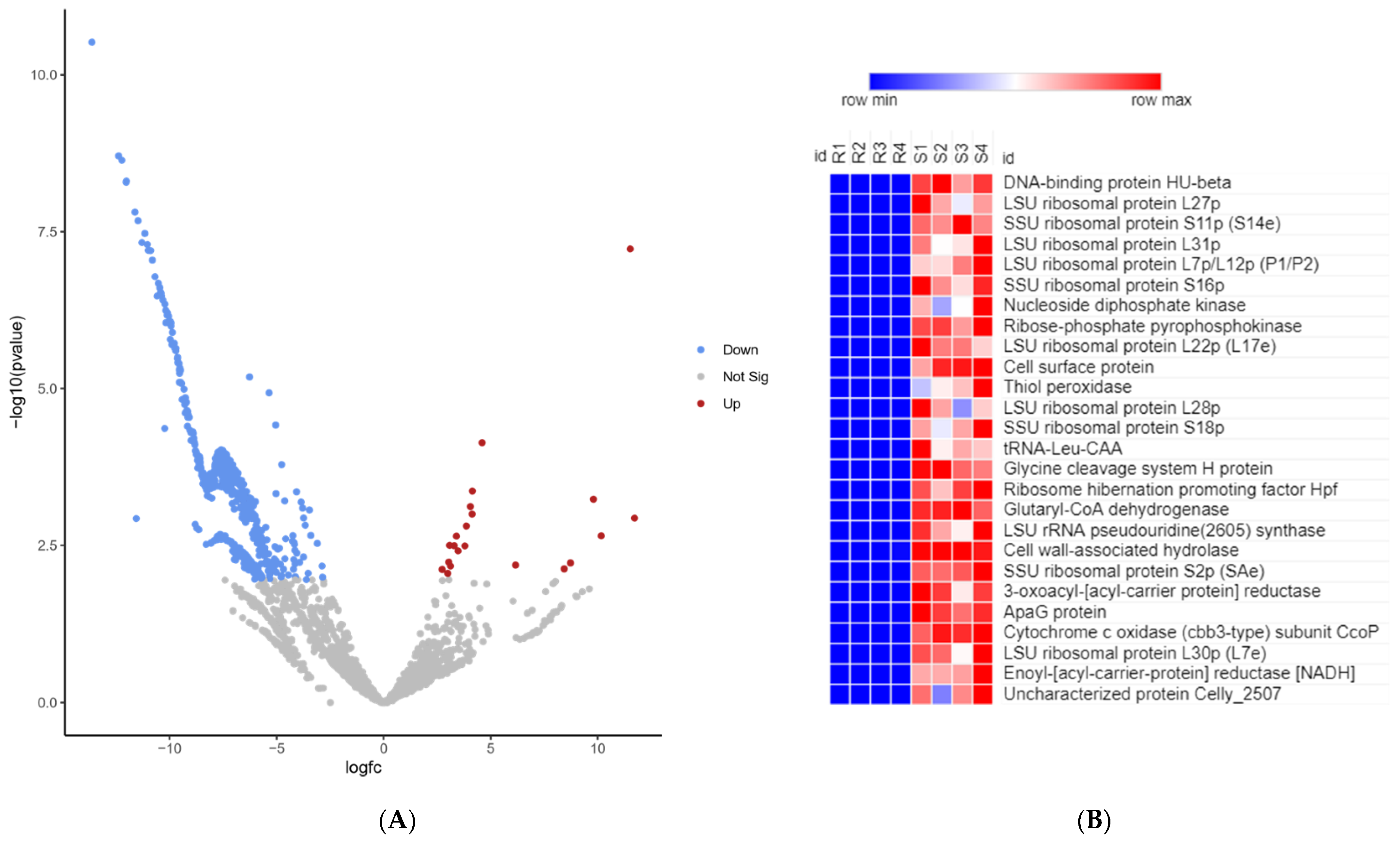
2.8. RNA-Seq of Pathogen on Day 5 Post-Infection of Fish from Resistant and Susceptible Genetic Lines
2.9. Cell Wall Hydrolase (CWH)
2.10. CWH Is Conserved among Many Strains of Fp
2.11. Genetic Manipulation of the Cell Wall Hydrolase Gene Failed in Fp
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strain and Growth Condition
4.2. Isolation of OMVs
4.3. Transmission Electron Microscopy (TEM) of OMVs
4.4. RNA Extraction, Library Preparation, and Sequencing
4.5. Data Processing and Functional Prediction of Transcripts
4.6. Bacterial Challenge of BCWD-Resistant and BCWD-Susceptible Fish Population
4.7. Sequencing and Differential Gene Expression Analysis of BCWD-Resistant and BCWD-Susceptible Fish
4.8. Conservation of CWH Transcripts
4.9. Genetic Manipulation of CWH Gene
Construction of the CWH Deletion Mutant
4.10. Conjugative Transfer of Plasmid into F. psychrophilum
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starliper, C.E. Bacterial coldwater disease of fishes caused by Flavobacterium psychrophilum. J. Adv. Res. 2011, 2, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Wiens, G.D.; LaPatra, S.E.; Welch, T.J.; Rexroad, C., 3rd; Call, D.R.; Cain, K.D.; LaFrentz, B.R.; Vaisvil, B.; Schmitt, D.P.; Kapatral, V. Complete Genome Sequence of Flavobacterium psychrophilum Strain CSF259-93, Used To Select Rainbow Trout for Increased Genetic Resistance against Bacterial Cold Water Disease. Genome Announc. 2014, 2, e00889-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, M.E. A review of Flavobacterium psychrophilum Biology, Clinical signs, and Bacterial Cold Water Disease Prevention and Treatment. Open Fish Sci. J. 2011, 4, 40–48. [Google Scholar] [CrossRef]
- Levipan, H.A.; Avendano-Herrera, R. Different Phenotypes of Mature Biofilm in Flavobacterium psychrophilum Share a Potential for Virulence That Differs from Planktonic State. Front. Cell Infect. Microbiol. 2017, 7, 76. [Google Scholar] [CrossRef]
- Nematollahi, A.; Decostere, A.; Pasmans, F.; Haesebrouck, F. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 2003, 26, 563–574. [Google Scholar] [CrossRef]
- Leeds, T.D.; Silverstein, J.T.; Weber, G.M.; Vallejo, R.L.; Palti, Y.; Rexroad, C.E., 3rd; Evenhuis, J.; Hadidi, S.; Welch, T.J.; Wiens, G.D. Response to selection for bacterial cold water disease resistance in rainbow trout. J. Anim. Sci. 2010, 88, 1936–1946. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Johnston, J.J.; van Baaren, J.M.; McBride, M.J. Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA. J. Bacteriol. 2013, 195, 3201–3212. [Google Scholar] [CrossRef] [Green Version]
- Duchaud, E.; Boussaha, M.; Loux, V.; Bernardet, J.F.; Michel, C.; Kerouault, B.; Mondot, S.; Nicolas, P.; Bossy, R.; Caron, C.; et al. Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat. Biotechnol. 2007, 25, 763–769. [Google Scholar] [CrossRef]
- Secades, P.; Alvarez, B.; Guijarro, J.A. Purification and properties of a new psychrophilic metalloprotease (Fpp2) in the fish pathogen Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2003, 226, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, B.; Alvarez, J.; Menendez, A.; Guijarro, J.A. A mutant in one of two exbD loci of a TonB system in Flavobacterium psychrophilum shows attenuated virulence and confers protection against cold water disease. Microbiology 2008, 154, 1144–1151. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, K.; Hampton, T.H.; Jarek, M.; Scharfe, M.; Gerber, S.A.; Mielcarz, D.W.; Demers, E.G.; Dolben, E.L.; Hammond, J.H.; Hogan, D.A.; et al. A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016, 12, e1005672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjostrom, A.E.; Sandblad, L.; Uhlin, B.E.; Wai, S.N. Membrane vesicle-mediated release of bacterial RNA. Sci. Rep. 2015, 5, 15329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyckoff, T.J.; Taylor, J.A.; Salama, N.R. Beyond growth: Novel functions for bacterial cell wall hydrolases. Trends Microbiol. 2012, 20, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, L.; Lepek, K.; Stasilojc, M.; Kosznik-Kwasnicka, K.; Zdrojewska, K.; Maciag-Dorszynska, M.; Wegrzyn, G.; Wegrzyn, A. Bacteriophage-encoded enzymes destroying bacterial cell membranes and walls, and their potential use as antimicrobial agents. Microbiol. Res. 2021, 248, 126746. [Google Scholar] [CrossRef]
- Eshwar, A.K.; Guldimann, C.; Oevermann, A.; Tasara, T. Cold-Shock Domain Family Proteins (Csps) Are Involved in Regulation of Virulence, Cellular Aggregation, and Flagella-Based Motility in Listeria monocytogenes. Front. Cell Infect. Microbiol. 2017, 7, 453. [Google Scholar] [CrossRef]
- Yan, W.; Wang, H.; Xu, W.; Wu, K.; Yao, R.; Xu, X.; Dong, J.; Zhang, Y.; Zhong, W.; Zhang, X. SP0454, a putative threonine dehydratase, is required for pneumococcal virulence in mice. J. Microbiol. 2012, 50, 511–517. [Google Scholar] [CrossRef]
- Stonehouse, E.; Kovacikova, G.; Taylor, R.K.; Skorupski, K. Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J. Bacteriol. 2008, 190, 4736–4748. [Google Scholar] [CrossRef] [Green Version]
- Poehlsgaard, J.; Douthwaite, S. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 2005, 3, 870–881. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, D.; Steitz, T.A.; Polikanov, Y.S.; Gagnon, M.G. Ribosome-Targeting Antibiotics: Modes of Action, Mechanisms of Resistance, and Implications for Drug Design. Annu. Rev. Biochem. 2018, 87, 451–478. [Google Scholar] [CrossRef] [Green Version]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular Mechanisms of Two-Component Signal Transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvage, E.; Terrak, M. Glycosyltransferases and Transpeptidases/Penicillin-Binding Proteins: Valuable Targets for New Antibacterials. Antibiotics 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- El Qaidi, S.; Zhu, C.; McDonald, P.; Roy, A.; Maity, P.K.; Rane, D.; Perera, C.; Hardwidge, P.R. High-Throughput Screening for Bacterial Glycosyltransferase Inhibitors. Front. Cell Infect. Microbiol. 2018, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y. Telling bacteria: Do not LytTR. Structure 2008, 16, 657–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crater, D.L.; Moran, C.P., Jr. Identification of a DNA binding region in GerE from Bacillus subtilis. J. Bacteriol. 2001, 183, 4183–4189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, R.; Jackowski, S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus 2007, 2. [Google Scholar] [CrossRef] [Green Version]
- Izard, T. A novel adenylate binding site confers phosphopantetheine adenylyltransferase interactions with coenzyme A. J. Bacteriol. 2003, 185, 4074–4080. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Sharma, P.; Singh, T.P.; Sharma, S. Phosphopantetheine Adenylyltransferase: A promising drug target to combat antibiotic resistance. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140566. [Google Scholar] [CrossRef]
- Yang, B.; Feng, L.; Wang, F.; Wang, L. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat. Commun. 2015, 6, 6592. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Jiang, L.; Wang, S.; Wang, L. Global transcriptional regulation by BirA in enterohemorrhagic Escherichia coli O157:H7. Future Microbiol. 2018, 13, 757–769. [Google Scholar] [CrossRef]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. A central role for aspartate in Mycobacterium tuberculosis physiology and virulence. Front. Cell Infect. Microbiol. 2013, 3, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferla, M.P.; Patrick, W.M. Bacterial methionine biosynthesis. Microbiology 2014, 160, 1571–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murima, P.; McKinney, J.D.; Pethe, K. Targeting bacterial central metabolism for drug development. Chem. Biol. 2014, 21, 1423–1432. [Google Scholar] [CrossRef]
- Husna, A.U.; Wang, N.; Cobbold, S.A.; Newton, H.J.; Hocking, D.M.; Wilksch, J.J.; Scott, T.A.; Davies, M.R.; Hinton, J.C.; Tree, J.J.; et al. Methionine biosynthesis and transport are functionally redundant for the growth and virulence of Salmonella Typhimurium. J. Biol. Chem. 2018, 293, 9506–9519. [Google Scholar] [CrossRef] [Green Version]
- Jurgenson, C.T.; Begley, T.P.; Ealick, S.E. The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem. 2009, 78, 569–603. [Google Scholar] [CrossRef]
- Maupin-Furlow, J.A. Vitamin B1 (Thiamine) Metabolism and Regulation in Archaea. In B Group Vitamins—Current Uses and Perspectives; LeBlanc, J.G., Ed.; IntechOpen Limited: London, UK, 2018; pp. 9–31. [Google Scholar]
- Liu, X.; Wang, X.; Sun, B.; Sun, L. The Involvement of Thiamine Uptake in the Virulence of Edwardsiella piscicida. Pathogens 2022, 11, 464. [Google Scholar] [CrossRef]
- Rolando, M.; Silvestre, C.D.; Gomez-Valero, L.; Buchrieser, C. Bacterial methyltransferases: From targeting bacterial genomes to host epigenetics. microLife 2022, 3, uqac014. [Google Scholar] [CrossRef]
- Marancik, D.; Gao, G.; Paneru, B.; Ma, H.; Hernandez, A.G.; Salem, M.; Yao, J.; Palti, Y.; Wiens, G.D. Whole-body transcriptome of selectively bred, resistant-, control-, and susceptible-line rainbow trout following experimental challenge with Flavobacterium psychrophilum. Front. Genet. 2014, 5, 453. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Castro, C.; Saini, A.; Outten, F.W. Fe-S cluster assembly pathways in bacteria. Microbiol. Mol. Biol. Rev. 2008, 72, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Lechardeur, D.; Bernardet, J.F.; Kerouault, B.; Guerin, C.; Rigaudeau, D.; Nicolas, P.; Duchaud, E.; Rochat, T. Two functionally distinct heme/iron transport systems are virulence determinants of the fish pathogen Flavobacterium psychrophilum. Virulence 2022, 13, 1221–1241. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Ma, J.; Sudheesh, P.S.; Cain, K.D. Quantification and comparison of gene expression associated with iron regulation and metabolism in a virulent and attenuated strain of Flavobacterium psychrophilum. J. Fish Dis. 2021, 44, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L. Biosynthesis of Glutamate, Aspartate, Asparagine, L-Alanine, and D-Alanine. EcoSal Plus 2004, 1. [Google Scholar] [CrossRef]
- Xie, K.; Bunse, C.; Marcus, K.; Leichert, L.I. Quantifying changes in the bacterial thiol redox proteome during host-pathogen interaction. Redox Biol. 2019, 21, 101087. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhang, L.; Wan, X.; Chen, J.; Hu, L.; Ding, X.; Li, L.; Karar, J.; Peng, H.; Chen, S.; et al. Structure and specificity of the bacterial cysteine methyltransferase effector NleE suggests a novel substrate in human DNA repair pathway. PLoS Pathog. 2014, 10, e1004522. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Zhao, M.; Huang, Y.; Li, J.; Liu, X.; Ren, Z.; Kan, B.; Liang, W. Integration Host Factor Modulates the Expression and Function of T6SS2 in Vibrio fluvialis. Front. Microbiol. 2018, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- UniProt. hupB—DNA-Binding Protein HU-Beta—Escherichia coli (Strain K12)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P0ACF4/entry (accessed on 2 September 2022).
- El Yacoubi, B.; Bonnett, S.; Anderson, J.N.; Swairjo, M.A.; Iwata-Reuyl, D.; de Crecy-Lagard, V. Discovery of a new prokaryotic type I GTP cyclohydrolase family. J. Biol. Chem. 2006, 281, 37586–37593. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Wouters, J.; Poulter, C.D. Isopentenyl diphosphate isomerase. Mechanism-based inhibition by diene analogues of isopentenyl diphosphate and dimethylallyl diphosphate. J. Am. Chem. Soc. 2005, 127, 17433–17438. [Google Scholar] [CrossRef] [Green Version]
- Heuston, S.; Begley, M.; Gahan, C.G.M.; Hill, C. Isoprenoid biosynthesis in bacterial pathogens. Microbiology 2012, 158, 1389–1401. [Google Scholar] [CrossRef]
- Drabinska, J.; Ziecina, M.; Modzelan, M.; Jagura-Burdzy, G.; Kraszewska, E. Individual Nudix hydrolases affect diverse features of Pseudomonas aeruginosa. MicrobiologyOpen 2020, 9, e1052. [Google Scholar] [CrossRef]
- Lu, W.; Tan, J.; Lu, H.; Wang, G.; Dong, W.; Wang, C.; Li, X.; Tan, C. Function of Rhs proteins in porcine extraintestinal pathogenic Escherichia coli PCN033. J. Microbiol. 2021, 59, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Page, J.E.; Walker, S. Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes. J. Biol. Chem. 2020, 295, 3347–3361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochat, T.; Perez-Pascual, D.; Nilsen, H.; Carpentier, M.; Bridel, S.; Bernardet, J.F.; Duchaud, E. Identification of a Novel Elastin-Degrading Enzyme from the Fish Pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2019, 85, e02535-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell Wall Hydrolases in Bacteria: Insight on the Diversity of Cell Wall Amidases, Glycosidases and Peptidases Toward Peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- van Heijenoort, J. Peptidoglycan hydrolases of Escherichia coli. Microbiol. Mol. Biol. Rev. 2011, 75, 636–663. [Google Scholar] [CrossRef] [Green Version]
- Kroniger, T.; Flender, D.; Schluter, R.; Kollner, B.; Trautwein-Schult, A.; Becher, D. Proteome analysis of the Gram-positive fish pathogen Renibacterium salmoninarum reveals putative role of membrane vesicles in virulence. Sci. Rep. 2022, 12, 3003. [Google Scholar] [CrossRef]
- Castillo, D.; Christiansen, R.H.; Dalsgaard, I.; Madsen, L.; Espejo, R.; Middelboe, M. Comparative Genome Analysis Provides Insights into the Pathogenicity of Flavobacterium psychrophilum. PLoS ONE 2016, 11, e0152515. [Google Scholar] [CrossRef] [Green Version]
- Jarau, M.; Di Natale, A.; Huber, P.E.; MacInnes, J.I.; Lumsden, J.S. Virulence of Flavobacterium psychrophilum isolates in rainbow trout Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2018, 41, 1505–1514. [Google Scholar] [CrossRef]
- Sundell, K.; Landor, L.; Nicolas, P.; Jorgensen, J.; Castillo, D.; Middelboe, M.; Dalsgaard, I.; Donati, V.L.; Madsen, L.; Wiklund, T. Phenotypic and Genetic Predictors of Pathogenicity and Virulence in Flavobacterium psychrophilum. Front. Microbiol. 2019, 10, 1711. [Google Scholar] [CrossRef]
- Gliniewicz, K.; Wildung, M.; Orfe, L.H.; Wiens, G.D.; Cain, K.D.; Lahmers, K.K.; Snekvik, K.R.; Call, D.R. Potential mechanisms of attenuation for rifampicin-passaged strains of Flavobacterium psychrophilum. BMC Microbiol. 2015, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Chodisetti, P.K.; Reddy, M. Peptidoglycan hydrolase of an unusual cross-link cleavage specificity contributes to bacterial cell wall synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 7825–7830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, P.; Rochat, T.; Mohammed, H.H.; Wiens, G.D.; Bernardet, J.F.; Halpern, D.; Duchaud, E.; McBride, M.J. The Type IX Secretion System Is Required for Virulence of the Fish Pathogen Flavobacterium psychrophilum. Appl. Envrion. Microbiol. 2020, 86, e01769-17. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Garcıa-Márquez, S.; Santos, Y. Improved growth of Flavobacterium psychrophilum using a new culture medium. Aquaculture 2004, 238, 75–82. [Google Scholar] [CrossRef]
- Silverstein, J.T.; Vallejo, R.L.; Palti, Y.; Leeds, T.D.; Rexroad, C.E., 3rd; Welch, T.J.; Wiens, G.D.; Ducrocq, V. Rainbow trout resistance to bacterial cold-water disease is moderately heritable and is not adversely correlated with growth. J. Anim. Sci. 2009, 87, 860–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneru, B.; Al-Tobasei, R.; Palti, Y.; Wiens, G.D.; Salem, M. Differential expression of long non-coding RNAs in three genetic lines of rainbow trout in response to infection with Flavobacterium psychrophilum. Sci. Rep. 2016, 6, 36032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Al-Tobasei, R.; Kenney, B.; Leeds, T.D.; Salem, M. Integrated analysis of lncRNA and mRNA expression in rainbow trout families showing variation in muscle growth and fillet quality traits. Sci. Rep. 2018, 8, 12111. [Google Scholar] [CrossRef] [Green Version]
- de Jong, A.; Kuipers, O.P.; Kok, J. FUNAGE-Pro: Comprehensive web server for gene set enrichment analysis of prokaryotes. Nucleic Acids Res. 2022, 50, W330–W336. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
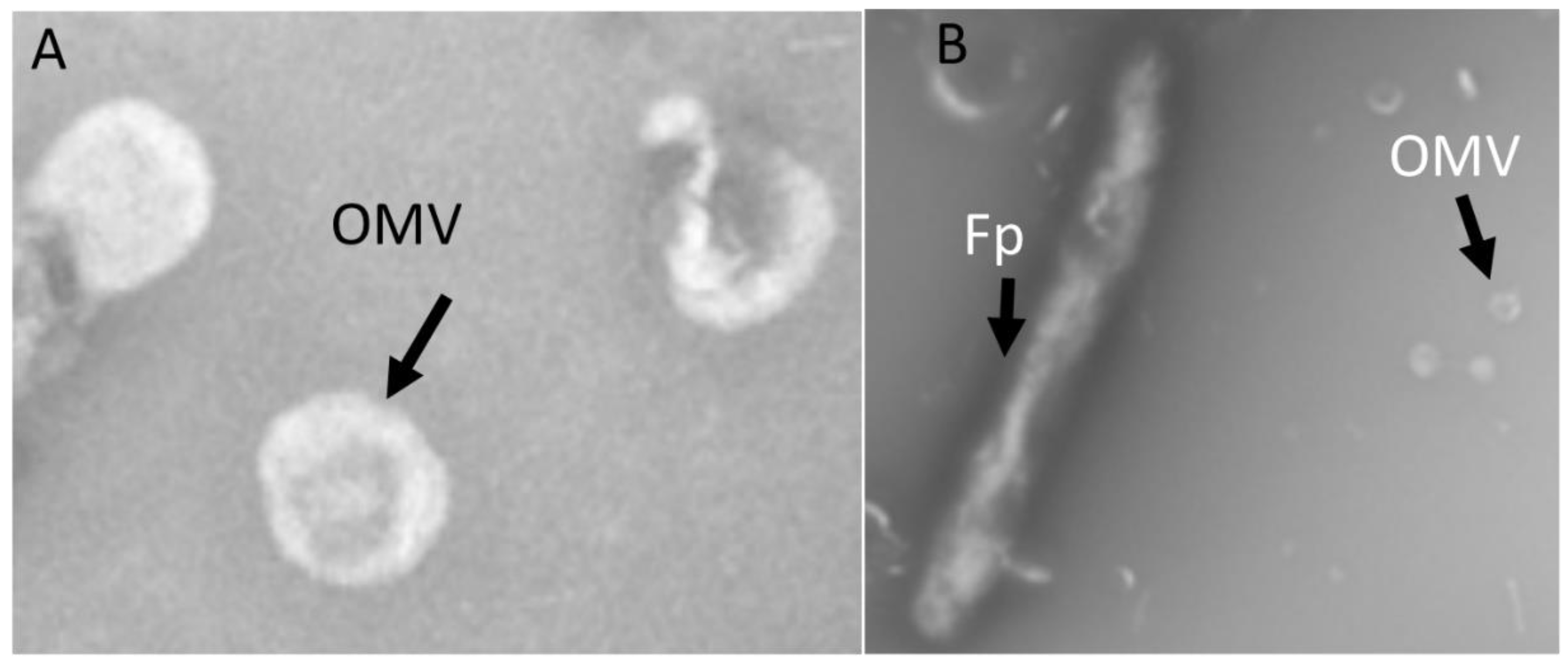


| Feature ID | Gene Description | Differential Abundance (OMVs/Fp Whole Cell) |
|---|---|---|
| FE46_RS03875 | Hypothetical protein IA03_02225 | 258.63 |
| FE46_RS01465 | Cold shock domain-containing protein | 236.83 |
| FE46_RS04325 | DUF3820 family protein | 40.87 |
| FE46_RS03890 | Co-chaperone GroES | 28.51 |
| FE46_RS08245 | Putative membrane spanning protein | 25.95 |
| FE46_RS05500 | Integration host factor subunit beta | 23.18 |
| FE46_RS05600 | CDP-alcohol phosphatidyltransferase family protein | 18.81 |
| FE46_RS02350 | Threonine ammonia-lyase IlvA | 18.17 |
| FE46_RS04330 | OsmC family protein | 15.54 |
| FE46_RS02255 | Cadmium-translocating P-type ATPase | −223.61 |
| FE46_RS12900 | Leucine-rich repeat protein | −193.17 |
| FE46_RS10250 | Family transcriptional regulator | −186.12 |
| FE46_RS11155 | Ketoacyl-ACP synthase III | −127.88 |
| FE46_RS02245 | Acetyl-hydrolase transferase family | −117.56 |
| FE46_RS12880 | Leucine-rich repeat protein | −100.47 |
| FE46_RS12920 | Leucine-rich repeat protein | −82.91 |
| FE46_RS12885 | Leucine-rich repeat protein | −79.44 |
| FE46_RS03485 | Division cell wall cluster transcriptional repressor | −60.93 |
| FE46_RS03530 | UDP-N-acetylmuramate--L-alanine ligase | −59.90 |
| FE46_RS11460 | NADH-quinone oxidoreductase subunit B | −53.92 |
| FE46_RS04540 | DUF2147 domain-containing | −51.66 |
| Feature ID | Gene Description | Differential Abundance (OMVs/Fp Whole Cell) |
|---|---|---|
| FE46_RS03770 | HU family DNA-binding protein | 250.39 |
| FE46_RS09040 | 30S ribosomal protein S20 | 236.37 |
| FE46_RS03985 | KTSC domain-containing protein | 124.10 |
| FE46_RS03320 | Family outer membrane | 61.13 |
| FE46_RS09965 | Type B 50S ribosomal L31 | 43.71 |
| FE46_RS09215 | 50S ribosomal L33 | 37.49 |
| FE46_RS12230 | 50S ribosomal L32 | 33.37 |
| FE46_RS01825 | 30S ribosomal S16 | 30.62 |
| FE46_RS01795 | Inorganic pyrophosphatase | 29.61 |
| FE46_RS12455 | Copper resistance | 28.99 |
| FE46_RS11385 | 50S ribosomal L27 | 23.06 |
| FE46_RS05880 | 3,4-Dihydroxy-2-butanone-4-phosphate synthase | 22.28 |
| FE46_RS04555 | 30S ribosomal S6 | 21.17 |
| FE46_RS04560 | 30S ribosomal S18 | 19.11 |
| FE46_RS02470 | Ribosome assembly cofactor | 16.53 |
| FE46_RS05480 | NAD(P) FAD-dependent oxidoreductase | −158.95 |
| FE46_RS10070 | Cytochrome-c cbb3-type subunit I | −149.66 |
| FE46_RS08120 | 4Fe-4S dicluster domain-containing | −125.13 |
| FE46_RS10110 | Aconitate hydratase | −59.32 |
| FE46_RS10085 | Cytochrome c oxidase accessory | −56.74 |
| FE46_RS01775 | Electron transfer flavo subunit alpha family | −40.12 |
| FE46_RS12380 | 2-Oxoglutarate dehydrogenase complex dihydrolipoyllysine-residue succinyltransferase | −37.85 |
| FE46_RS01970 | 4Fe-4S dicluster domain-containing | −33.28 |
| FE46_RS12375 | 2-Oxoglutarate dehydrogenase E1 component | −26.94 |
| FE46_RS02175 | L-glutamate gamma-semialdehyde dehydrogenase | −25.98 |
| FE46_RS06595 | Succinate--ligase subunit alpha | −24.98 |
| FE46_RS01780 | Electron transfer flavo subunit beta family | −24.12 |
| FE46_RS05145 | Dihydrolipoyl dehydrogenase | −23.63 |
| FE46_RS08480 | FAD-binding | −22.90 |
| FE46_RS05800 | Class II fumarate hydratase | −22.35 |
| FE46_RS11950 | Aldehyde dehydrogenase family | −21.72 |
| FE46_RS00480 | Succinate dehydrogenase/fumarate reductase iron-sulfur subunit | −17.80 |
| Feature ID | Fold Change (R/S) | p-Value FDR | Gene Description |
|---|---|---|---|
| FE46_RS09020 | −1204.81 | 0.002 | Cell wall-associated hydrolase |
| FE46_RS07385 | −348.46 | 0.003 | TIGR00730 family Rossman fold protein |
| FE46_RS11855 | −306.96 | 0.003 | Rhs-family protein |
| FE46_RS04860 | −291.40 | 0.003 | Isopentenyl-diphosphate delta-isomerase |
| FE46_RS08365 | −288.91 | 0.003 | GTP cyclohydrolase |
| FE46_RS08015 | −116.46 | 0.022 | NUDIX domain-containing protein |
| FE46_RS08160 | −84.13 | 0.006 | GTP cyclohydrolase I |
| FE46_RS11250 | −8.59 | 0.018 | Cell wall-associated hydrolase |
| Restriction Enzyme | Primers | Tm (Degree C) |
|---|---|---|
| CWH1us(Bam) | 5′ actactGGATCCTAAAAGACAAAATATGCTAGATGG 3′ | 61 |
| CWH1us(Sal) | 3′ actactGTCGACTTATGTACACACTTTTCCCGAG 5′ | 62 |
| CWH1ds(Pst) | 5′ actactCTGCAGTTTCTAGCCATTAGCCATTAG 3′ | 60 |
| CWH1ds(Pst) | 3′ actactCTGCAGTTATCAAATCCGTGTCATCTG 5′ | 60 |
| CWH1ko(scrn) | 5′ GAATTTAGAAATATTTATGAAGAAAC 3′ | 60 |
| CWH1ko(scrn) | 3′ TCTCGTAGCTCAGCTGGTTAG 5′ | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapagain, P.; Ali, A.; Salem, M. Dual RNA-Seq of Flavobacterium psychrophilum and Its Outer Membrane Vesicles Distinguishes Genes Associated with Susceptibility to Bacterial Cold-Water Disease in Rainbow Trout (Oncorhynchus mykiss). Pathogens 2023, 12, 436. https://doi.org/10.3390/pathogens12030436
Chapagain P, Ali A, Salem M. Dual RNA-Seq of Flavobacterium psychrophilum and Its Outer Membrane Vesicles Distinguishes Genes Associated with Susceptibility to Bacterial Cold-Water Disease in Rainbow Trout (Oncorhynchus mykiss). Pathogens. 2023; 12(3):436. https://doi.org/10.3390/pathogens12030436
Chicago/Turabian StyleChapagain, Pratima, Ali Ali, and Mohamed Salem. 2023. "Dual RNA-Seq of Flavobacterium psychrophilum and Its Outer Membrane Vesicles Distinguishes Genes Associated with Susceptibility to Bacterial Cold-Water Disease in Rainbow Trout (Oncorhynchus mykiss)" Pathogens 12, no. 3: 436. https://doi.org/10.3390/pathogens12030436
APA StyleChapagain, P., Ali, A., & Salem, M. (2023). Dual RNA-Seq of Flavobacterium psychrophilum and Its Outer Membrane Vesicles Distinguishes Genes Associated with Susceptibility to Bacterial Cold-Water Disease in Rainbow Trout (Oncorhynchus mykiss). Pathogens, 12(3), 436. https://doi.org/10.3390/pathogens12030436










