‘Candidatus Rickettsia andeanae’ and Probable Exclusion of Rickettsia parkeri in Ticks from Dogs in a Natural Area of the Pampa Biome in Brazil
Abstract
1. Introduction
2. Materials and Methods
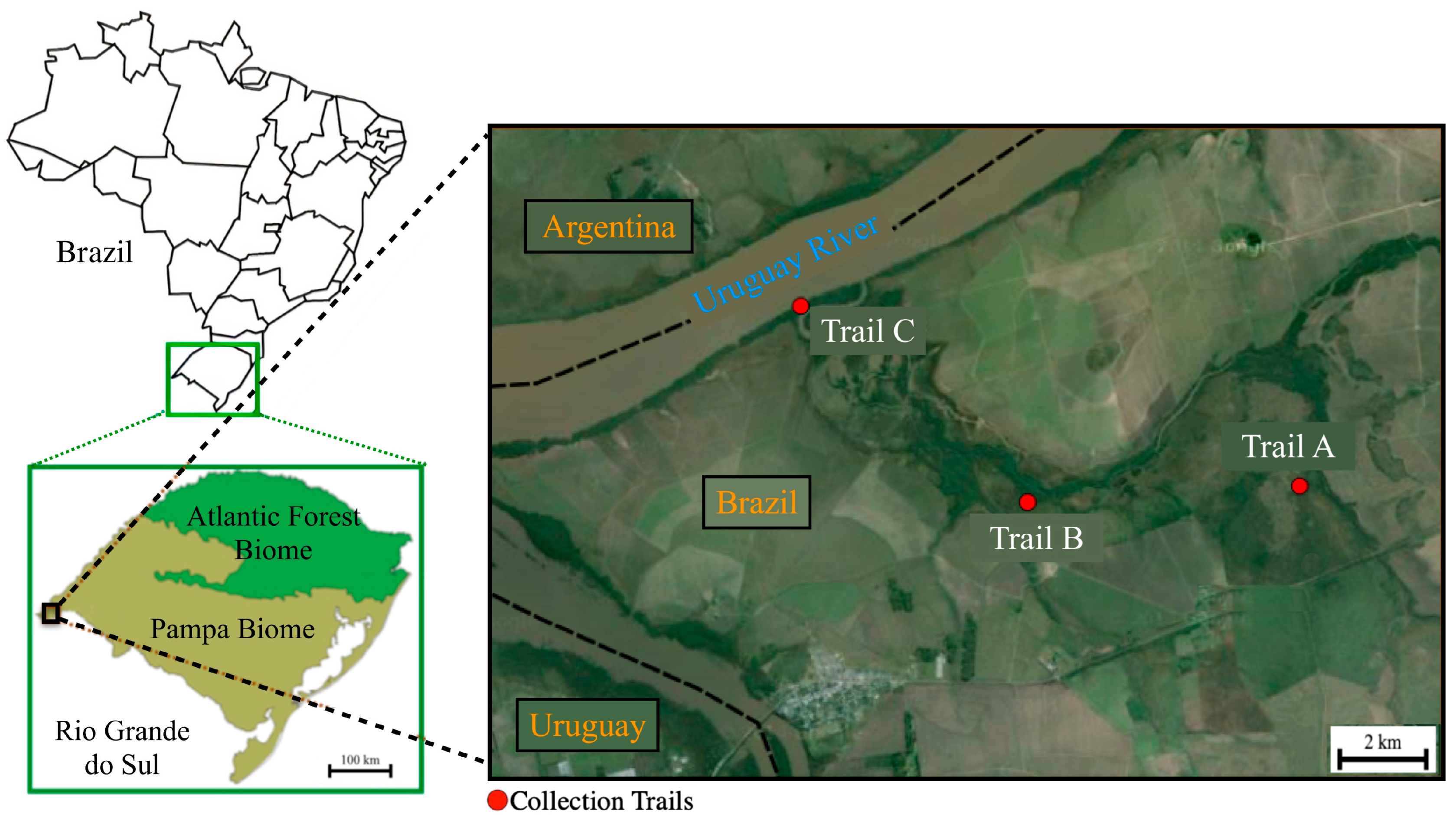
2.1. Study Area
2.2. Domestic Dogs
2.3. Small Mammals
2.4. Host-Seeking Ticks
2.5. Tick Identification and Rickettsial Infection in Ticks
2.6. Serology
2.7. Correlation of Rickettsial Infection in A. tigrinum Populations
3. Results
3.1. Ticks from Domestic Dogs
3.2. Ticks from Wild Mammals and Vegetations
3.3. Rickettsial Infection in Ticks
3.4. Serology
3.5. Accession Numbers
3.6. Correlation of Rickettsial Infection in A. tigrinum Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Morales, A.J.; Bonilla-Aldana, D.K.; Idarraga-Bedoya, S.E.; Garcia-Bustos, J.J.; Cardona-Ospina, J.A.; Faccini-Martínez, Á.A. Epidemiology of zoonotic tick-borne diseases in Latin America: Are we just seeing the tip of the iceberg? F1000Research 2018, 7, 1988. [Google Scholar] [CrossRef]
- Labruna, M.B.; Kamakura, O.; Moraes-Filho, J.; Horta, M.C.; Pacheco, R.C. Rocky Mountain spotted fever in dogs, Brazil. Emerg. Infect. Dis. 2009, 15, 458–460. [Google Scholar] [CrossRef]
- Faccini-Martínez, Á.A.; Krawczak, F.S.; Oliveira, S.V.; Labruna, M.B.; Angerami, R.N. Rickettsioses in Brazil: Distinct diseases and new paradigms for epidemiological surveillance. Rev. Soc. Bras. Med. Trop. 2021, 54, e07322020. [Google Scholar] [CrossRef] [PubMed]
- Weck, B.; Dall’Agnol, B.; Souza, U.; Webster, A.; Stenzel, B.; Klafke, G.; Martins, J.R.; Reck, J. Spotted fever group Rickettsia in the pampa biome, Brazil, 2015–2016. Emerg. Infect. Dis. 2016, 22, 2014–2016. [Google Scholar] [CrossRef]
- Moo-Llanes, D.A.; Oca-Aguilar, A.C.M.; Romero-Salas, D.; Sánchez-Montes, S. Inferring the Potential Distribution of an Emerging Rickettsiosis in America: The Case of Rickettsia parkeri. Pathogens 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schunemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A fragile biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Ministério da Saúde. Casos confirmados de febre maculosa. Brasil, Grandes Regiões e Unidades Federadas. 2007 a 2022*. Sistema de Informação de Agravos de Notificação. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa/situacao-epidemiologica/casos-confirmados-de-febre-maculosa-brasil-grandes-regioes-e-unidades-federadas-infeccao-2007-2022/view (accessed on 24 November 2022).
- Krawczak, F.S.; Binder, L.C.; Oliveira, C.S.; Costa, F.B.; Moraes-Filho, J.; Martins, T.F.; Sponchiado, J.; Melo, G.L.; Gregori, F.; Polo, G.; et al. Ecology of a tick-borne spotted fever in southern Brazil. Exp. Appl. Acarol. 2016, 70, 219–229. [Google Scholar] [CrossRef]
- Evans, D.E.; Martins, J.R.; Guglielmone, A.A. A review of the ticks (Acari, Ixodida) of Brazil, their hosts and geographic distribution—1. The State of Rio Grande do Sul, Southern Brazil. Mem. Inst. Oswaldo Cruz. 2000, 95, 453–470. [Google Scholar] [CrossRef]
- Nava, S.; Elshewany, Y.; Eremeeva, M.E.; Sumner, J.W.; Mastropaolo, M.; Paddock, C.D. Rickettsia parkeri in Argentina. Emerg. Infect. Dis. 2008, 14, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Venzal, J.M.; Portillo, A.; Estrada-Peña, A.; Castro, O.; Cabrera, P.A.; Oteo, J.Á. Rickettsia parkeri in Amblyomma triste from Uruguay. Emerg. Infect. Dis. 2004, 10, 1493–1495. [Google Scholar] [CrossRef]
- Romer, Y.; Seijo, A.C.; Crudo, F.; Nicholson, W.L.; Varela-Stokes, A.; Lash, R.R.; Paddock, C.D. Rickettsia parkeri rickettsiosis, Argentina. Emerg. Infect. Dis. 2011, 17, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Denison, A.M.; Dryden, M.W.; Noden, B.H.; Lash, R.R.; Abdelghani, S.S.; Evans, A.E.; Kelly, A.R.; Hecht, J.A.; Karpathy, S.E.; et al. High prevalence of “Candidatus Rickettsia andeanae” and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculatum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks Tick Borne Dis. 2015, 6, 297–302. [Google Scholar] [CrossRef]
- Lado, P.; Castro, O.; Labruna, M.B.; Venzal, J.M. First molecular detection of Rickettsia parkeri in Amblyomma tigrinum and Amblyomma dubitatum ticks from Uruguay. Ticks Tick Borne Dis. 2014, 5, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Souza, U.; Dall’Agnol, B.; Michel, T.; Webster, A.; Weck, B.; Doyle, R.; Kasper, C.B.; Soares, J.; Martins, J.R.; Trigo, T.C.; et al. Molecular survey of Rickettsia spp. in the Neotropical deer tick Haemaphysalis juxtakochi from Brazilian pampa. Parasitol Res. 2018, 117, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Weck, B.; Dall’Agnol, B.; Souza, U.; Webster, A.; Stenzel, B.; Klafke, G.; Martins, J.R.; Reck, J. Rickettsia parkeri in Amblyomma dubitatum ticks in a spotted fever focus from the Brazilian Pampa. Acta Trop. 2017, 28, 182–185. [Google Scholar] [CrossRef]
- Weck, B.; Krawczak, F.S.; Costa, F.B.; Dall’Agnol, B.; Marcili, A.; Reck, J.; Labruna, M.B. Rickettsia parkeri in the Pampa biome of southern Brazil: Isolation, molecular characterization, and serological evidence of canine infection. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100448. [Google Scholar] [CrossRef]
- Dall’Agnol, B.; Souza, U.A.; Weck, B.; Trigo, T.C.; Jardim, M.M.A.; Costa, F.B.; Labruna, M.B.; Peters, F.B.; Favarini, M.O.; Mazim, F.D.; et al. Rickettsia parkeri in free-ranging wild canids from Brazilian Pampa. Transbound. Emerg. Dis. 2018, 65, e224–e230. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.B.; Cunha-Filho, N.A.; Pacheco, R.C.; Ferreira, F.; Pappen, F.G.; Farias, N.A.; Larsson, C.E.; Labruna, M.B. Canine infection by rickettsiae and ehrlichiae in southern Brazil. Am. J. Trop. Med. Hyg. 2008, 79, 102–108. [Google Scholar] [CrossRef]
- Rio Grande do Sul. Plano de Manejo do Parque Estadual do Espinilho; Secretaria Estadual do Meio Ambiente, Departamento de Florestas e Áreas Protegidas: Porto Alegre, Brazil, 2009; pp. 1–238. Available online: https://www.sema.rs.gov.br/parque-estadual-do-espinilho (accessed on 7 February 2023).
- Krawczak, F.S.; Binder, L.C.; Sobotyk, C.; Costa, F.B.; Gregori, F.; Martins, T.F.; Pádua, G.T.; Sponchiado, J.; Melo, G.L.; Polo, G.; et al. Rickettsial infection in ticks from a natural area of Atlantic Forest biome in southern Brazil. Exp. Appl. Acarol. 2022, 88, 371–386. [Google Scholar] [CrossRef]
- Soto, F.R.M.; Ferreira, F.; Pinheiro, S.R.; Nogari, F.; Risseto, M.R.; de Souza, O.; Amaku, M. Dinâmica populacional canina no Município de Ibiúna-SP estudo retrospectivo. Braz. J. Vet. Res. Anim. Sci. 2006, 43, 178. [Google Scholar] [CrossRef]
- Labruna, M.B.; Horta, M.C.; Aguiar, D.M.; Cavalcante, G.T.; Pinter, A.; Gennari, S.M.; Camargo, L.M.A. Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro Municipality, Western Amazon, Brazil. Vector-Borne Zoonotic Dis. 2007, 7, 249–255. [Google Scholar] [CrossRef]
- Arya, R.; Antonisamy, B.; Kumar, S. Sample size estimation in prevalence studies. Indian J. Pediatr. 2012, 79, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, F.; Naxara, L.; Pardini, R. Evaluating the efficiency of pitfall traps for sampling small mammals in the neotropics. J. Mammal. 2006, 87, 757–765. [Google Scholar] [CrossRef]
- Bonvicino, C.R.; Oliveira, J.A.; D’Andrea, P.S. Guia dos Roedores do Brasil, Com Chaves Para Gêneros Baseadas em Caracteres Externos. Organização Pan-Americana da Saúde. 2008. 120p. Available online: http://www.fiocruz.br/ioc/media/livro%20roedores.pdf (accessed on 24 November 2022).
- Melo, G.L.; Sponchiado, J.; Machado, A.F.; Cáceres, N.C. Small-mammal community structure in a South American deciduous Atlantic Forest. Community Ecol. 2011, 12, 58–66. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Borges, L.M.F.; Lopes, C.M.L.; Leite, R.C. Population dynamics of the free-living stages of Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) on pastures of Pedro Leopoldo, Minas Gerais State, Brazil. Vet. Parasitol. 2000, 92, 295–301. [Google Scholar] [CrossRef]
- Terassini, F.A.; Barbieri, F.S.; Albuquerque, S.; Szabó, M.P.J.; Camargo, L.M.A.; Labruna, M.B. Comparison of two methods for collecting free-living ticks in the Amazonian Forest. Ticks Tick Borne Dis. 2010, 1, 194–196. [Google Scholar] [CrossRef]
- Martins, T.F.; Onofrio, V.C.; Barros-Battesti, D.M.; Labruna, M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification key. Ticks Tick-Borne Dis. 2010, 1, 75–99. [Google Scholar] [CrossRef]
- Barros-Battesti, D.M.; Arzua, M.; Bechara, G.H. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um Guia Ilustrado para Identificação de Espécies; Vox/ICTTD-3/ Butantan: São Paulo, Brazil, 2006; pp. 1–223. [Google Scholar]
- Nava, S.; Beati, L.; Venzal, J.M.; Labruna, M.B.; Szabó, M.P.J.; Petney, T.; Saracho-Bottero, M.N.; Tarragona, E.L.; Dantas-Torres, F.; Silva, M.M.S.; et al. Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick-Borne Dis. 2018, 9, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the State of São Paulo, Brazil, where Brazilian Spotted Fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sangioni, L.A.; Horta, M.C.; Vianna, M.C.B.; Gennari, S.M.; Soares, R.M.; Galvão, M.A.M.; Schumaker, T.T.S.; Ferreira, F.; Vidotto, O.; Labruna, M.B. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg. Infect. Dis. 2005, 11, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Horta, M.C.; Labruna, M.B.; Pinter, A.; Linardi, P.M.; Schumaker, T.T.S. Rickettsia infection in five areas of the state of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2007, 102, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Guedes, E.; Leite, R.C.; Prata, M.C.A.; Pacheco, R.C.; Walker, D.H.; Labruna, M.B. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever-endemic area in the state of Minas Gerais. Mem. Inst. Oswaldo Cruz 2005, 100, 841–845. [Google Scholar] [CrossRef]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996, 34, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and othertick genera among Metastriata (Acari: Ixodidae). Parasitol. Res. 1998, 84, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.P.; Nieri-Bastos, F.A.; Spolidorio, M.G.; Martins, T.F.; Barbieri, A.M.; Labruna, M.B. In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecological aspects of the Atlantic rainforest Rickettsia, the causative agent of a novel spotted fever rickettsiosis in Brazil. Parasitology 2013, 140, 719–728. [Google Scholar] [CrossRef]
- Romer, Y.; Nava, S.; Govedic, F.; Cicuttin, G.; Denison, A.M.; Singleton, J.; Kelly, A.J.; Kato, C.Y.; Paddock, C.D. Rickettsia parkeri rickettsiosis in different ecological regions of Argentina and its association with Amblyomma tigrinum as a potential vector. Am. J. Trop. Med. Hyg. 2014, 91, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; Conte, V.; Parrilla, G.; De Meneghi, D. Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma tigrinum ticks, Cochabamba Department, Bolivia. Vector Borne Zoonotic Dis. 2010, 10, 953–958. [Google Scholar] [CrossRef]
- Arrais, R.C.; Paula, R.C.; Martins, T.F.; Nieri-Bastos, F.A.; Marcili, A.; Labruna, M.B. Survey of ticks and tick-borne agents in maned wolves (Chrysocyon brachyurus) from a natural landscape in Brazil. Ticks Tick-Borne Dis. 2021, 12, 101639. [Google Scholar] [CrossRef]
- Saracho Bottero, M.N.; Tarragona, E.L.; Nava, S. Spotted fever group rickettsiae in Amblyomma ticks likely to infest humans in rural areas from northwestern Argentina. Medicina (Buenos Aires) 2015, 75, 391–395. [Google Scholar]
- Abarca, K.; Lopez, J.; Acosta-Jamett, G.; Martinez-Valdebenito, C. Detection of Rickettsia andeanae in two regions of Chile. Rev. Chilena Infectol. 2013, 30, 388–394. [Google Scholar] [CrossRef]
- Eberhardt, A.T.; Fernandez, C.; Fargnoli, L.; Beldomenico, P.M.; Monje, L.D. A putative novel strain of Ehrlichia infecting Amblyomma tigrinum associated with Pampas fox (Lycalopex gymnocercus) in Esteros del Iberá ecoregion, Argentina. Ticks Tick-Borne Dis. 2020, 11, 101318. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Venzal, J.M.; González-Acuña, D.; Martins, T.F.; Guglielmone, A.A. Ticks of the Southern Cone of America: Diagnosis, Distribution and Hosts with Taxonomy, Ecology and Sanitary Importance; Elsevier: London, UK, 2017. [Google Scholar]
- Ogrzewalska, M.; Saraiva, D.G.; Moraes-Filho, J.; Martins, T.F.; Costa, F.B.; Pinter, A.; Labruna, M.B. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of São Paulo, Brazil. Parasitology 2012, 139, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Mangold, A.J.; Guglielmone, A.A. The natural hosts of larvae and nymphs of Amblyomma tigrinum Koch, 1844 (Acari: Ixodidae). Vet. Parasitol. 2006, 140, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Luz, H.R.; Costa, F.B.; Benatti, H.R.; Ramos, V.N.; Serpa, M.C.; Martins, T.F.; Acosta, I.C.L.; Ramirez, D.G.; Muñoz-Leal, S.; Ramirez-Hernandez, A.; et al. Epidemiology of capybara-associated Brazilian spotted fever. PLoS Negl. Trop. Dis. 2019, 13, e0007734. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Goddard, J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol. 2015, 52, 230–252. [Google Scholar] [CrossRef]
- Budachetri, K.; Browning, R.B.; Adamson, S.W.; Dowd, S.E.; Chao, C.C.; Ching, W.M.; Karim, S. An insight into the microbiome of Amblyomma maculatum (Acari: Ixodidae). J. Med. Entomol. 2014, 51, 119–129. [Google Scholar] [CrossRef]
- Grasperge, B.J.; Morgan, T.W.; Paddock, C.D.; Peterson, K.E.; Macaluso, K.R. Feeding by Amblyomma maculatum (Acari: Ixodidae) enhances Rickettsia parkeri (Rickettsiales: Rickettsiaceae) infection in the skin. J. Med. Entomol. 2014, 51, 855–863. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Hayes, S.F.; Mavros, A.J. Nonpathogenic rickettsiae in Dermacentor andersoni: A limiting factor for the distribution of Rickettsia rickettsii. In Rickettsiae and Rickettsial Diseases; Burgdorfer, W., Anacker, R.L., Eds.; Academic Press: New York, NY, USA, 1981; pp. 585–594. [Google Scholar]
- Macaluso, K.R.; Sonenshine, D.E.; Ceraul, S.M.; Azad, A.F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002, 39, 809–813. [Google Scholar] [CrossRef]
- Levin, M.L.; Schumacher, L.B.M.; Snellgrove, A. Effects of Rickettsia amblyommatis infection on the vector competence of Amblyomma americanum ticks for Rickettsia rickettsii. Vector-Borne Zoonotic Dis. 2018, 18, 579–587. [Google Scholar] [CrossRef]
- Wright, C.L.; Sonenshine, D.E.; Gaff, H.D.; Hynes, W.L. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J. Med. Entomol. 2015, 52, 1090–1095. [Google Scholar] [CrossRef]
- Sakai, R.K.; Costa, F.B.; Ueno, T.E.; Ramirez, D.G.; Soares, J.F.; Fonseca, A.H.; Labruna, M.B.; Barros-Battesti, D.M. Experimental infection with Rickettsia rickettsii in an Amblyomma dubitatum tick colony, naturally infected by Rickettsia bellii. Ticks Tick Borne Dis. 2014, 5, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Fournier, P.E.; Sumner, J.W.; Goddard, J.; Elshenawy, Y.; Metcalfe, M.G.; Loftis, A.D.; Varela–Stokes, A. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 2010, 76, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Luce–Fedrow, A.; Wright, C.; Gaff, H.D.; Sonenshine, D.E.; Hynes, W.L.; Richards, A.L. In vitro propagation of Candidatus Rickettsia andeanae isolated from Amblyomma maculatum. FEMS Immunol. Med. Microbiol. 2011, 64, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.A.; Goddard, J.; Moraru, G.M.; Smith, W.E.; Varela-Stokes, A.S. Isolation of “Candidatus Rickettsia andeanae” (Rickettsiales: Rickettsiaceae) in embryonic cells of naturally infected Amblyomma maculatum (Ixodida: Ixodidae). J. Med. Entomol. 2013, 50, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
| Tick Species | Tick Stage | Source | No. Ticks with Rickettsial DNA/No. Tested Ticks (%) | Rickettsia Species Identified by DNA Sequencing |
|---|---|---|---|---|
| Amblyomma aureolatum | Nymphs | Vegetation | 0/1 (0) | |
| Adults | Vegetation/Dogs | 1/20 (5) | ‘Candidatus R. andeanae’ | |
| Amblyomma ovale | Adults | Cerdocyon thous | 0/1 (0) | |
| Amblyomma tigrinum | Adults | Vegetation/Dogs | 50/61 (82) * | ‘Candidatus R. andeanae’ |
| Rhipicephalus sanguineus | Adults | Dogs | 0/19 (0) | |
| TOTAL | 51/102 (50) |
| Dogs and Small Mammal Species (No. Tested Specimens) | No. Seroreactive Animals to Each Rickettsia Species a (% Seroreactivity) | No. Animals with PAIHR b | |||||
|---|---|---|---|---|---|---|---|
| R.pa | R.ri | R.am | R.rh | R.fe | R.be | ||
| Dogs (36) | 5 (14) | 5 (14) | 4 (11) | 4 (11) | 4 (11) | 2 (6) | 4 R.pa |
| Akodon azarae (21) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cavia aperea (1) | 0 | 1 (100) | 0 | 1 (100) | 0 | 0 | |
| Cryptonanus chacoensis (1) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Oligoryzomys nigripes (11) | 0 | 0 | 0 | 0 | 0 | 0 | |
| TOTAL (70) | 5 (7) | 6 (9) | 4 (6) | 5 (7) | 4 (6) | 2 (3) | |
| No. Tested Ticks | Infection Rates (%) of A. tigrinum Adult Ticks | Country | Reference | |
|---|---|---|---|---|
| R. parkeri | ‘Ca. R. andeanae’ | |||
| 6 | 67 | 0 | Uruguay | [14] |
| 13 | 46 | 0 | Brazil | [17] |
| 47 | 28 | 0 | Brazil | [4] |
| 81 | 3 | 0 | Argentina | [40] |
| 41 | 54 | 2 a | Bolivia | [41] |
| 63 | 5 b | 17 b | Brazil | [42] |
| 44 | 0 | 48 | Argentina | [43] |
| 31 | 0 | 65 | Chile | [44] |
| 12 | 0 | 100 | Argentina | [45] |
| 61 | 0 | 34 | Brazil | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczak, F.S.; Binder, L.C.; Gregori, F.; Martins, T.F.; Pádua, G.T.; Sponchiado, J.; Melo, G.L.; Polo, G.; Labruna, M.B. ‘Candidatus Rickettsia andeanae’ and Probable Exclusion of Rickettsia parkeri in Ticks from Dogs in a Natural Area of the Pampa Biome in Brazil. Pathogens 2023, 12, 446. https://doi.org/10.3390/pathogens12030446
Krawczak FS, Binder LC, Gregori F, Martins TF, Pádua GT, Sponchiado J, Melo GL, Polo G, Labruna MB. ‘Candidatus Rickettsia andeanae’ and Probable Exclusion of Rickettsia parkeri in Ticks from Dogs in a Natural Area of the Pampa Biome in Brazil. Pathogens. 2023; 12(3):446. https://doi.org/10.3390/pathogens12030446
Chicago/Turabian StyleKrawczak, Felipe S., Lina C. Binder, Fábio Gregori, Thiago F. Martins, Gracielle T. Pádua, Jonas Sponchiado, Geruza L. Melo, Gina Polo, and Marcelo B. Labruna. 2023. "‘Candidatus Rickettsia andeanae’ and Probable Exclusion of Rickettsia parkeri in Ticks from Dogs in a Natural Area of the Pampa Biome in Brazil" Pathogens 12, no. 3: 446. https://doi.org/10.3390/pathogens12030446
APA StyleKrawczak, F. S., Binder, L. C., Gregori, F., Martins, T. F., Pádua, G. T., Sponchiado, J., Melo, G. L., Polo, G., & Labruna, M. B. (2023). ‘Candidatus Rickettsia andeanae’ and Probable Exclusion of Rickettsia parkeri in Ticks from Dogs in a Natural Area of the Pampa Biome in Brazil. Pathogens, 12(3), 446. https://doi.org/10.3390/pathogens12030446







