Febrile Children with Pneumonia Have Higher Nasopharyngeal Bacterial Load Than Other Children with Fever
Abstract
:1. Introduction
2. Subjects, Materials, and Methods
2.1. Participants and Clinical Definitions
2.2. Clinical Data, Sampling, and PCR Analysis
2.3. Statistical Analysis
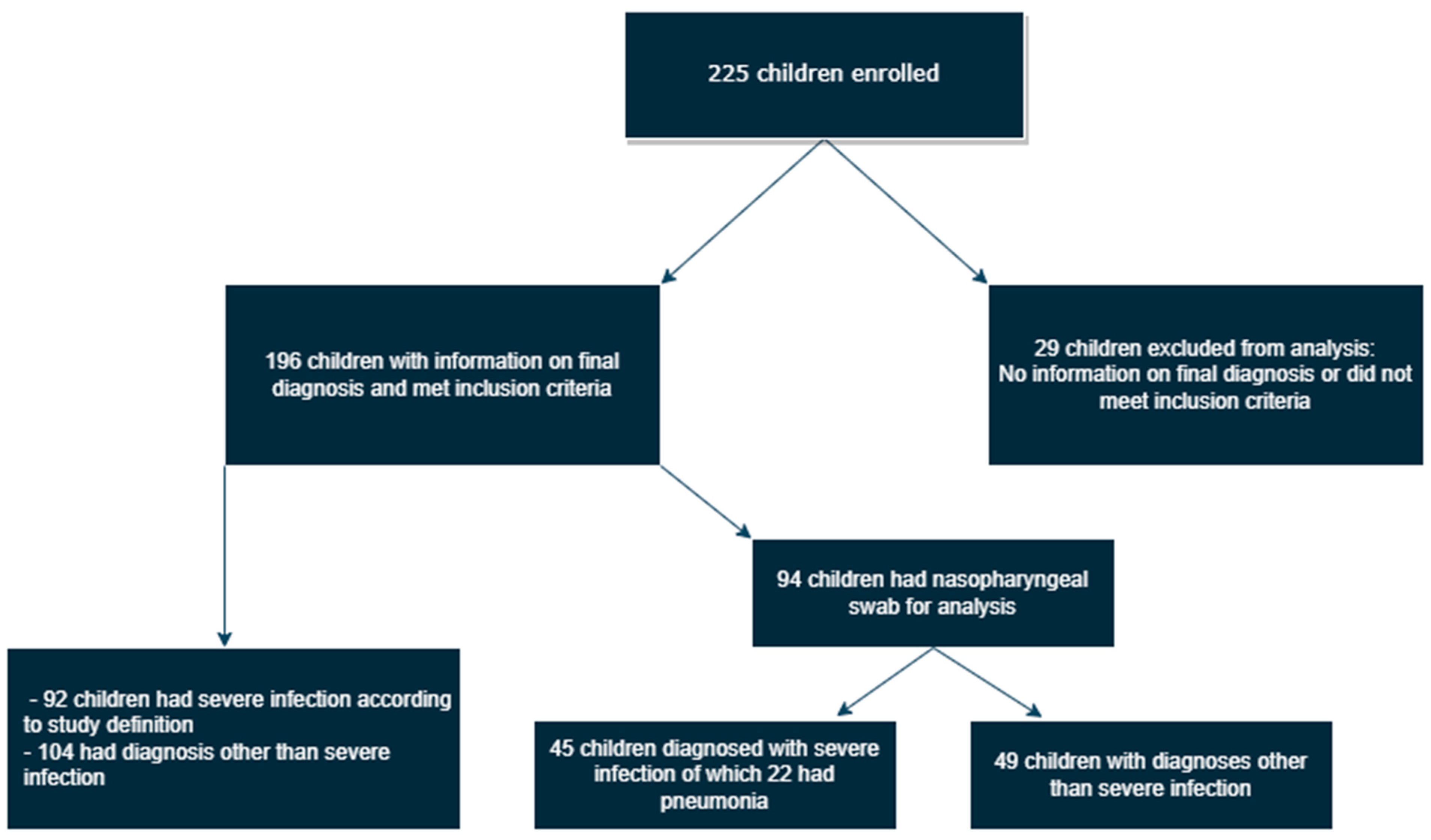
3. Results
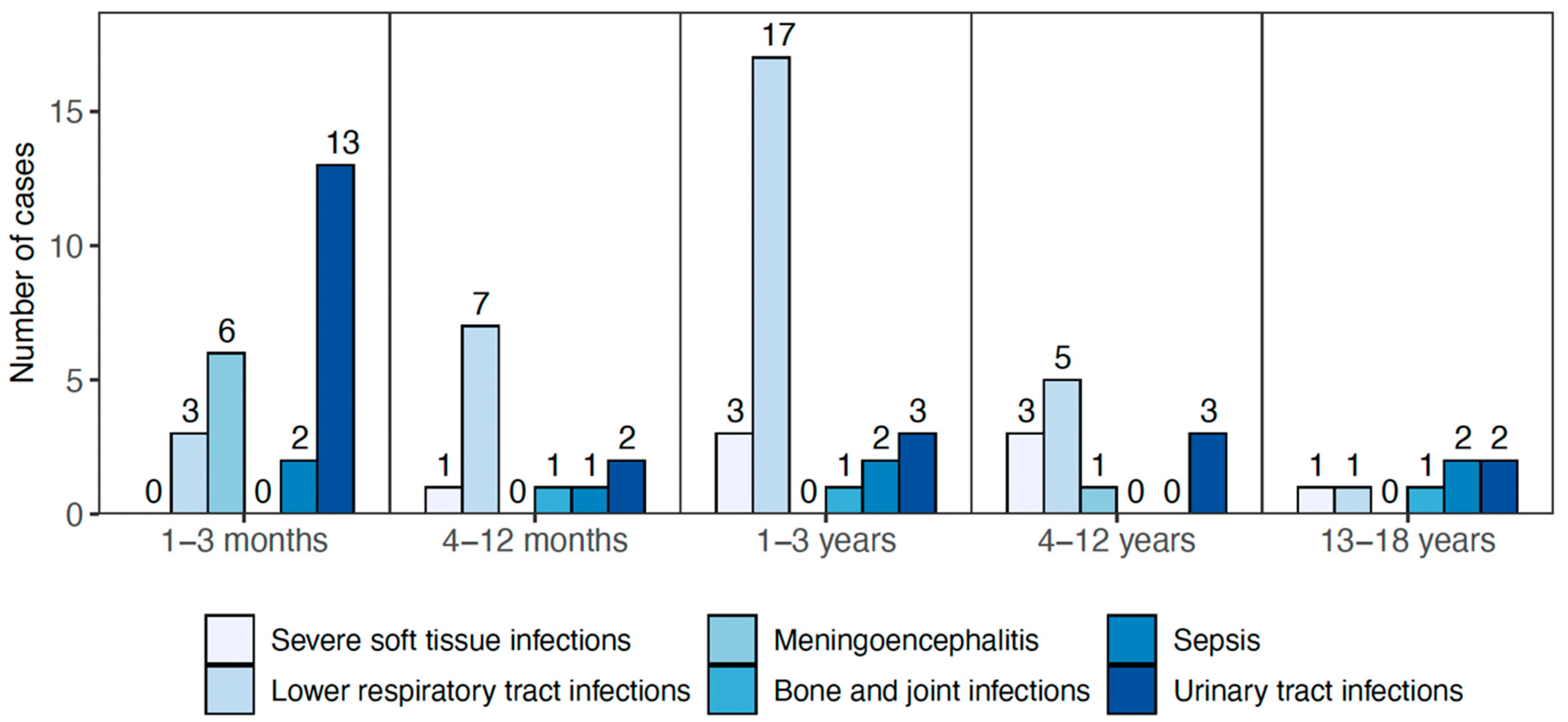
3.1. Demographic Information
3.2. Clinical Outcomes and Treatment
3.3. PCR Analysis of Nasopharyngeal Samples
3.4. Relationship between Nasopharyngeal Microorganisms and Pneumonia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Olarte, L.; Barson, W.J.; Barson, R.M.; Romero, J.R.; Bradley, J.S.; Tan, T.Q.; Givner, L.B.; Hoffman, J.A.; Lin, P.L.; Hultén, K.G.; et al. Pneumococcal Pneumonia Requiring Hospitalization in US Children in the 13-Valent Pneumococcal Conjugate Vaccine Era. Clin. Infect. Dis. 2017, 64, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, P.; Carnovale, C.; Perrone, V.; Salvati, D.; Gentili, M.; Brusadelli, T.; Antoniazzi, S.; Pozzi, M.; Radice, S.; Clementi, E. Epidemiological analysis on two decades of hospitalisations for meningitis in the United States. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Snaebjarnardóttir, K.; Erlendsdóttir, H.; Reynisson, I.K.; Kristinsson, K.; Halldórsdóttir, S.; Hardardottir, H.; Gudnason, T.; Gottfredsson, M.; Haraldsson, A. Bacterial meningitis in children in Iceland, 1975–2010: A nationwide epidemiological study. Scand. J. Infect. Dis. 2013, 45, 819–824. [Google Scholar] [CrossRef]
- Griffin, M.R.; Zhu, Y.; Moore, M.R.; Whitney, C.G.; Grijalva, C.G. U.S. Hospitalizations for Pneumonia after a Decade of Pneumococcal Vaccination. N. Engl. J. Med. 2013, 369, 155–163. [Google Scholar] [CrossRef]
- Memar, M.Y.; Varshochi, M.; Shokouhi, B.; Asgharzadeh, M.; Kafil, H.S. Procalcitonin: The marker of pediatric bacterial infection. Biomed. Pharmacother. 2017, 96, 936–943. [Google Scholar] [CrossRef]
- Paydar-Darian, N.; Kimia, A.A.; Monuteaux, M.C.; Michelson, K.A.; Landschaft, A.; Maulden, A.B.; Chenard, R.L.; Nigrovic, L.E. C-reactive protein or erythrocyte sedimentation rate results reliably exclude invasive bacterial infections. Am. J. Emerg. Med. 2019, 37, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, M.R.; Biesbroek, G.; Rossen, J.W.A.; de Steenhuijsen Piters, W.A.A.; Bosch, A.A.T.M.; van Gils, E.J.M.; Wang, X.; Boonacker, C.W.; Veenhoven, R.H.; Bruin, J.P.; et al. Associations between pathogens in the upper respiratory tract of young children: Interplay between viruses and bacteria. PLoS ONE 2012, 7, e47711. [Google Scholar] [CrossRef] [PubMed]
- Shak, J.R.; Vidal, J.E.; Klugman, K.P. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol. 2012, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdóttir, H.; Björnsdóttir, J.B.; Erlendsdóttir, H.; Hjálmarsdóttir, M.; Hrafnkelsson, B.; Haraldsson, Á.; Kristinsson, K.G.; Haraldsson, G. The Effect of the 10-Valent Pneumococcal Nontypeable Haemophilus influenzae Protein D Conjugate Vaccine on H. influenzae in Healthy Carriers and Middle Ear Infections in Iceland. J. Clin. Microbiol. 2019, 57, e00116-19. [Google Scholar] [CrossRef]
- Thors, V.; Christensen, H.; Morales-Aza, B.; Oliver, E.; Sikora, P.; Vipond, I.; Muir, P.; Finn, A. High-density Bacterial Nasal Carriage in Children Is Transient and Associated With Respiratory Viral Infections—Implications for Transmission Dynamics. Pediatr. Infect. Dis. J. 2019, 38, 533–538. [Google Scholar] [CrossRef]
- Thors, V.; Morales-Aza, B.; Pidwill, G.; Vipond, I.; Muir, P.; Finn, A. Population density profiles of nasopharyngeal carriage of 5 bacterial species in pre-school children measured using quantitative PCR offer potential insights into the dynamics of transmission. Hum. Vaccines Immunother. 2015, 12, 375–382. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; McAuley, J.L.; Browall, S.; Iverson, A.R.; Boyd, K.L.; Normark, B.H. Influenza Enhances Susceptibility to Natural Acquisition of and Disease due to Streptococcus pneumoniae in Ferrets. J. Infect. Dis. 2010, 202, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Chochua, S.; D’Acremont, V.; Hanke, C.; Alfa, D.; Shak, J.; Kilowoko, M.; Kyungu, E.; Kaiser, L.; Genton, B.; Klugman, K.P.; et al. Increased Nasopharyngeal Density and Concurrent Carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis Are Associated with Pneumonia in Febrile Children. PLoS ONE 2016, 11, e0167725. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.T.; Yoshida, L.M.; Suzuki, M.; Nguyen, H.A.T.; Nguyen, C.D.L.; Nguyen, A.T.T.; Oishi, K.; Yamamoto, T.; Watanabe, K.; Vu, T.D.; et al. Association Between Nasopharyngeal Load of Streptococcus pneumoniae, Viral Coinfection, and Radiologically Confirmed Pneumonia in Vietnamese Children. Pediatr. Infect. Dis. J. 2011, 30, 11–18. [Google Scholar] [CrossRef]
- Cherian, T.; Mulholland, E.K.; Carlin, J.B.; Ostensen, H.; Amin, R.; De Campo, M.; Greenberg, D.; Lagos, R.; Lucero, M.; Madhi, S.A.; et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 2005, 83, 353–359. [Google Scholar]
- Kaltoft, M.S.; Sørensen, U.B.S.; Slotved, H.-C.; Konradsen, H.B. An easy method for detection of nasopharyngeal carriage of multiple Streptococcus pneumoniae serotypes. J. Microbiol. Methods 2008, 75, 540–544. [Google Scholar] [CrossRef]
- Gunson, R.N.; Carman, W.F. During the summer 2009 outbreak of “swine flu” in Scotland what respiratory pathogens were diagnosed as H1N1/2009? BMC Infect. Dis. 2011, 11, 192. [Google Scholar] [CrossRef]
- Carr, M.; Gunson, R.; Maclean, A.; Coughlan, S.; Fitzgerald, M.; Scully, M.; O’Herlihy, B.; Ryan, J.; O’Flanagan, D.; Connell, J.; et al. Development of a real-time RT-PCR for the detection of Swine-lineage Influenza A (H1N1) virus infections. J. Clin. Virol. 2009, 45, 196–199. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef]
- Mettelman, R.C.; Allen, E.K.; Thomas, P.G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 2022, 55, 749–780. [Google Scholar] [CrossRef]
- Yao, W.; Pan, J.; Liu, Z.; Dong, Z.; Liang, M.; Xia, S.; Xiao, Y.; Cai, X.; Peng, T.; Zhou, X.; et al. The Cellular and Viral circRNAome Induced by Respiratory Syncytial Virus Infection. Mbio 2021, 12, e03075-21. [Google Scholar] [CrossRef]
- Bertolini, M.; Ranjan, A.; Thompson, A.; Diaz, P.I.; Sobue, T.; Maas, K.; Dongari-Bagtzoglou, A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019, 15, e1007717. [Google Scholar] [CrossRef]
- Verkaik, N.; Nguyen, D.; de Vogel, C.; Moll, H.; Verbrugh, H.; Jaddoe, V.; Hofman, A.; van Wamel, W.; Hoogen, B.V.D.; Buijs-Offerman, R.; et al. Streptococcus pneumoniae exposure is associated with human metapneumovirus seroconversion and increased susceptibility to in vitro HMPV infection. Clin. Microbiol. Infect. 2011, 17, 1840–1844. [Google Scholar] [CrossRef]
- Man, W.H.; De Steenhuijsen Piters, W.A.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Prevaes, S.M.; de Winter-de Groot, K.M.; Janssens, H.M.; Piters, W.A.A.D.S.; Tramper-Stranders, G.A.; Wyllie, A.L.; Hasrat, R.; Tiddens, H.A.; Van Westreenen, M.; Van Der Ent, C.K.; et al. Development of the Nasopharyngeal Microbiota in Infants with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 193, 504–515. [Google Scholar] [CrossRef]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early Respiratory Microbiota Composition Determines Bacterial Succession Patterns and Respiratory Health in Children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Thors, V.; Christensen, H.; Morales-Aza, B.; Vipond, I.; Muir, P.; Finn, A. The Effects of Live Attenuated Influenza Vaccine on Nasopharyngeal Bacteria in Healthy 2 to 4 Year Olds. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 193, 1401–1409. [Google Scholar] [CrossRef]
- DeMuri, G.P.; Gern, J.E.; Eickhoff, J.C.; Lynch, S.V.; Wald, E.R. Dynamics of Bacterial Colonization With Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis During Symptomatic and Asymptomatic Viral Upper Respiratory Tract Infection. Clin. Infect. Dis. 2017, 66, 1045–1053. [Google Scholar] [CrossRef]
- Van den Bruel, M.T.A.; Buntinx, F.; Mant, D.; Haj-Hassan, T.; Oostenbrink, R.; Moll, H.; Aertgeerts, B.; Lakhanpaul, M. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: A systematic review. Lancet 2010, 375, 834–845. [Google Scholar] [CrossRef]
- da Silva, A.A.; Dias, D.A.D.A.; Marques, A.; di Biase, C.B.; Murni, I.; Dramowski, A.; Sharland, M.; Huebner, J.; Zingg, W. Role of antimicrobial stewardship programmes in children: A systematic review. J. Hosp. Infect. 2017, 99, 117–123. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Eythorsson, E.; Erlendsdóttir, H.; Hrafnkelsson, B.; Kristinsson, K.G.; Haraldsson, Á. Impact of the 10-valent pneumococcal conjugate vaccine on hospital admissions in children under three years of age in Iceland. Vaccine 2020, 38, 2707–2714. [Google Scholar] [CrossRef]
| All Children (n = 196) | Children Diagnosed with Severe Infection (n = 92) | |
|---|---|---|
| Age | ||
| 1–3 months | 72 (37%) | 43 (32%) |
| 4–11 months | 26 (13%) | 15 (16%) |
| 1–3 years | 49 (25%) | 27 (29%) |
| 4–12 years | 32 (16, 3%) | 14 (15%) |
| 13–18 years | 17 (9%) | 7 (8%) |
| Boys | 109 (56%) | 49 (53%) |
| Siblings in pre-school | 107 (55%) | 51 (55%) |
| Household smoking | 36 (18%) | 17 (19%) |
| Vaccination status | ||
| General vaccination schedule up to date | 194 (99%) | 91 (99%) |
| Received annual influenza vaccine | 9 (5%) | 4 (4%) |
| Any underlying health condition | 41 (21%) | 24 (26%) |
| Severe Infection Type | Number of Cases (% of Severe Infections) |
|---|---|
| Radiologically confirmed pneumonia [15] | 44 (47.8%) |
| Pyelonephritis | 23 (25.0%) |
| Severe soft tissue infection | 9 (9.8%) |
| Clinical sepsis/bacteraemia * | 7 (7.6%) |
| Meningoencephalitis ** | 6 (6.5%) |
| Bone and joint infections | 3 (3.3%) |
| Nasopharyngeal Swab | Pneumonia n (%) | Non-Pneumonia n (%) | p-Value |
|---|---|---|---|
| S. pneumoniaepositive | 13/22 (59.1%) | 19/72 (26.4%) | 0.009 |
| Gene copy number, median (IQR) | 352.30 (0–5370) | 0 (0–5.42) | 0.0013 |
| H. influenzaepositive | 15/22 (68.1%) | 22/72 (30.6%) | 0.002 |
| Gene copy number, median (IQR) | 27,905 (0–196,419) | 0 (0–357) | 0.0001 |
| M. catarrhalispositive | 13/22 (59.1%) | 33/72 (45.8%) | 0.334 |
| Gene copy number, median (IQR) | 430 (0–53,065) | 0 (0–12,944) | 0.340 |
| Presence of respiratory virus | 18/22 (81.8%) | 46/72 (63.9%) | 0.190 |
| Explanatory Variable: | Adjusted Odds Ratio | p-Value | 95% Confidence Interval |
|---|---|---|---|
| S. pneumoniae density (log10) | 1.34 | 0.019 | 1.05–1.72 |
| H. influenzae density (log10) | 1.26 | 0.009 | 1.06–1.50 |
| M. catarrhalis density (log10) | 0.85 | 0.12 | 0.69–1.05 |
| Presence of respiratory virus | 3.69 | 0.067 | 0.91–14.9 |
| Age | 0.99 | 0.71 | 0.98–1.01 |
| Male sex | 0.89 | 0.85 | 0.28–2.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjornsdottir, B.; Benitez Hernandez, U.; Haraldsson, A.; Thors, V. Febrile Children with Pneumonia Have Higher Nasopharyngeal Bacterial Load Than Other Children with Fever. Pathogens 2023, 12, 517. https://doi.org/10.3390/pathogens12040517
Bjornsdottir B, Benitez Hernandez U, Haraldsson A, Thors V. Febrile Children with Pneumonia Have Higher Nasopharyngeal Bacterial Load Than Other Children with Fever. Pathogens. 2023; 12(4):517. https://doi.org/10.3390/pathogens12040517
Chicago/Turabian StyleBjornsdottir, Bryndis, Ubaldo Benitez Hernandez, Asgeir Haraldsson, and Valtyr Thors. 2023. "Febrile Children with Pneumonia Have Higher Nasopharyngeal Bacterial Load Than Other Children with Fever" Pathogens 12, no. 4: 517. https://doi.org/10.3390/pathogens12040517
APA StyleBjornsdottir, B., Benitez Hernandez, U., Haraldsson, A., & Thors, V. (2023). Febrile Children with Pneumonia Have Higher Nasopharyngeal Bacterial Load Than Other Children with Fever. Pathogens, 12(4), 517. https://doi.org/10.3390/pathogens12040517







