Avian Influenza: Strategies to Manage an Outbreak
Abstract
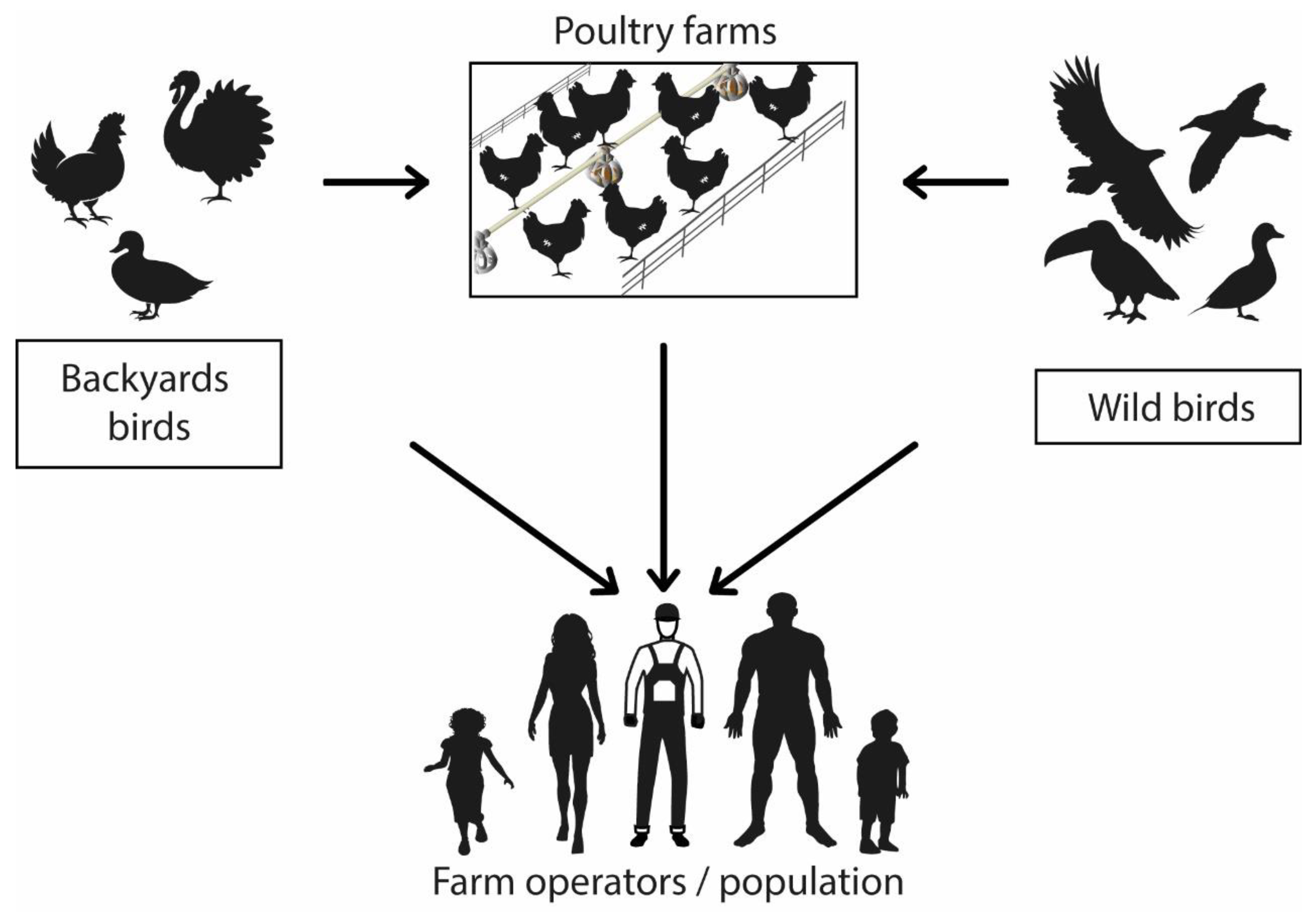
:1. Introduction
2. Avian Influenza Virus
2.1. Etiology
2.2. Pathogenicity and Virulence
2.2.1. Low Pathogenic Avian Influenza Virus (LPAI)
2.2.2. Highly Pathogenic Avian Influenza Virus (HPAI)
2.3. Transmission Mechanisms
Bird-to-Bird Transmission
2.4. Interspecies Transmission
2.4.1. Transmission to Mammals
2.4.2. Zoonotic Transmission
2.5. Virus Reservoirs
2.6. Virus Survival
3. Avian Influenza in Poultry
3.1. Clinical Diagnosis
3.1.1. Highly Pathogenic Avian Influenza (HPAI)
3.1.2. Low Pathogenic Avian Influenza (LPAI)
3.2. Laboratory Diagnosis
3.2.1. Sampling
3.2.2. Viral Isolation
3.2.3. Antigen Detection
3.2.4. Antibody Detection
3.2.5. Viral RNA Detection
4. Management of a Possible Avian Influenza Outbreak
4.1. Strategies to Deal with a Suspected Case
- Whenever trained personnel are available, carry out a clinical diagnosis and, if possible, a necropsy of the dead animals.
- Initiate an epidemiological investigation to determine possible forms of transmission and contact with other birds.
- Proceed immediately with sample collection and shipment to specialized laboratories.
- Create a register of farms, poultry, and domestic fowl within the suspected outbreak area.
- Create communication channels between the owners of the affected animals and the sanitary authorities so that they understand the situation until the diagnosis is confirmed.
- Report to international institutions, such as the World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO), so they can take inter-institutional coordination measures.
4.2. Strategies for a Confirmed Case
- Generate poultry records of each nearby farm, including backyard poultry.
- Conduct an epidemiological investigation to identify the mode of transmission and possible contacts to prevent new cases.
- The epidemiological fence should be managed as follows (Figure 2):
- Infected Zone: It will be integrated by poultry farms and domestic breeding animals within a radius of 1 km around case 0; in this zone, the recommendations indicate drastic measures, such as the depopulation of flocks and sanitary culling in infected sectors. Additionally, the elimination of carcasses, products, and by-products of all poultry, executing cleaning and disinfection procedures of all infected material, should occur.
- Observation Zone: Poultry farms and domestic livestock within a radius of 3 km around case 0; in this zone, the transport of poultry products should be prohibited, and health sensors should monitor a possible outbreak.
- Surveillance Zone: Poultry farms and domestic livestock within a 7 to 10 km radius around case 0; a safety margin should be established by closing stores and markets selling poultry and eggs within a 10 km radius around the infected outbreak.
5. Containment Measures for Confirmed Cases
5.1. Sanitary Slaughter of Infected Poultry
5.1.1. Environment Saturation with CO2
5.1.2. Carbon Dioxide Foam
5.1.3. Cervical Dislocation
5.2. Disposal
5.2.1. Burial
5.2.2. Incineration
5.3. Infected Poultry Farms’ Disinfection
- The first step is to spray all surfaces that may have been in contact with the affected birds with cationic surfactants, oxidizing agents, aldehydes, or acids and leave them for one day [84].
- The second step is general cleaning with hot water or steam using degreasing and sulfating agents, finishing with disinfectant application for one week.
- The third step involves the same procedure as the second step but, optimally, leaving the disinfectant for 21 days [1].
6. Avian Influenza Control and Surveillance
- a
- Vaccination
- b
- Active surveillance for early warning of HPAI in poultry.
- Strengthening surveillance systems for timely identification of highly pathogenic AI and isolation of infected animals.
- Permanent monitoring of animals, health sensors, and the population that has contact with domestic poultry will allow the health authorities to carry out rapid AI infection detection.
- All suspected cases of highly pathogenic avian influenza should be reported to the animal health authorities for investigation, and samples should be taken and sent for laboratory analysis.
- A consensus should be reached between owners and authorities for periodic diagnostic testing for birds at high risk of infection.
- Increase biosecurity measures in poultry production and train personnel in good animal husbandry and manufacturing practices.
- Generate strategies for the identification of poultry workers, operators of poultry slaughter centers, and exposed persons within the food chain who should remain in isolation for approximately ten days from the last contact.
- c
- Passive surveillance of wild birds
- Surveillance of wild birds should be carried out according to the seasonality of the virus and the periods in which birds migrate to certain places, reinforcing surveillance in periods with more cases.
- When mortality in wild birds is observed, health authorities should be alerted and initiate the process of collection and testing for viral identification.
- When positive cases of HPAI are detected in a country or region, surveillance protocols for wild birds should be initiated, as the movement of migratory waterfowl is considered a potential risk for virus transmission into non-infected areas.
7. Discussion
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Center for Control and Prevention of the Unites States of America |
| RT-PCR | Reverse Transcriptase Polymerase Chain Reaction |
| FAO | Food and Agriculture Organization of the United Nations |
| WOAH | World Organization for Animal Health |
| HPAI | Highly pathogenic avian influenza |
| ECDC | The European Center for Disease Prevention and Control |
| WHO | World Health Organization |
| PAHO | Pan American Health Organization |
| LPAI | Low pathogenic avian influenza virus |
| EMPRES | Emergency Prevention System for Transboundary Animal and Plant Pests and Diseases |
| EIA | Enzyme Immunoassay |
References
- Caría, D.; Ferrer, M.E.; Chuard, N. Manual de Procedimientos para la Contingencia de la Influenza Aviar; SENASA: Buenos Aires, Argentina, 2017. Available online: https://www.argentina.gob.ar/sites/default/files/manual_de_procedimientos_-_plan_de_contingencia_de_ia_res._ndeg_73.2010.pdf (accessed on 10 January 2023).
- Smith, G.J.D.; Donis, R.O. Continued evolution of highly pathogenic avian influenza A (H5N1): Updated nomenclature. Influenza Other Respir. Viruses 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Saha, S.; Davis, W.W. The need for a One Health approach for influenza surveillance. Lancet Glob. Health 2022, 10, e1078–e1079. [Google Scholar] [CrossRef] [PubMed]
- Organización Panamericana de la Salud. Influenza Aviar; OPS/OMS: Buenos Aires, Argentina, 2023; Available online: https://www.paho.org/en/topics/avian-influenza (accessed on 15 January 2023).
- Swayne, D.E. Avian Influenza; Blackwell Publishing: Ames, IO, USA, 2008; Available online: https://books.google.com.ec/books?hl=es&lr=&id=eZjFavPmuxAC&oi=fnd&pg=PP2&ots=jyiRjnKa6Q&sig=tbhpc0dDVC45dPzGpFokIANkasU&redir_esc=y#v=onepage&q&f=false (accessed on 21 February 2023).
- Lee, D.H.; Criado, M.F.; Swayne, D.E. Pathobiological Origins and Evolutionary History of Highly Pathogenic Avian Influenza Viruses. Cold Spring Harb. Perspect. Med. 2021, 11, a038679. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Aparición y evolución de la gripe aviar H5N1|Influenza aviar (gripe); CDC: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/flu/avianflu/communication-resources/bird-flu-origin-infographic.html (accessed on 15 January 2023).
- Food and Agriculture Organization of the United Nations. Global Programme for The Prevention and Control of Highly Pathogenic Avian Influenza Third Report; FAO: Rome, Italy, 2008; pp. 1–114. [Google Scholar]
- Chatziprodromidou, I.P.; Arvanitidou, M.; Guitian, J.; Apostolou, T.; Vantarakis, G.; Vantarakis, A. Global avian influenza outbreaks 2010–2016: A systematic review of their distribution, avian species and virus subtype. Syst. Rev. 2018, 7, 17. [Google Scholar] [CrossRef]
- Organización Mundial de Sanidad Animal. Influenza Aviar—WOAH—Organización Mundial de Sanidad Animal; OMSA: Paris, France, 2023; Available online: https://www.woah.org/en/disease/avian-influenza/#ui-id-2 (accessed on 26 February 2023).
- Organización Mundial de Sanidad Animal. Distribution of HPAI new outbreaks in poultry, and corresponding subtypes; OMSA: Paris, France, 2023; Available online: https://www.woah.org/app/uploads/2023/02/hpai-situation-report-20230216.pdf (accessed on 26 February 2023).
- Agüero, M.; Monne, I.; Sanchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; del Valle Arrojo, M.; Fernandez-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance 2023, 28, 2300001. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al. Avian influenza overview June–September 2022. EFSA J. 2022, 20, e07597. [Google Scholar] [CrossRef]
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.; McCauley, J.W.; Peiris, J.M.; Webby, R.J.; Fouchier, R.A.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Pappas, C.; Pulit-Penaloza, J.A.; Brock, N.; Zeng, H.; Creager, H.M.; Le, S.; Wilson, M.; Lewis, A.; et al. Risk Assessment of Fifth-Wave H7N9 Influenza A Viruses in Mammalian Models. J. Virol. 2019, 93, e01740.18. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud Animal. Notification of positive HPAI results in wild mammalian species (UK); OMSA: Paris, France, 2022; Available online: https://www.woah.org/app/uploads/2023/01/notification-of-positive-hpai-results-in-wild-mammalian-species.pdf (accessed on 26 February 2023).
- Bui, C.; Bethmont, A.; Chughtai, A.A.; Gardner, L.; Sarkar, S.; Hassan, S.; Seale, H.; MacIntyre, C.R. A Systematic Review of the Comparative Epidemiology of Avian and Human Influenza A H5N1 and H7N9—Lessons and Unanswered Questions. Transbound. Emerg. Dis. 2016, 63, 602–620. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Human Infection with Avian Influenza A(H5) Viruses Human Infection with Avian Influenza A(H5N1) Virus; OMS: Geneva, Switzerland, 2023; Available online: https://www.who.int/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20230217.pdf?sfvrsn=22ea0816_24 (accessed on 26 February 2023).
- Organización Mundial de Salud. Resumen de Casos Humanos Confirmados por Influenza Aviar (H5N1) 2003–2022; OMS: Geneva, Switzerland, 2022; Available online: https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk-assessments/2022_nov_tableh5n1.pdf?sfvrsn=babfcad1_1&download=true (accessed on 26 February 2023).
- Li, C.; Chen, H. H7N9 Influenza Virus in China. Cold Spring Harb. Perspect. Med. 2021, 11, a038349. [Google Scholar] [CrossRef]
- Esquerra, F. Gripe aviar: Brotes, impacto y cómo la enfrenta México. Anim. Político 2022. Available online: https://www.animalpolitico.com/elsabueso/gripe-aviar-brotes-impacto-y-como-la-enfrenta-mexico/ (accessed on 8 December 2022).
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al.; European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza Avian influenza overview December 2021–March 2022. EFSA J. 2022, 20, e07289. [Google Scholar] [CrossRef]
- BNO Noticias. Niña Muere de Gripe Aviar H5N1 en Camboya. 2023. Available online: https://bnonews.com/index.php/2023/02/young-girl-dies-of-h5n1-bird-flu-in-cambodia/ (accessed on 23 February 2023).
- Nuñez, I.A.; Ross, T.M. A Review of H5Nx Avian Influenza Viruses. Therapeutic Advances in Vaccines and Immunotherapy; SAGE Publications Ltd.: New York, NY, USA, 2019; Volume 7, p. 2515135518821625. [Google Scholar] [CrossRef]
- Wahlgren, J. Influenza A viruses: An ecology review. Infect. Ecol. Epidemiol. 2011, 1, 6004. [Google Scholar] [CrossRef]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef]
- Stallknecht, D.E.; Shane, S.M. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 1988, 12, 125–141. [Google Scholar] [CrossRef]
- Parvin, R.; Hossain, I.; Hasan, A.; Afrin, S.Z.; Shehata, A.A. Influenza and coronavirus zoonoses: An overview on pandemic events, viral genome, replication and emergency preparedness. Ger. J. Microbiol. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Sendor, A.; Weerasuriya, D.; Sapra, A. Influenza Aviar; National Library of Medicine: Ottawa, IL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553072/#article-18048.s4 (accessed on 6 December 2022).
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a Novel Influenza A Virus Hemagglutinin Subtype (H16) Obtained from Black-Headed Gulls. J. Virol. 2005, 79, 2814. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef]
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Pantin-Jackwood, M. Pathogenicity of avian influenza viruses in poultry. Dev. Biol. 2006, 124, 61–67. [Google Scholar]
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vetereinary Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Zitzow, L.A.; Rowe, T.; Morken, T.; Shieh, W.-J.; Zaki, S.; Katz, J.M. Pathogenesis of Avian Influenza A (H5N1) Viruses in Ferrets. J. Virol. 2002, 76, 4420. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Lu, X.; Maines, T.R.; Smith, C.; Li, Y.; Donis, R.O.; Katz, J.M.; Tumpey, T.M. Pathogenesis of Avian Influenza (H7) Virus Infection in Mice and Ferrets: Enhanced Virulence of Eurasian H7N7 Viruses Isolated from Humans. J. Virol. 2007, 81, 11139. [Google Scholar] [CrossRef]
- Oficina Internacional de Epizootias (OIE). Influenza Aviar (Incluida la Infección por los Virus de la Influenza Aviar Altamente Patógenos). Capítulo 3.3.4. 2021. Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.03.04_AI.pdf (accessed on 22 February 2023).
- Rabadan, R.; Robins, H. Evolution of the Influenza A Virus: Some New Advances. Evol. Bioinform. 2007, 3, 299. [Google Scholar] [CrossRef]
- To, K.K.W.; Chan, J.F.W.; Chen, H.; Li, L.; Yuen, K.Y. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: A tale of two cities. Lancet Infect. Dis. 2013, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, R.; Bora, I.; Ratho, R.K.; Thakur, V.; Mohi, G.K.; Thakur, P. Avian influenza revisited: Concerns and constraints. VirusDisease 2022, 33, 456–465. [Google Scholar] [CrossRef]
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J. Virol. 2015, 89, 8671–8676. [Google Scholar] [CrossRef]
- Horimoto, T.; Kawaoka, Y. Pandemic Threat Posed by Avian Influenza A Viruses. Clin. Microbiol. Rev. 2001, 14, 129. [Google Scholar] [CrossRef]
- Herfst, S.; Mok, C.K.P.; van den Brand, J.M.A.; van der Vliet, S.; Rosu, M.E.; Spronken, M.I.; Yang, Z.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; et al. Human Clade 2.3.4.4 A/H5N6 Influenza Virus Lacks Mammalian Adaptation Markers and Does Not Transmit via the Airborne Route between Ferrets. mSphere 2018, 3, e00405.17. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, S.-A.; Matrosovich, M.-N. What Adaptative is Hemagglutinin and Neuraminidase are Necessary for Emergence of Pandemic Influenza Virus from Its Avian Precursor? Biochemistry 2015, 80, 872–880. [Google Scholar] [CrossRef]
- Kuiken, T.; Holmes, E.C.; McCauley, J.; Rimmelzwaan, G.F.; Williams, C.S.; Grenfell, B.T. Host Species Barriers to Influenza Virus Infections. Science 2006, 312, 394–397. [Google Scholar] [CrossRef]
- Cáceres, C.J.; Rajao, D.S.; Perez, D.R. Airborne Transmission of Avian Origin H9N2 Influenza A Viruses in Mammals. Viruses 2021, 13, 1919. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, X.; Wang, T.; Li, Y.; Li, Y.; Xu, Y.; Chu, D.; Sun, H.; Wu, C.; Li, S.; et al. Fatal H5N6 Avian Influenza Virus Infection in a Domestic Cat and Wild Birds in China. Sci. Rep. 2015, 5, 10704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, Y.; Yang, J.; Guo, J.; He, J.; Guo, J.; Weng, S.; Jia, Y.; Liu, B.; Li, X.; et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect. Genet. Evol. 2015, 36, 462–466. [Google Scholar] [CrossRef]
- Cao, X.; Yang, F.; Wu, H.; Xu, L. Genetic characterization of novel reassortant H5N6-subtype influenza viruses isolated from cats in eastern China. Arch. Virol. 2017, 162, 3501–3505. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Cooper, V.L.; Schwartz, K.J.; Harmon, K.M.; Kim, W.I.; Janke, B.H.; Strohbehn, J.; Butts, D.; Troutman, J. Influenza Virus Infection in Racing Greyhounds. Emerg. Infect. Dis. 2005, 11, 1974. [Google Scholar] [CrossRef]
- Amonsin, A.; Payungporn, S.; Theamboonlers, A.; Thanawongnuwech, R.; Suradhat, S.; Pariyothorn, N.; Tantilertcharoen, R.; Damrongwantanapokin, S.; Buranathai, C.; Chaisingh, A.; et al. Genetic characterization of H5N1 influenza A viruses isolated from zoo tigers in Thailand. Virology 2006, 344, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian Influenza H5N1 in Tigers and Leopards. Emerg. Infect. Dis. 2004, 10, 2189. [Google Scholar] [CrossRef]
- Marchenko, V.; Goncharova, N.; Susloparov, I.; Kolosova, N.; Gudymo, A.; Svyatchenko, S.; Danilenko, A.; Durymanov, A.; Gavrilova, E.; Maksyutov, R.; et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology 2018, 525, 216–223. [Google Scholar] [CrossRef]
- Kwon, H.I.; Kim, E.H.; Kim, Y.I.; Park, S.J.; Si, Y.J.; Lee, I.W.; Nguyen, H.D.; Yu, K.M.; Yu, M.A.; Jung, J.H.; et al. Comparison of the pathogenic potential of highly pathogenic avian influenza (HPAI) H5N6, and H5N8 viruses isolated in South Korea during the 2016–2017 winter season. Emerg. Microbes Infect. 2018, 7, 29. [Google Scholar] [CrossRef]
- Nabi, G.; Wang, Y.; Lü, L.; Jiang, C.; Ahmad, S.; Wu, Y.; Li, D. Bats and birds as viral reservoirs: A physiological and ecological perspective. Sci. Total Environ. 2021, 754, 142372. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, W.; Wang, Y.; Han, G.Z.; Chen, H.; Wang, X. A unique feature of swine ANP32A provides susceptibility to avian influenza virus infection in pigs. PLoS Pathog. 2020, 16, e1008330. [Google Scholar] [CrossRef]
- Roguski, K.; Fry, A. Travel-related infectious diseasesChapter 4; Travelers’ HealthCenters for Disease Control and Prevention: Atlanta, GA, USA, 2019; Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/influenza#:~:text=The (accessed on 25 February 2023).
- Chan, J.F.W.; To, K.K.W.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Gripe (Aviar y Otras Zoonóticas); OMS: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-other-zoonotic) (accessed on 25 February 2023).
- Chin, J. El control de las enfermedades transmisibles. Organ. Panam. De La Salud 2001, 17, 1–673. Available online: www.paho.org (accessed on 25 February 2023). [CrossRef]
- Daoust, P.Y.; van de Bildt, M.; van Riel, D.; van Amerongen, G.; Bestebroer, T.; Vanderstichel, R.; Fouchier, R.A.; Kuiken, T. Replication of 2 Subtypes of Low-Pathogenicity Avian Influenza Virus of Duck and Gull Origins in Experimentally Infected Mallard Ducks. Vet. Pathol. 2013, 50, 548–559. [Google Scholar] [CrossRef]
- Capua, I.; Alexander, D.J. Avian influenza: Recent developments. Avian Pathol. 2004, 33, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Meseko, C.A.; Oluwayelu, D.O. Avian influenza. In Transboundary Animal Diseases in Sahelian Africa and Connected Regions; Springer: Berlin/Heidelberg, Germany, 2019; pp. 345–374. [Google Scholar] [CrossRef]
- Dubey, S.C.; Dahal, N.; Nagarajan, S.; Tosh, C.; Murugkar, H.V.; Rinzin, K.; Sharma, B.; Jain, R.; Katare, M.; Patil, S.; et al. Isolation and characterization of influenza A virus (subtype H5N1) that caused the first highly pathogenic avian influenza outbreak in chicken in Bhutan. Vet. Microbiol. 2012, 155, 100–105. [Google Scholar] [CrossRef]
- El-Zoghby, E.F.; Aly, M.M.; Nasef, S.A.; Hassan, M.K.; Arafa, A.S.; Selim, A.A.; Kholousy, S.G.; Kilany, W.H.; Safwat, M.; Abdelwhab, E.M.; et al. Surveillance on A/H5N1 virus in domestic poultry and wild birds in Egypt. Virol. J. 2013, 10, 1–10. [Google Scholar] [CrossRef]
- Centros para el Control y la Prevención de Enfermedades. Directrices Provisionales Sobre Pruebas y Recolección de Muestras para Pacientes con Sospecha de Infección por Nuevos Virus de Influenza A con el Potencial de Causar Enfermedades Graves en Humanos|Influenza Aviar (gripe); CDC: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/flu/avianflu/severe-potential.htm (accessed on 8 December 2022).
- Organización Mundial de Sanidad Animal. Código Sanitario Para Los Animales Terrestres. Capítulo 7.6. 2011. Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahc/2011/es_chapitre_1.7.6.htm (accessed on 9 December 2022).
- Centro Regionale Epidemiologia Veterinaria. Manuale Operativo in Caso di Influenza Aviaria; OIE/FAO: Rome, Italy, 2006; Volume 1, pp. 1–41. [Google Scholar]
- David, M. Influenza Aviar Altamente Patógena: Desafíos encontrados y medidas para prevenir su diseminación. Américas-Com. Reg. OIE 2016, 1–11. [Google Scholar] [CrossRef]
- Slomka, M.J.; To, T.L.; Tong, H.H.; Coward, V.J.; Mawhinney, I.C.; Banks, J.; Brown, I.H. Evaluation of lateral flow devices for identification of infected poultry by testing swab and feather specimens during H5N1 highly pathogenic avian influenza outbreaks in Vietnam. Influenza Other Respir. Viruses 2012, 6, 318. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Recomendaciones y procedimientos de laboratorio para la detección del virus A(H5N1)de la influenza aviar; OMS: Geneva, Switzerland, 2007; Available online: http://www.who.int/csr/resources/publications/surveillance/WHO_CDS_EPR_ARO_2006_1/en/index.html (accessed on 24 February 2023).
- Centro de Vigilancia Veterinaria. Niveles de Bioseguridad en Laboratorios y Animalarios; BIOSLab—Universidad Complutense de Madrid: Madrid, Spain, 2023; Available online: https://www.visavet.es/es/bioslab/niveles-de-bioseguridad.php (accessed on 26 February 2023).
- Ministerio de Salud España. Prevención, Detección Precoz y Actuaciones Ante la Gripe Aviar. 2022. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/20220304_Vigilancia_prevencion_gripe_aviar.pdf (accessed on 6 December 2022).
- Ministerio de Agricultura Pesca y Alimentación—España. Manual Práctico de Operaciones en la Lucha Contra La Influenza Aviar; Ministerio De Agricultura, Pesca Y Alimentación: Madrid, Spain, 2022. Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/manualiaabril2022_tcm30-437988.pdf (accessed on 6 December 2022).
- Agencia de Regulación y Control Fito y zoosanitario. Bienestar Animal Faenamiento de Animales de Producción; Lineamientos Técnicos—Agrocalidad: Quito, Ecuador, 2020; pp. 1–52. Available online: https://www.agrocalidad.gob.ec/wp-content/uploads/2020/05/ll3.pdf (accessed on 9 December 2022).
- Ministerio de Agricultura y Ganadería—Ecuador. Reglamento General de la Ley Orgánica de Sanidad Agropecuaria; Ministerio de Agricultura y Ganadería: Quito, Ecuador, 2019. Available online: http://www.epmrq.gob.ec/images/servicios/Reglamento_LOSA.pdf (accessed on 9 December 2022).
- Berg, C.; Raj, A.B.M. Procesado de Carne Métodos de Aturdido para las Aves: Aspectos del Bienestar Animal. Selecciones Avícolas 2014, 1, 142–152. [Google Scholar]
- Benson, E.R.; Alphin, R.L.; Rankin, M.K.; Caputo, M.P.; Hougentogler, D.P.; Johnson, A.L. Mass Emergency Water-Based Foam Depopulation of Poultry. Avian Diseases. 2012, 56, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Alphin, R.L.; Rankin, M.K.; Johnson, K.J.; Benson, E.R. Comparison of Water-Based Foam and Inert-Gas Mass Emergency Depopulation Methods. Avian Diseases. 2010, 54, 757–762. [Google Scholar] [CrossRef]
- Fuenzalida, S.E. Análisis del sacrificio de pollos de carne desde el punto de vista del Bienestar Animal; Universidad De Las Américas: Santiago, Chile, 2017; Available online: https://repositorio.udla.cl/xmlui/bitstream/handle/udla/283/a40840.pdf?sequence=1&isAllowed=y (accessed on 9 December 2022).
- Aziz, J. Manual for the Control of Highly Pathogenic Avian Influenza (HPAI) Malaysia; MOA-INCORPORATED: Compton, CA, USA, 2005; pp. 1–55. Available online: https://www.woah.org/fileadmin/database/ASIA/Malaysia/Manual_for_the_control_of_Highly_Pathogenic_Avian_Influenza_(HPAI)_Malaysia.pdf (accessed on 9 December 2022).
- Jesus, R. Bioseguridad, Vacío sanitario. In Programas 3D (Desinfección, Desinsectación y Desratización); Ceva Salud Animal S. A.: Barcelona, Spain, 2017; pp. 1–18. Available online: https://www.wpsa-aeca.es/aeca_imgs_docs/09_04_46_Bioseguridad.pdf (accessed on 10 December 2022).
- Dey, P.; Ahuja, A.; Panwar, J.; Choudhary, P.; Rani, S.; Kaur, M.; Sharma, A.; Kaur, J.; Yadav, A.K.; Sood, V.; et al. Immune Control of Avian Influenza Virus Infection and Its Vaccine Development. Vaccines 2023, 11, 593. [Google Scholar] [CrossRef]
- Liu, S.; Zhuang, Q.; Wang, S.; Jiang, W.; Jin, J.; Peng, C.; Hou, G.; Li, J.; Yu, J.; Yu, X.; et al. Control of avian influenza in China: Strategies and lessons. Transbound. Emerg. Dis. 2020, 67, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Welte, V.R.; Terán, M.V. Emergency prevention system (EMPRES) for transboundary animal and plant pests and diseases—The EMPRES-Livestock: An FAO Initiative. Ann. N. Y. Acad. Sci. 2004, 1026, 19–31. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud; Organización Mundial de la Salud. Alerta Epidemiológica: Brotes de Influenza Aviar e infección humana causada por influenza A(H5). Implicaciones Para la Salud Pública en la Alerta Epidemiológica, January 2023; OPS: Washington, DC, USA. Available online: www.paho.org (accessed on 7 December 2022).
- Centros Para el Control y la Prevención de Enfermedades. CDC—La Gripe Aviar—Temas de Salud y Seguridad de NIOSH; Instituto Nacional Para la Seguridad y Salud Ocupacional (NIOSH): Washington, DC, USA, 2017 June 2017. Available online: https://www.cdc.gov/spanish/niosh/topics/aviar.html (accessed on 6 December 2022).
- Organización Mundial de Sanidad Animal. Acceso en Línea al Código Terrestre; OMSA: Paris, France, 2023; Available online: https://www.woah.org/es/que-hacemos/normas/codigos-y-manuales/acceso-en-linea-al-codigo-terrestre/?id=169&L=1&htmfile=chapitre_avian_influenza_viruses.htm (accessed on 27 February 2023).
- Organización de las Naciones Unidas para la Agricultura y la Alimentación. Lucha Contra las Enfermedades de los Animales; FAO: Rome, Italy, 2007; Available online: www.fao.org (accessed on 27 February 2023).
- Raj, M. Welfare during stunning and slaughter of poultry. Poult. Sci. 1998, 77, 1815–1819. [Google Scholar] [CrossRef]
- Centers Disease Control and Prevention. Situación Actual de la Gripe Aviar en las Aves Silvestres|Influenza Aviar (Gripe); CDC: Atlanta, GA, USA, 11 March 2022. Available online: https://www.cdc.gov/flu/avianflu/wildbirds.htm (accessed on 15 January 2023).
- Centro para el Control y Prevención de Enfermedades. Recolección y análisis de muestras en pacientes con los nuevos virus de influenza tipo A; CDC: Atlanta, GA, USA, 14 January 2022.
- King, J.; Staubach, C.; Lüder, C.; Koethe, S.; Günther, A.; Stacker, L.; Rubbenstroth, D.; Dietze, K.; Grund, C.; Conraths, F.J.; et al. Connect to Protect: Dynamics and Genetic Connections of Highly Pathogenic Avian Influenza Outbreaks in Poultry from 2016 to 2021 in Germany. Viruses 2022, 14, 1849. [Google Scholar] [CrossRef]
- McKeegan, D.E.F.; Sparks, N.H.C.; Sandilands, V.; Demmers, T.G.M.; Boulcott, P.; Wathes, C.M. Physiological responses of laying hens during whole-house killing with carbon dioxide. British Poultry Science. 2012, 52, 645–657. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; de Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- Yang, R.; Sun, H.; Gao, F.; Luo, K.; Huang, Z.; Tong, Q.; Song, H.; Han, Q.; Liu, J.; Lan, Y.; et al. Human infection of avian influenza A H3N8 virus and the viral origins: A descriptive study. Lancet Microbe 2022, 3, e824–e834. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Krog, J.S.; Ryt-Hansen, P.; Pedersen, A.G.; Kvisgaard, L.K.; Holm, E.; Nielsen, P.D.; Hammer, A.S.; Madsen, J.J.; Thorup, K.; et al. Molecular Characterization of Highly Pathogenic Avian Influenza Viruses H5N6 Detected in Denmark in 2018–2019. Viruses 2021, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Raj, M. Humane Killing of Nonhuman Animals for Disease Control Purposes. J. Appl. Anim. Welf. Sci. 2008, 11, 112–124. [Google Scholar] [CrossRef]
- Gerritzen, M.A.; Lambooij, E.; Hillebrand, S.J.W.; Lankhaar, J.A.C.; Pieterse, C. Behavioral Responses of Broilers to Different Gaseous Atmospheres. Poult. Sci. 2000, 79, 928–933. [Google Scholar] [CrossRef]
- Webster, A.B.; Fletcher, D.L. Reactions of Laying Hens and Broilers to Different Gases Used for Stunning Poultry. Poult. Sci. 2001, 80, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- McKeegan, D.E.F.; Reimert, H.G.; Hindle, V.A.; Boulcott, P.; Sparrey, J.M.; Wathes, C.M.; Demmers, T.G.; Gerritzen, M.A. Physiological and behavioral responses of poultry exposed to gas-filled high expansion foam. Poultry Sci. 2013, 92, 1145–1154. [Google Scholar] [CrossRef]
- Woolcott, C.R.; Torrey, S.; Turner, P.V.; Chalmers, H.; Levison, L.J.; Schwean-Lardner, K.; Widowski, T.M. Assessing a Method of Mechanical Cervical Dislocation as a Humane Option for On-Farm Killing Using Anesthetized Poults and Young Turkeys On-Farm Killing of Turkeys. Front. Vet. Sci. 2018, 1, 275. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Sandercock, D.A.; Sandilands, V.; Sparrey, J.; Baker, L.; Sparks, N.H.C.; McKeegan, D.E.F. Welfare Risks of Repeated Application of On-Farm Killing Methods for Poultry. Animals 2018, 8, 39. [Google Scholar] [CrossRef]
- Trapp, A.L.; Taylor, R.F. Methods of Euthanasia in Poultry and Food-Producing Animals. Vet. Clin. N. Am. Food Anim. Pract. 1986, 2, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Sandilands, V.; Sparrey, J.; Baker, L.; Dixon, L.M.; McKeegan, D.E.F. Welfare assessment of novel on-farm killing methods for poultry. PLoS ONE 2019, 14, e0212872. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; James, F.; Torrey, S.; Widowski, T.; Schwean-Lardner, K.; Monteith, G.; Turner, P.V. Electroencephalographic, physiologic and behavioural responses during cervical dislocation euthanasia in turkeys. BMC Vet. Res. 2019, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de Sanidad Animal. Terrestrial Animal Health Code; Organización Mundial de Sanidad Animal: Paris, Francia, 2019; Available online: https://rr-europe.woah.org/wp-content/uploads/2020/08/oie-terrestrial-code-1_2019_en.pdf (accessed on 11 January 2023).
- Alfonso, P.; Percedo, M.I.; Abeledo, M.A.; Fernández, A. Algunas Consideraciones para el Sacrificio Sanitario masivo de Aves de Corral en Brotes de Influenza Aviar. Rev. De Salud Anim. 2007, 29, 69–77. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-570X2007000200001&lng=es&nrm=iso&tlng=es (accessed on 11 January 2023).
- Hill, D.D.; Owens, W.E.; Tchounwou, P.B. Impact of Animal Waste Application on Runoff Water Quality in Field Experimental Plots. Int. J. Environ. Res. Public Health 2005, 2, 314. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Forman, A.; Lubroth, J. Animal Production and Health Food and Agriculture Organization of The United Nations Manual Preparing for Highly Pathogenic Avian Influenza Preparing for Highly Pathogenic Avian Influenza; FAO: Rome, Italy, 2006. [Google Scholar]
- Catalán, J.F. Procedimientos Para Enfrentar un Brote de Influenza Aviar. WATTPoultry. January 2007. Available online: https://www.wattagnet.com/articles/3190-procedimientos-para-enfrentar-un-brote-de-influenza-aviar (accessed on 11 January 2023).
- Agencia de Regulación y Control Fito y Zoosanitaria. Programa Nacional Sanitario Avícola; Agencia de Regulación y Control Fito y Zoosanitaria: Quito, Ecuador, 2013 August 2013; Available online: https://www.woah.org/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Self-declarations/Annexes/ANEXO_3.pdf (accessed on 9 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simancas-Racines, A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Zambrano, A.K.; Simancas-Racines, D. Avian Influenza: Strategies to Manage an Outbreak. Pathogens 2023, 12, 610. https://doi.org/10.3390/pathogens12040610
Simancas-Racines A, Cadena-Ullauri S, Guevara-Ramírez P, Zambrano AK, Simancas-Racines D. Avian Influenza: Strategies to Manage an Outbreak. Pathogens. 2023; 12(4):610. https://doi.org/10.3390/pathogens12040610
Chicago/Turabian StyleSimancas-Racines, Alison, Santiago Cadena-Ullauri, Patricia Guevara-Ramírez, Ana Karina Zambrano, and Daniel Simancas-Racines. 2023. "Avian Influenza: Strategies to Manage an Outbreak" Pathogens 12, no. 4: 610. https://doi.org/10.3390/pathogens12040610
APA StyleSimancas-Racines, A., Cadena-Ullauri, S., Guevara-Ramírez, P., Zambrano, A. K., & Simancas-Racines, D. (2023). Avian Influenza: Strategies to Manage an Outbreak. Pathogens, 12(4), 610. https://doi.org/10.3390/pathogens12040610





