Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 had reported over 676 million cases by March 2023. The main aim of this study is to investigate whether the levels of anti-S and anti-N antibodies could precisely indicate the degree of protection against SARS-CoV-2 and affect the probability or time of contracting COVID-19. In this study, a serosurveillance study was conducted in healthcare workers (HCWs) at a regional hospital in Taiwan to evaluate their antibody levels based on infection and vaccination status. Of 245 HCWs enrolled, all have been vaccinated prior to infection. Of these, 85 participants were infected by SARS-CoV-2, while 160 participants were not infected at the time of blood sample collection. The level of anti-SARS-CoV-2 S antibody was significantly higher in the infected HCWs than in the non-infected participants (p < 0.001). It is worth noting that the mean duration between the administration of the last dose of the vaccine and the occurrence of SARS-CoV-2 infection was 5.61 ± 2.95 months. Our follow-up survey revealed that the non-infected group had significantly higher levels of antibodies compared to the infected group (all p < 0.001). In conclusion, this study suggests that the level of antibodies could serve as a reflection of the protective efficacy against SARS-CoV-2 infection. It has the implication for vaccine decision-making policies in the future.
1. Introduction
As of March 2023, the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had reported over 676 million confirmed cases and 6 million deaths worldwide [1]. In Taiwan, by March 2023, over 10 million cases had been recorded [2], with its first significant wave occurring from May to August 2021 due to the alpha variant [3]. Healthcare workers (HCWs) are at an increased risk of viral exposure due to prolonged contact with infected patients and aerosol generation in the medical environment [4].
The infectious virion of the SARS-CoV-2 virus contains four structural proteins: envelope protein (E), matrix protein (M), spike protein (S), and nucleocapsid protein (N). Only the N and S proteins have been observed to induce high levels of antibodies in patients who were infected naturally. Nevertheless, it is unlikely that antibodies to N can effectively neutralize SARS-CoV-2 [5]. Therefore, the majority of COVID-19 vaccines prioritize the generation of T cells that produce neutralizing antibodies against the spike protein, which contains the receptor binding domain (RBD) [5,6].
To curb the spread of the disease, many countries have developed vaccines to provide herd immunity [7]. Several vaccines have been proven effective against COVID-19, including ChAdOx1 (AstraZeneca, AZ), BNT162b2 (Pfizer), and mRNA1273 (Moderna) [8]. Taiwan’s vaccination program started in March 2021, with most HCWs receiving two doses of the ChAdOx1 vaccine [3]. As additional vaccines became available in Taiwan, Moderna and BNT162b2 were also administrated to HCWs [9]. In July 2021, Taiwan Food and Drug Administration authorized the emergency use of MVC-COV1901, a CpG1018 and aluminum hydroxide-adjuvanted SARS-CoV-2 pre-fusion-stabilized spike protein S-2P vaccine developed by Medigen Vaccine Biologics Corporation. MVC-COV1901 is one of two vaccines included in the WHO Solidarity Trial [3].
The question of passive immunity has arisen despite efforts to maximize vaccine uptake and coverage. Studies have shown that active immunization does not necessarily produce antibodies, and in some cases, the antibody count drops several months after completing the vaccination schedule [10,11]. Terms such as infection-induced immunity, vaccine-induced immunity, and hybrid immunity have been introduced [12]. Neutralizing antibodies are a crucial tool in monitoring an individual’s immune reaction to both vaccination and infection [13]. Assays that measure antibodies against the nucleocapsid protein have demonstrated high sensitivity and specificity in many studies [14]. The first studies show the antibody response after a single dose of SARS-CoV-2 vaccine [15,16].
It is also worth noting that the majority of the studies investigating the levels of SARS-CoV-2 antibodies in HCW were conducted in the Americas or Europe. There was also a lack of data in certain WHO regions, namely the Eastern Mediterranean Region, South-East Asia region and the African region where resources are lower. More prevalence studies need to be conducted in these areas in order to properly estimate the overall seroprevalence of SARS-CoV-2 antibodies in the population. The extent of vaccine-induced protection varies greatly when combined with SARSCoV-2 infections [17], and the clinical implications of immunogenicity for hospital staff are still unclear. Therefore, the main aim of this study is to investigate whether the levels of anti-S and anti-N antibodies could precisely indicate the degree of protection against SARS-CoV-2 and affect the probability or time of contracting COVID-19. To address this, a serosurveillance study was conducted in HCWs at a regional hospital in Taiwan to evaluate their antibody levels based on infection and vaccination status, as well as the different vaccine brands used.
2. Material and Methods
2.1. Study Design and Participants
The HCWs who had been vaccinated were invited to participate in this prospective study in August 2022. In addition, the hospital staff aged 20~70 years who were not infected with SARS-CoV-2 or had been infected with SARS-CoV-2 were enrolled. The status of infection was determined at the time of blood sample collection. The clinical data and sera of hospital staff in a Regional Hospital in Central Taiwan were collected in this study. A follow-up survey on the infection status of the participants was conducted in January 2023.
2.2. Serological Tests
10 mL of blood sample was collected from each participant and serum was separated for anti-N and anti-S antibody serological tests. The humoral immunity response of the vaccines was examined by measuring total immunoglobulin levels to the RBD of the SARS-CoV-2 spike protein using the anti-SARS-CoV-2 S enzyme immunoassay (Elecsys, Roche Diagnostics International Ltd., Mannheim, Germany), according to the manufacturer’s instructions. The assay result ranged from 0.8 to 250 U/mL. The samples with results <0.8 U/mL were regarded as negative for anti-SARS-CoV-2 S. Following vaccination, antibodies against the SARS-CoV-2 N protein were determined using the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Mannheim, Germany). The assay results were interpreted as nonreactive/negative (cutoff index < 1) and reactive/positive (cutoff index ≥ 1). The pre-existing SARS-CoV-2 infection was defined as positivity for anti-nucleocapsid at any point, anti-spike before vaccination, and/or a history of positive PCR results on the nasopharyngeal swab [18,19].
2.3. Statistical Analysis
The interval between the time of sample obtained and the last dose of vaccination was calculated and grouped into three groups: <3 months, ≥3~<6 months, and ≥6 months. The mean, median, standard deviation and quartiles of the interval between the first-time infection date and the last dose of vaccination were presented. Fisher’s exact test was performed to compare the gender, vaccination status, and the combination of vaccine brand for the first three doses between the infected and non-infected individuals. The independent 2-sample t-test was used to compare the distribution of age, antibody titer, laboratory assessments, the interval between the time of laboratory testing and the last dose of vaccination, and the interval between the laboratory testing and the subsequent infections between the two groups. Pearson correlation coefficients were calculated to examine the linear associations between time from the last dose of vaccination and SARS-CoV-2 S/N antibodies with natural logarithm transformation for non-infected individuals and various combinations of the vaccine brands. The interval between the date of a positive test and the last dose of the vaccination, and the interval between the laboratory testing and the subsequent infections were calculated and the association between the intervals and SARS-CoV-2 S/N antibodies with/without natural logarithm transformation were also evaluated using the Pearson correlation coefficient for infected HCWs. All statistical analyses were performed using SPSS Version 18.0 for Windows (IBM SPSS Statistics, Chicago, IL, USA) and the level of significance was set at 0.05.
3. Results
3.1. Demographic Data
Of 245 HCWs enrolled, all have been vaccinated prior to infection. Of these, 85 participants were infected by SARS-CoV-2, while 160 participants were not infected at the time of blood sample collection (Table 1). The mean ages were 37.5 ± 10.2 and 37.1 ± 9.3 years old for the non-infected and infected participants, respectively. Most participants for both groups were female (90.6% and 95.3% for the non-infected and infected participants, respectively) and were predominantly nurses. All of them were vaccinated by 2, 3 or 4 doses of different brands or different combinations. Within each of the two groups, only one participant had received 2 vaccine doses. In the non-infected group, the majority of individuals received 4 doses for <3 months (49.4%, Table 1), and most HCWs received the vaccine combination of 2 AZ + 2 Moderna (n = 63, 39.4%). In contrast, in the infected group, most people received 3 doses for ≥6 months (82.4%, Table 1), and the majority of HCWs received a combination of 2 AZ + Moderna (n = 56, 65.9%). There were no significant differences in age, sex, vaccination status and the combination of vaccine brands between the infected and non-infected participants (Table 1).

Table 1.
Demographic data of the participants.
3.2. Antibody Level and Other Factor Analysis
Our results revealed that the levels of anti-SARS-CoV-2 S antibodies were significantly higher in the infected HCWs than in those in the non-infected participants (27,885.05 U/mL vs. 11,488.69 U/mL, p < 0.001, Table 2). The infected HCWs also showed significantly higher levels of anti-SARS-CoV-2 N antibodies than the non-infected participants (29.93 COI vs. 0.38 COI, p < 0.001, Table 2). The mean interval between the last dose of vaccine and the time when the antibody levels were measured in this study were 4.04 months and 6.24 months for the non-infected and infected groups, respectively (Table 2).

Table 2.
The amount of anti-SARS-CoV-2 antibodies and other clinical data for the participants.
It is worthwhile to note that the levels of white blood cells (WBC) were significantly higher in the non-infected participants than those in the infected HCWs (6771.50/μL vs. 6305.65/μL, p = 0.050, Table 2), whereas the levels of cholesterol (184.01 mg/dL vs. 194.12 mg/dL, p = 0.014) and high-density lipoprotein cholesterol (HDL-C) (62.93 mg/dL vs. 68.04 mg/dL, p = 0.008) for the non-infected participants were significantly lower than those for the infected HCWs.
3.3. Correlation of Antibody Level and Vaccination or Infection
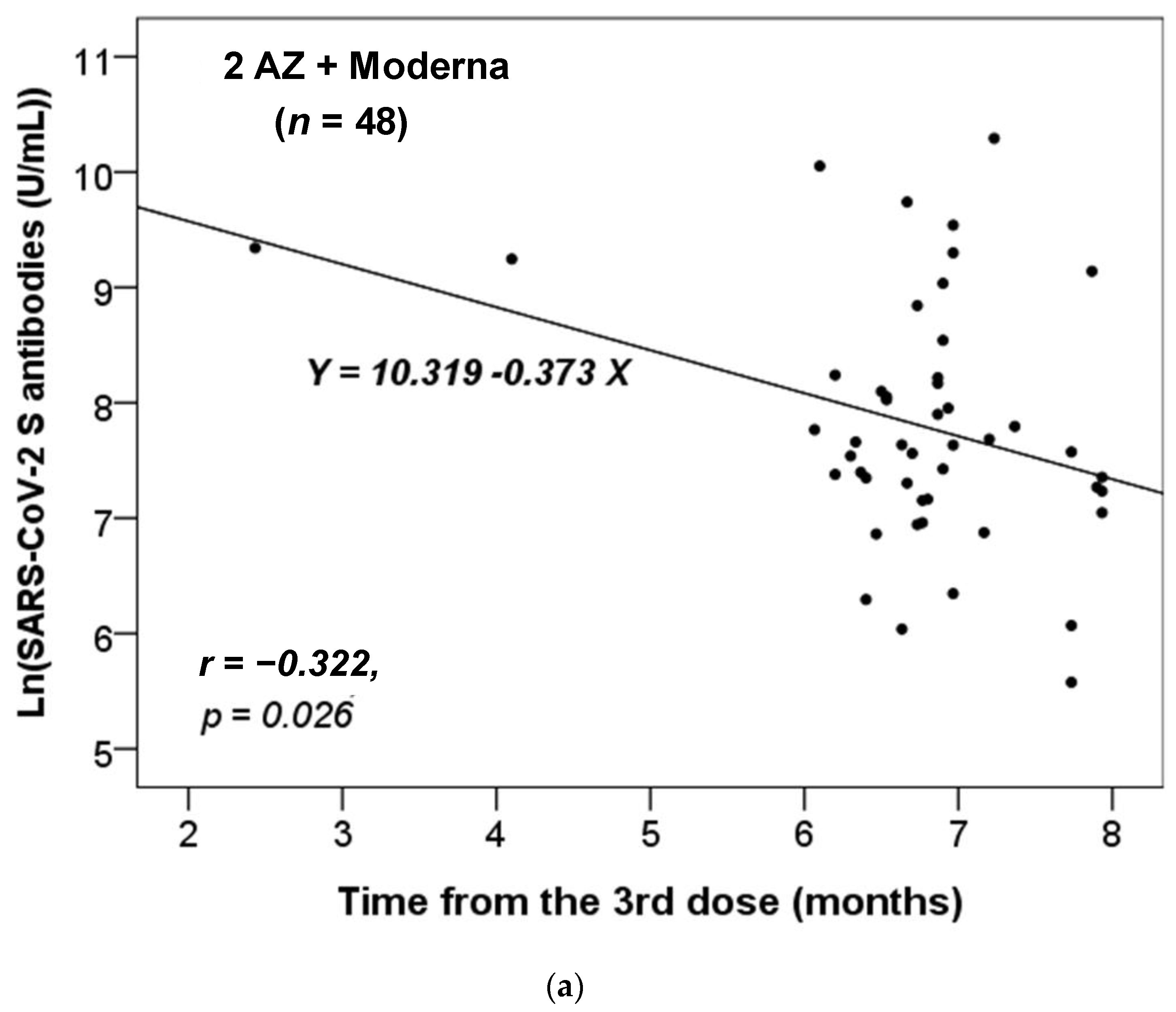
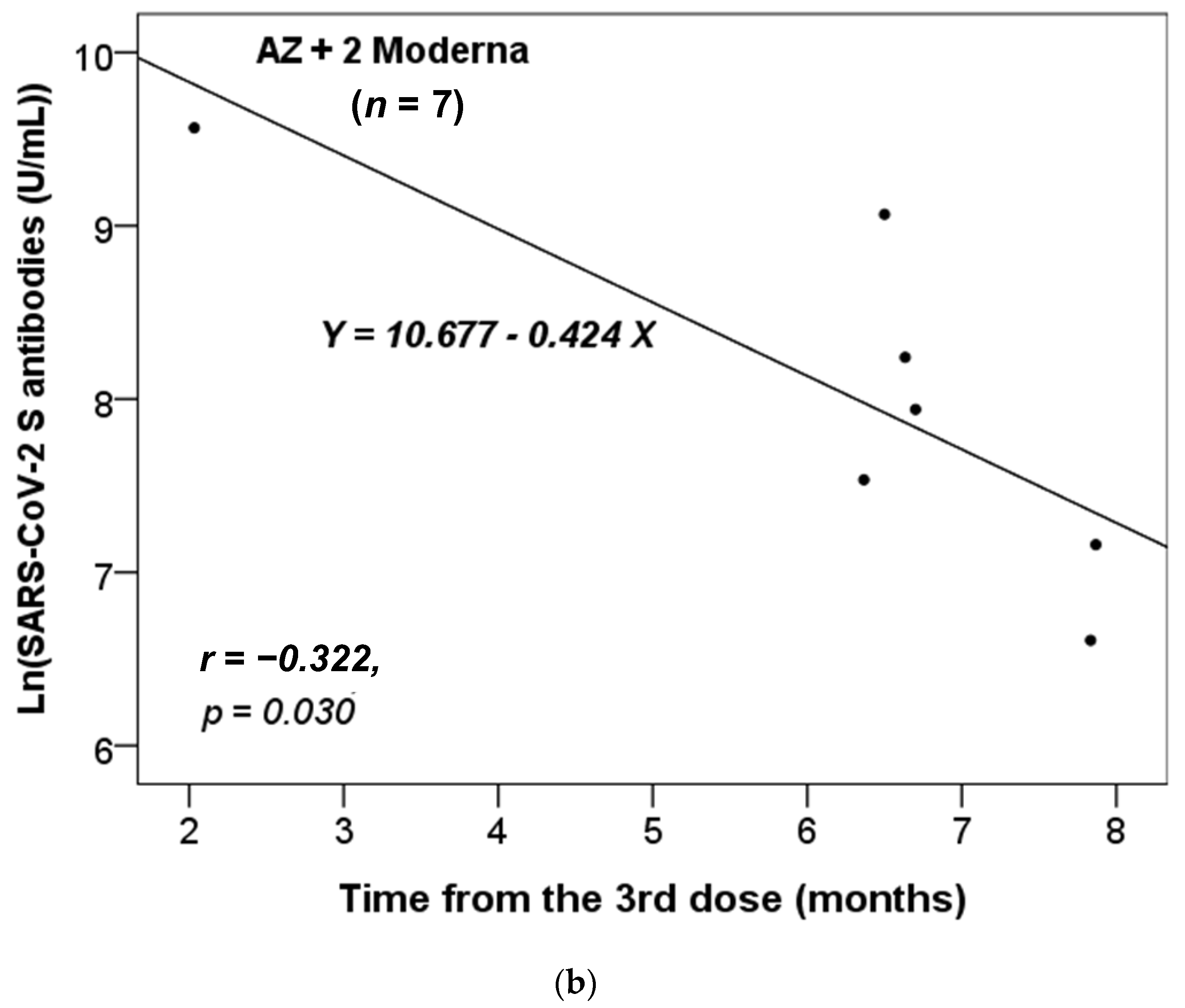
We further assessed whether there was a correlation between the time interval from the last administrated vaccine dose to the time of antibody testing and the titers of the anti-SARS-CoV-2 S antibody. We stratified these participants based on the different combinations of vaccines received. As there was only one participant who had received two vaccine doses in each of the non-infected and infected groups (Table 1), we focused our analysis on those who had received three or four doses. Additionally, we only analyzed the results for the vaccine combinations received by more than five participants. Figure 1 shows that only the anti-S antibody levels in the non-infected group received the vaccine combinations of 2 AZ + 1 Moderna (n = 48) or AZ + 2 Moderna (n = 7) were significantly and inversely correlated with the time from the third dose of vaccination. Thus, the concentration of anti-S antibody level decreased significantly over time from the last dose (p = 0.023 and 0.030, respectively).
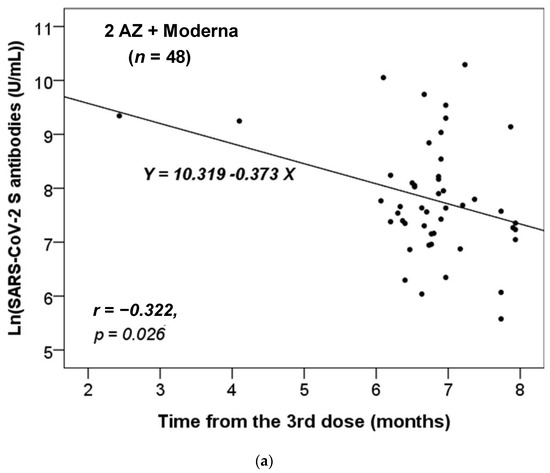
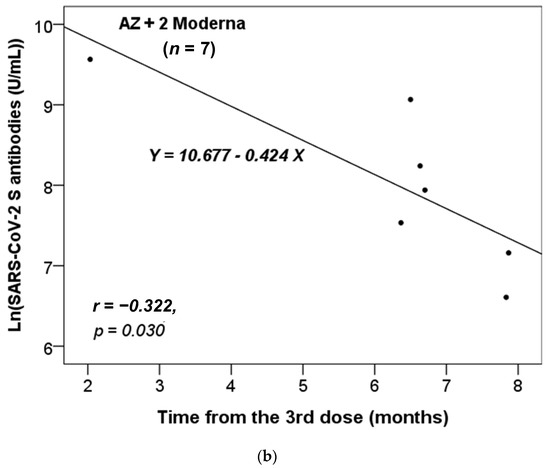
Figure 1.
The correlation between the time interval from the last administrated vaccine dose to the time of antibody testing and the level of anti-SARS-CoV-2 S antibody in the non-infected cases vaccinated with the combinations of (a) 2 AZ + 1 Moderna and (b) AZ + 2 Moderna. Pearson correlation coefficient (r) and p value were indicated.
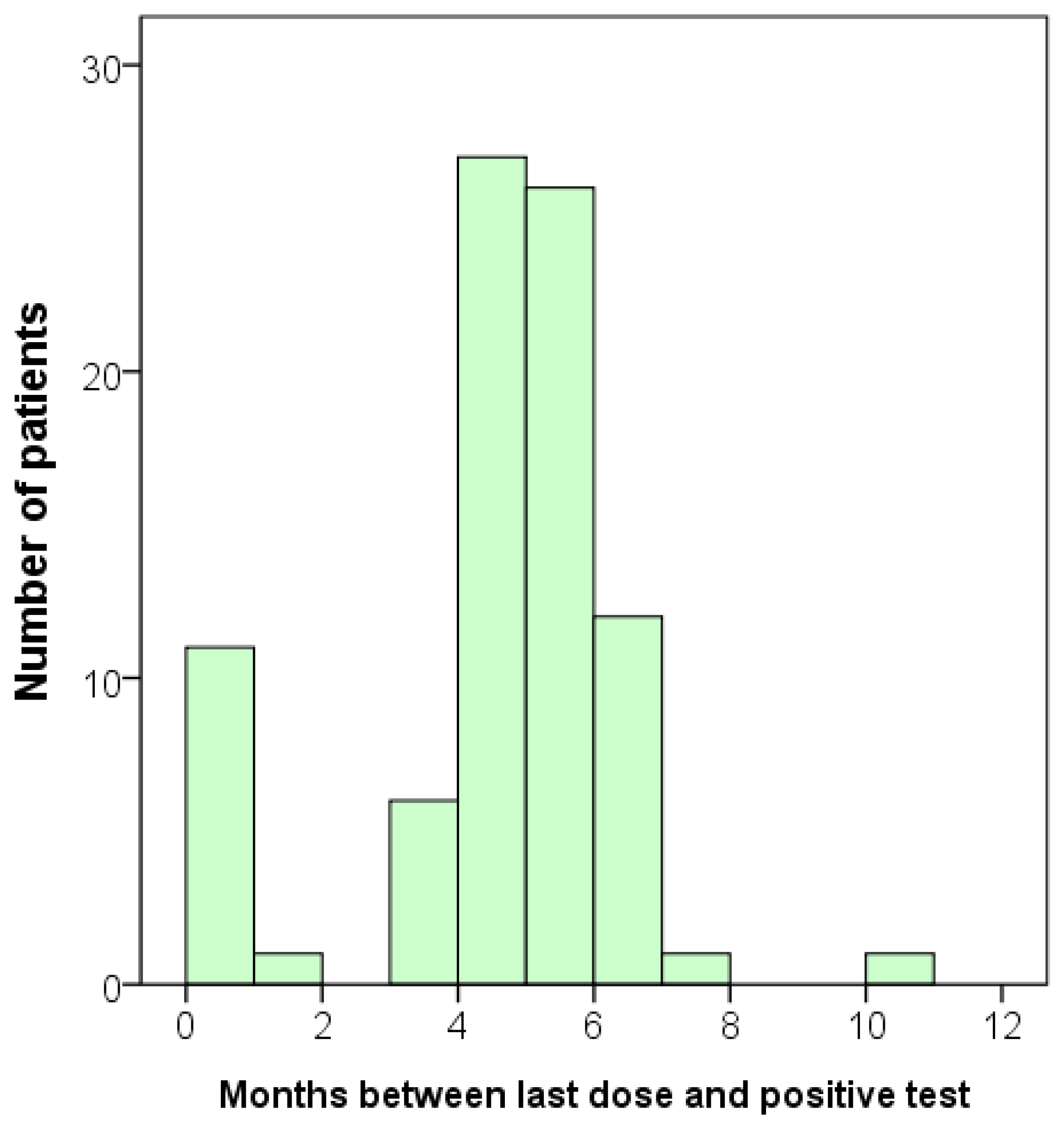
In the infected group, we also investigated whether there was a correlation between the interval from the onset of infection to the time of antibody testing and the concentrations of anti-SARS-CoV-2 S and N antibodies. Again, we concentrated our analysis on those who had received three or four vaccine doses. None of the vaccine combinations showed a significant correlation. However, since all the infected individuals were also vaccinated, it is challenging to observe any trend. Although we did not observe any correlation between the onset of infection and the antibody levels, we did identify a trend that most participants became infected after five or six months following vaccination (Figure 2).
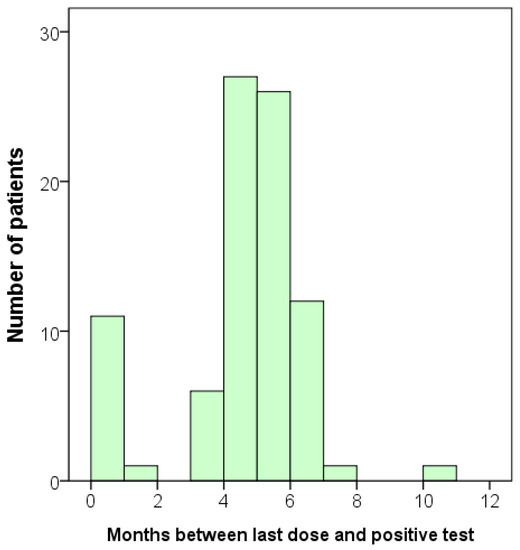
Figure 2.
Bar graph displaying the duration between the last dose of vaccination and the time of infection for each participant. Total n = 85.
3.4. A Follow-Up Survey on the Infection Status
After measuring the concentrations of antibodies, we conducted a follow-up survey on the infection status of the participants. An additional total of 79 participants were infected, including one with a second infection. When we combined the initial ‘infected’ group with the newly identified positive cases, the total number of infected participants increased to 163 (Table 3). Consequently, we obtained additional data that allowed us to analyze the relationship between vaccination and the time of infection or the antibody level in greater detail. The mean duration between the administration of the last dose of the vaccine and the occurrence of SARS-CoV-2 infection was 5.61 ± 2.95 months, with a wide range of 0.03 to 16.47 months (Table 3). This follow-up result was consistent with the findings shown in Figure 2.

Table 3.
Time of infection since the last dose of vaccination.
Next, we examined the correlation between the level of anti-SARS-CoV-2 S or N antibody and the likelihood of future infection. Our analysis revealed that neither of the anti-S and anti-N antibody levels were significantly correlated with the duration between the time measuring the antibody level and a positive infection (Table 4).

Table 4.
Correlation between the levels of antibodies and the interval (months) between the time point measuring the antibody level and a positive infection for cases that became infected after the laboratory tests were conducted.
As there was no significant correlation between the levels of antibodies and the occurrence of infection, we proceeded to investigate whether the levels of antibodies could predict the likelihood of future infection. Therefore, we compared the levels of antibodies in participants who remained uninfected (n = 166) with those who became infected (n = 79) after the measurement of antibodies. Our analysis revealed a significant difference between these two groups. The non-infected group had significantly higher levels of antibodies compared to the infected group (p < 0.001, Table 5).

Table 5.
Cases infected versus non-infected after the antibody evaluations.
4. Discussion
In this research, we assessed the present state of SARS-CoV-2 antibodies among hospital staff and its impact on the degree of protection against SARS-CoV-2 and affect the probability or time of contracting COVID-19. All hospital staff at our facility were vaccinated as they belong to high-risk groups for COVID-19. The anti-S antibodies were higher in infected HCWs compared to non-infected participants (Table 2). The low anti-N antibody response following vaccination in the non-infected group has been noted as expected. It is known that the level of anti-N antibody is not indicative of the individual’s vaccination status, but rather their infection status [18,20]. Moreover, the combination of infection and vaccination significantly increased the levels of anti-SARS-CoV-2 S and anti-SARS-CoV-2 N antibodies (p < 0.001, Table 2). Prior evidence has shown that a previous SARS-CoV-2 infection before primary vaccination can efficiently prime individuals for the COVID-19 vaccination [21,22,23]. Moreover, individuals who recovered from COVID-19 and received a dose of the mRNA vaccine showed an increase in all components of the humoral reaction. Bone marrow plasma cells (BMPCs) expressing specific antibodies are long-lasting and have serum neutralizing activity against new variants of concern. These BMPCs are cleared and produced extensively after vaccination, suggesting that immunity in convalescent persons will be long-lasting [24].
Our data revealed that the titer of the anti-S antibody in the non-infected group with only the vaccine combinations of 2 AZ + 1 Moderna and AZ + 2 Moderna decreased over time (Figure 1). This suggests that the protective effects of vaccination combinations of AZ and Moderna may wane over time. Furthermore, our data showed that most participants became infected after five or six months of vaccination (Table 2 and Table 3, and Figure 2), which is consistent with previous studies [25,26]. According to the study by Matusali G. et al. [27], the levels of anti-RBD IgG and anti-trimeric S IgG persisted for up to six months following the second dose of the COVID-19 vaccine. However, the anti-RBD IgG antibodies showed a more rapid decline compared to the anti-trimeric S IgG antibodies [27]. Despite observing continuous seropositivity beyond six months following vaccination, Sarrigeorgiou et al. [28] noted a gradual decline in antibody levels. Thus, our results are consistent with numerous global studies, indicating that racial variances have no substantial impact on the humoral response to vaccines. The immune response following COVID-19 vaccination involves two processes: cellular response—which generates diverse T-cell lineages, interferons, and interleukins—and the generation of IgG antibodies that target viral antigens, including protein S, triggered by the first process [29]. Generally, these antibodies remain detectable for a period of approximately six months, after which they decrease by 5 to 10-fold [30,31,32,33]. As a result, it is necessary to administer a booster dose approximately six months after the initial vaccination, especially if new variants are circulating. It is noteworthy that new variants of concern (VOCs) of SARS-CoV-2 have the potential to increase virus transmissibility and/or disease severity, as well as result in diagnostic and/or treatment failures [34,35,36]. Taiwan experienced a COVID-19 outbreak in May 2021 and later in June 2021 due to the emergence of the Alpha-lineage (B.1.1.7) [37]. The first cases of the Delta-lineage B.1.617.2 were later reported by the Taiwan Central Epidemic Command Center (CECC), which then led to community transmission outbreaks [38].
Our results did not show any correlation between the antibody level and infection (Section 3.3 and Table 4). However, several studies have demonstrated that following infection, there is a decrease in serum anti-SARSCoV-2 antibodies. The decline occurs rapidly in the first 120 days after infection, followed by a slower decline in the subsequent 210 days. Nevertheless, significant antibody levels are maintained for at least 11 months after infection [39,40,41]. S-specific anti-SARS-CoV-2 bone marrow plasma cells (BMPC) were detected in bone marrow aspirates obtained approximately 7 and 11 months post-infection [42]. A correlation was found between the concentration of anti-S IgG BMPC present in bone marrow aspirate and the circulating anti-S IgG titers at 210–240 days after symptom onset in convalescent individuals [43].
There is a need for immunologic surveillance and follow-up among health personnel, particularly in high-risk groups. This surveillance can provide evidence-based data to determine the appropriate timing for administering a booster shot to sustain heightened levels of antibodies and supply enhanced protection against SARS-CoV-2 and its mutated strains [44]. Our research suggests that the quantity of antibodies present may serve as an indicator of protection against SARS-CoV-2 infection (Table 5). These findings are crucial in making decisions regarding vaccination policy in the future. For instance, hospital staff could receive vaccinations every six months, and those with high levels of antibodies against SARS-CoV-2 may not require frequent vaccinations, such as once a year. Therefore, it may be more prudent to assess antibody levels prior to determining the optimal vaccination frequency. These results may inform policymakers, researchers, and clinicians regarding the optimal time to administer booster shots. Our study provides important insights into individual antibody responses to vaccination/infection that can aid clinicians in identifying populations that may require follow-up immunization.
In addition to these findings, non-infected participants had significantly higher levels of WBC than infected HCWs (p = 0.050, Table 2), while cholesterol (p = 0.014) and HDL-C (p = 0.008) levels were significantly lower in non-infected participants compared to infected HCWs. These results suggest a potential association between COVID-19 infection and dyslipidemia. Similarly, a study by Tang et al. [45] proved that high BMI or a low Adp:Lep, indicative of adipose tissue dysfunction and poor metabolic health, was associated with higher cross-reactive IgG titers, potentially linked to increased disease severity. Following vaccination, obesity was associated with significantly lower antibody levels against RBD from Wuhan Hu-1 and B.1.1.7, but not B.1.351, P.1, or B.1.617.2 [45]. Conversely, analysis of individual dyslipidemia components found that hypertriglyceridemia was associated with lower antibody titers in men after the second and third doses [46]. This finding is in line with the previous research indicating that individuals with obesity may exhibit weaker immune responses to other vaccines, including those for influenza [47]. Dyslipidemia has been linked to a higher risk of severe illness and death from COVID-19 [48]. This could be due to dyslipidemia’s negative impact on the immune system, such as activation of inflammasomes [49], overproduction of the pro-inflammatory cytokine interleukin-6 [50], and reduction in serum immunoglobulin levels [51]. These results provide important insights into the potential factors affecting immune response to COVID-19 infection and vaccination, which could inform clinicians and policymakers in developing personalized strategies for disease prevention and management.
Previous studies also attempted to identify the factors that contribute to the variability in this antibody response. A statistically significant reduction in post-vaccine antibody response was observed among smokers compared to nonsmokers. Among individuals with an antibody titer greater than 250 U/mL, the proportion of nonsmokers was 72.5%, whereas the proportion of smokers in the same group was only 27.5% [52]. Similarly, the study by Moncunill et al. [53] demonstrated that smokers and those with underlying comorbidities, such as autoimmune and other immunological disorders, chronic respiratory and renal diseases, heart and liver diseases, diabetes, and cancers, displayed markedly reduced antibody levels and decreased plasma neutralizing capacity. Yamamoto et al.’s study [54] with a large sample size revealed that both heated tobacco product users and exclusive cigarette smokers had significantly lower titers of anti-S IgG antibodies compared with non-smokers. An evident decline in antibody levels was also noticeable as alcohol consumption increased, even with moderate alcohol intake [54]. This finding is consistent with the study by Kageyama et al. [55]. In addition, Kageyama et al. demonstrated that patients who were taking immunosuppressive drugs or glucocorticoids showed decreased antibody responses. However, medication prescribed for an allergy was found to be a factor significantly correlated with higher levels of antibodies [55]. In contrast, the findings of Wratil et al. suggest that smoking behavior may be protective against the SARS-CoV-2 infection [56].
This study predominantly consisted of females (90.6% and 95.3% for the non-infected and infected participants, respectively) as most participants enrolled were female nurses. Similarly, other studies investigating HCWs also reported higher proportions of female participants [19,57,58]. Several studies have shown that women generally exhibit a more substantial antibody response to vaccine as compared to men [55,57,58,59,60].
There are several limitations to this study that should be noted. Firstly, due to the use of binding antibodies as the only measure, it was not possible to confirm the immunogenicity of the vaccine, such as the T-cell response. Secondly, this study did not involve a real follow-up design, and there were no regular blood tests taken after vaccination to monitor changes in antibody levels over time. Additionally, we did not collect blood samples prior to vaccination. Thirdly, our sample size was small, and there was a gender distribution bias towards female healthcare personnel, and most were nurses. As this study only included HCWs, there were no elderly participants. Finally, since all participants received the vaccine, it was not possible to compare the effects of vaccination and natural infection on antibody levels. Therefore, further studies with larger sample sizes and follow-up designs are necessary.
5. Conclusions
In conclusion, we demonstrated that the antibody levels could reflect the protective effectiveness against SARS-CoV-2 infection; the non-infected group exhibited significantly elevated levels of antibodies compared to the infected group. The protective effects of vaccination may wane over time. The mean period from the last vaccine dose to the onset of SARS-CoV-2 infection was 5.61 ± 2.95 months. Therefore, the finding of this study has implications for vaccine decision-making policies in the future. It suggests that hospital staff may benefit from receiving a vaccination every six months and testing their antibody levels against SARS-CoV-2 prior to vaccination. Those with high antibody levels may not require frequent vaccination, such as once a year.
Author Contributions
Conceptualization and methodology, K.-S.L. and H.-J.W.; formal analysis, K.-S.L., Y.-Y.Y., K.-L.H. and H.-J.W.; investigation, K.-S.L., Y.-Y.Y. and H.-J.W.; data curation, K.-S.L., Y.-Y.Y., K.-L.H. and H.-J.W.; writing—original draft preparation, H.-J.W.; writing—review and editing, K.-S.L.; supervision, K.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the institutional review board of Show Chwan Memorial Hospital, Taiwan, approval code No 1110713.
Informed Consent Statement
Informed consent was obtained from all participants involved in this study.
Data Availability Statement
All data in this study are included in the article.
Acknowledgments
The authors thank the staff from the Division of Infectious Diseases, Department of Internal Medicine, as well as the Department of Laboratory Medicine, Show Chwan Memorial Hospital for the technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- John Hopkins Coronavirus Resource Center. COVID-19 Dashboard. Available online: https://coronavirus.jhu.edu/map.html (accessed on 15 March 2023).
- Taiwan Centers for Disease Control. COVID-19 (SARS-CoV-2 Infection). Available online: https://www.cdc.gov.tw/En (accessed on 9 March 2023).
- Chuang, C.H.; Huang, C.G.; Huang, C.T.; Chen, Y.C.; Kung, Y.A.; Chen, C.J.; Chuang, T.C.; Liu, C.C.; Huang, P.W.; Yang, S.L.; et al. Titers and breadth of neutralizing antibodies against SARS-CoV-2 variants after heterologous booster vaccination in health care workers primed with two doses of ChAdOx1 nCov-19: A single-blinded, randomized clinical trial. J. Clin. Virol. 2022, 157, 105328. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; King, C.C.; Yen, M.Y. Protecting Healthcare Workers During the Coronavirus Disease 2019 (COVID-19) Outbreak: Lessons From Taiwan’s Severe Acute Respiratory Syndrome Response. Clin. Infect. Dis. 2020, 71, 858–860. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Bae, J.Y.; Jun, K.I.; Kim, J.; Son, H.J.; Chung, H.S.; Kim, S.K.; Kim, S.; Minn, D.; Choi, H.J. Evaluation of the Efficacy of COVID-19 Booster Vaccinations in Healthcare Personnel. Vaccines 2022, 10, 1797. [Google Scholar] [CrossRef]
- Shao, P.L.; Tu, H.C.; Gong, Y.N.; Shu, H.Y.; Kirby, R.; Hsu, L.Y.; Yeo, H.Y.; Kuo, H.Y.; Huang, Y.C.; Lin, Y.F.; et al. Emergence and Persistent Dominance of SARS-CoV-2 Omicron BA.2.3.7 Variant, Taiwan. Emerg. Infect. Dis. 2023, 29, 792–796. [Google Scholar] [CrossRef]
- He, Z.; Ren, L.; Yang, J.; Guo, L.; Feng, L.; Ma, C.; Wang, X.; Leng, Z.; Tong, X.; Zhou, W.; et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: A longitudinal, population-level, cross-sectional study. Lancet 2021, 397, 1075–1084. [Google Scholar] [CrossRef]
- Meng, H.; Mao, J.; Ye, Q. Booster vaccination strategy: Necessity, immunization objectives, immunization strategy, and safety. J. Med. Virol. 2022, 94, 2369–2375. [Google Scholar] [CrossRef]
- World Health Organization. Interim Statement on Hybrid Immunity and Increasing Population Seroprevalence Rates. Available online: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates (accessed on 15 December 2022).
- U.S. Food & Drug Administration. Coronavirus Update: FDA Authorizes First Test to Detect Neutralizing Antibodies from Recent or Previous SARS-CoV-2 Infection. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-test-detects-neutralizing-antibodies-recent-or (accessed on 15 December 2022).
- Tan, S.S.; Saw, S.; Chew, K.L.; Wang, C.; Pajarillaga, A.; Khoo, C.; Wang, W.; Ali, Z.M.; Yang, Z.; Chan, Y.H.; et al. Comparative Clinical Evaluation of the Roche Elecsys and Abbott Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Serology Assays for Coronavirus Disease 2019 (COVID-19). Arch. Pathol. Lab. Med. 2021, 145, 32–38. [Google Scholar] [CrossRef]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Milne, G.; Hames, T.; Scotton, C.; Gent, N.; Johnsen, A.; Anderson, R.M.; Ward, T. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir. Med. 2021, 9, 1450–1466. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response following Vaccination with BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Hyun, J.; Park, Y.; Song, Y.G.; Han, S.H.; Park, S.Y.; Kim, S.H.; Park, J.S.; Jeon, S.Y.; Lee, H.S.; Lee, K.H. Reactogenicity and Immunogenicity of the ChAdOx1 nCOV-19 Coronavirus Disease 2019 Vaccine in South Korean Healthcare Workers. Yonsei Med. J. 2022, 63, 1078–1087. [Google Scholar] [CrossRef]
- Follmann, D.; Janes, H.E.; Buhule, O.D.; Zhou, H.; Girard, B.; Marks, K.; Kotloff, K.; Desjardins, M.; Corey, L.; Neuzil, K.M.; et al. Antinucleocapsid Antibodies after SARS-CoV-2 Infection in the Blinded Phase of the Randomized, Placebo-Controlled mRNA-1273 COVID-19 Vaccine Efficacy Clinical Trial. Ann. Intern. Med. 2022, 175, 1258–1265. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef]
- Urlaub, D.; Wolfsdorff, N.; Durak, D.; Renken, F.; Watzl, C. SARS-CoV-2 infection shortly after BNT162b2 vaccination results in high anti-spike antibody levels in nursing home residents and staff. Immun. Inflamm. Dis. 2021, 9, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 Infection and Antibody Levels after Vaccination. Front. Public Health 2021, 9, 778243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Pierobon, A.; Zotto, A.D.; Antico, A.; De Antoni, M.E.; Vianello, L.; Gennari, M.; Di Caprio, A.; Russo, F.; Brambilla, G.; Saugo, M. Outbreak of SARS-CoV-2 B.1.617.2 (delta) variant in a nursing home 28 weeks after two doses of mRNA anti-COVID-19 vaccines: Evidence of a waning immunity. Clin. Microbiol. Infect. 2022, 28, 614.e5–614.e7. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Sberna, G.; Meschi, S.; Gramigna, G.; Colavita, F.; Lapa, D.; Francalancia, M.; Bettini, A.; Capobianchi, M.R.; Puro, V.; et al. Differential Dynamics of SARS-CoV-2 Binding and Functional Antibodies upon BNT162b2 Vaccine: A 6-Month Follow-up. Viruses 2022, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Sarrigeorgiou, I.; Moschandreou, D.; Dimitriadis, A.; Tsinti, G.; Sotiropoulou, E.; Ntoukaki, E.; Eliadis, P.; Backovic, M.; Labropoulou, S.; Escriou, N.; et al. Combined monitoring of IgG and IgA anti-Spike and anti-Receptor binding domain long term responses following BNT162b2 mRNA vaccination in Greek healthcare workers. PLoS ONE 2022, 17, e0277827. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, R.M.; Dennehy, J.J.; Poon, L.L.M.; Saif, L.J.; Enquist, L.W. Are COVID-19 Vaccine Boosters Needed? The Science behind Boosters. J. Virol. 2022, 96, e0197321. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Boehm, E.; Kronig, I.; Neher, R.A.; Eckerle, I.; Vetter, P.; Kaiser, L.; Geneva Centre for Emerging Viral, D. Novel SARS-CoV-2 variants: The pandemics within the pandemic. Clin. Microbiol. Infect. 2021, 27, 1109–1117. [Google Scholar] [CrossRef]
- Bal, A.; Destras, G.; Gaymard, A.; Stefic, K.; Marlet, J.; Eymieux, S.; Regue, H.; Semanas, Q.; d’Aubarede, C.; Billaud, G.; et al. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, August to December 2020. Euro. Surveill. 2021, 26, 2100008. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Jarvis, C.I.; Group, C.C.-W.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.J.; Perng, C.L.; Chung, H.Y.; Chang, C.K.; Lin, J.C.; Yeh, K.M.; Chen, C.W.; Hsieh, S.S.; Pan, P.C.; Chang, H.T.; et al. Clinical assessment of SARS-CoV-2 antigen rapid detection compared with RT-PCR assay for emerging variants at a high-throughput community testing site in Taiwan. Int. J. Infect. Dis. 2022, 115, 30–34. [Google Scholar] [CrossRef]
- Yen, A.M.-F.; Chen, T.H.-H.; Chang, W.-J.; Lin, T.-Y.; Jen, G.H.-H.; Hsu, C.-Y.; Wang, S.-T.; Dang, H.; Chen, S.L.-S. Epidemic Surveillance Models for Containing the Spread of SARS-CoV-2 Variants: Taiwan Experience. medRxiv 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Prion-like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms 2022, 10, 280. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; D’Ettorre, E.; Nico, A.; Inchingolo, F.; Dipalma, G.; Tomassone, D.; Serlenga, E.M.; Dalagni, G.; et al. New Translational Trends in Personalized Medicine: Autologous Peripheral Blood Stem Cells and Plasma for COVID-19 Patient. J. Pers. Med. 2022, 12, 85. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 Vaccines vs. Variants-Determining How Much Immunity Is Enough. JAMA 2021, 325, 1241–1243. [Google Scholar] [CrossRef]
- Tang, L.; Cherry, S.; Tuomanen, E.I.; Kirkpatrick Roubidoux, E.; Lin, C.Y.; Allison, K.J.; Gowen, A.; Freiden, P.; Allen, E.K.; Su, Y.; et al. Host Predictors of Broadly Cross-Reactive Antibodies against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants of Concern Differ between Infection and Vaccination. Clin. Infect. Dis. 2022, 75, e705–e714. [Google Scholar] [CrossRef]
- Islam, Z.; Yamamoto, S.; Mizoue, T.; Oshiro, Y.; Inamura, N.; Konishi, M.; Ozeki, M.; Sugiura, W.; Ohmagari, N. Dyslipidemia and SARS-CoV-2 spike antibody titres after the second and third doses of the BNT162b2 vaccine among healthcare workers in Japan. Diabetes Metab. Res. Rev. 2023, 39, e3606. [Google Scholar] [CrossRef]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Y.; Yin, Y.; Chen, W.; Li, X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): A meta-analysis. Virol. J. 2021, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Rajamaki, K.; Lappalainen, J.; Oorni, K.; Valimaki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Gabriele, M.; Puntoni, M.R.; Longo, V.; Pucci, L. Relationship among IL-6, LDL cholesterol and lipid peroxidation. Cell. Mol. Biol. Lett. 2015, 20, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Bridgeman, M.B.; Brunetti, L. Evaluation of alterations in serum immunoglobulin concentrations in components of metabolic syndrome, obesity, diabetes, and dyslipidemia. BMC Cardiovasc. Disord 2019, 19, 319. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gumus, S.; Bektore, B.; Bozkurt, H.; Gozalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef]
- Moncunill, G.; Aguilar, R.; Ribes, M.; Ortega, N.; Rubio, R.; Salmeron, G.; Molina, M.J.; Vidal, M.; Barrios, D.; Mitchell, R.A.; et al. Determinants of early antibody responses to COVID-19 mRNA vaccines in a cohort of exposed and naive healthcare workers. EBioMedicine 2022, 75, 103805. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tanaka, A.; Ohmagari, N.; Yamaguchi, K.; Ishitsuka, K.; Morisaki, N.; Kojima, M.; Nishikimi, A.; Tokuda, H.; Inoue, M.; et al. Use of heated tobacco products, moderate alcohol drinking, and anti-SARS-CoV-2 IgG antibody titers after BNT162b2 vaccination among Japanese healthcare workers. Prev. Med. 2022, 161, 107123. [Google Scholar] [CrossRef]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, 1861.e1–1861.e5. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Schmacke, N.A.; Osterman, A.; Weinberger, T.; Rech, J.; Karakoc, B.; Zeilberger, M.; Steffen, J.; Mueller, T.T.; Spaeth, P.M.; et al. In-depth profiling of COVID-19 risk factors and preventive measures in healthcare workers. Infection 2022, 50, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.F.; Berenguer-Llergo, A.; Julia, G.; Navarro, G.; Espasa, M.; Rodriguez, S.; Sanchez, N.; Van Den Eynde, E.; Navarro, M.; Calvet, J.; et al. Antibody Response Induced by BNT162b2 and mRNA-1273 Vaccines against the SARS-CoV-2 in a Cohort of Healthcare Workers. Viruses 2022, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic-Vega, A.; Urrunaga-Pastor, D.; Soto-Becerra, P.; Figueroa-Montes, L.E.; Fernandez-Bolivar, L.; Alvizuri-Pastor, S.; Oyanguren-Miranda, M.; Neyra-Vera, I.; Carrillo-Ramos, E.; Sagastegui, A.; et al. Post-vaccination seropositivity against SARS-CoV-2 in peruvian health workers vaccinated with BBIBP-CorV (Sinopharm). Travel Med. Infect. Dis. 2023, 52, 102514. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.D.; Liacos, C.I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef]
- Bayart, J.L.; Morimont, L.; Closset, M.; Wieers, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response following Administration of BNT162b2. Microorganisms 2021, 9, 1340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).