Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host
Abstract
1. Introduction
2. Chemotherapy in Leishmaniasis: Current Drugs, Limitations, and Challenges
| Drugs | Structure | Comments | Efficacy | Resistance | Uses | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|
| Meglumine antimoniate | 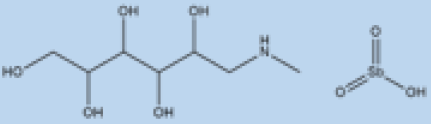 | i.v. or i.m. First-line treatment. | Varies between 35 and 95% based on area. | High resistance in some regions of India. | VL, CL | Cardiotoxicity arthralgia, anorexia, fever, urticaria and significant toxicity to the liver, kidneys, and spleen. Hospitalization and constant monitoring of patients during treatment are needed. | [57,58] |
| Paromomycin | 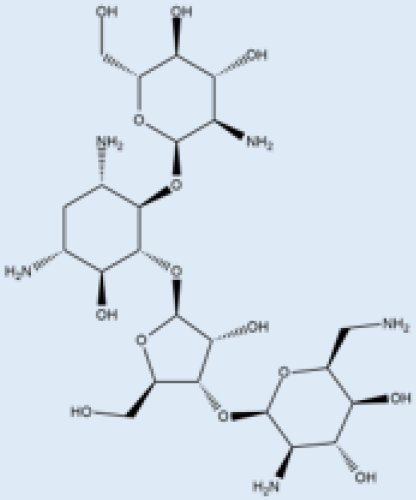 | i.m. | A Phase III trial of Paromomycin (15 mg kg−1 (11 mg base) for 21 days showed 95% cure rate. Effective against PKDL. | No effective resistance. | CL, PKDL | Pain at the injection site, kidney toxicity, liver toxicity, and hearing toxicity. | [25,59] |
| Amphotericin B | 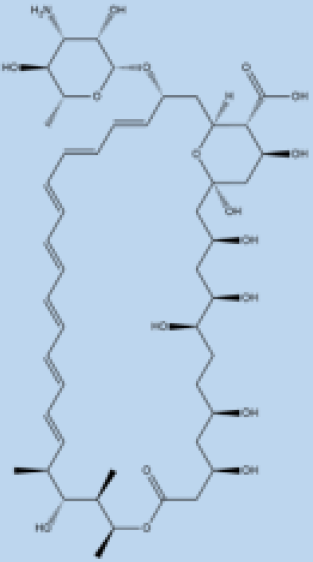 | i.v. Very effective in regions with resistance. | >90% | No effective resistance. | VL | Infusion-related reactions, anemia, nephrotoxicity, myocarditis, and even death of the patient. | [14,60] |
| Pentamidine |  | Pentamidine is a second-line leishmaniasis treatment that is mostly used for CL. | With cure rates ranging from 35% with L. braziliensis in Peru to 90% with L. guyanensis in Suriname, efficacy is very variable. | Yes | CL, VL | Heart damage, joint pain, loss of appetite, fever, urticaria, and serious liver, kidneys, and spleen damage. During treatment, patients must be hospitalized and constantly watched. | [55,61] |
| Miltefosine |  | p.o. Teratogenic. Increasing treatment failures. | 93–95% in India, 65–85% in Africa. | No effective resistance described. | CL, VL | It can cause birth defects, stomach problems, kidney damage, and liver damage and cannot be given to pregnant women. | [62,63] |
2.1. Antimonials
2.2. Amphotericin
2.3. Miltefosine
2.4. Pentamidine
2.5. Paromomycin
3. Drug Resistance and Significance of Combination Therapy
Combination Therapy
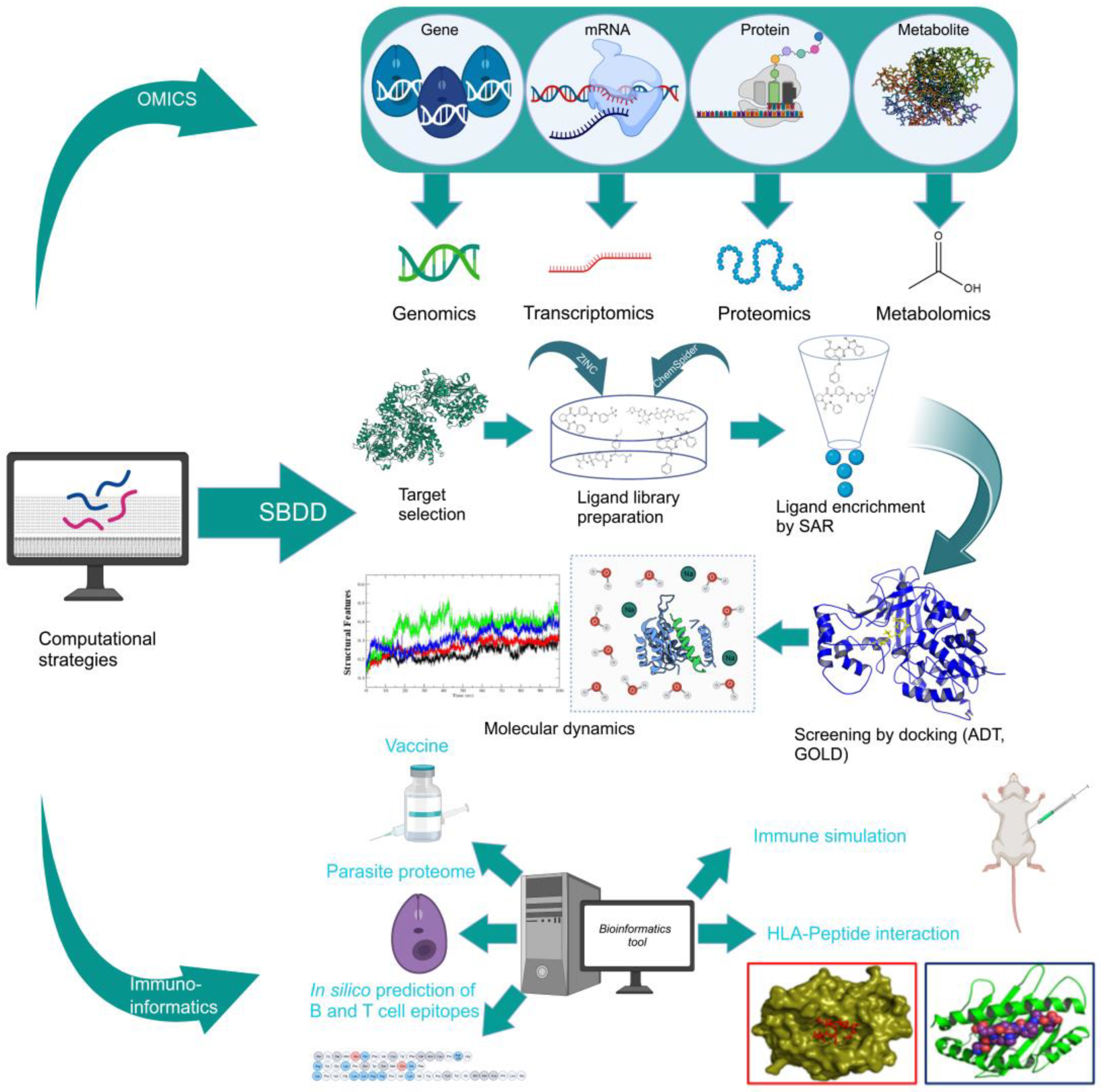
4. Structure- and Ligand-Based Drug Design: Antileishmanial Drug Discovery
4.1. Structure-Based Drug Design (SBDD)
4.2. Ligand-Based Drug Design
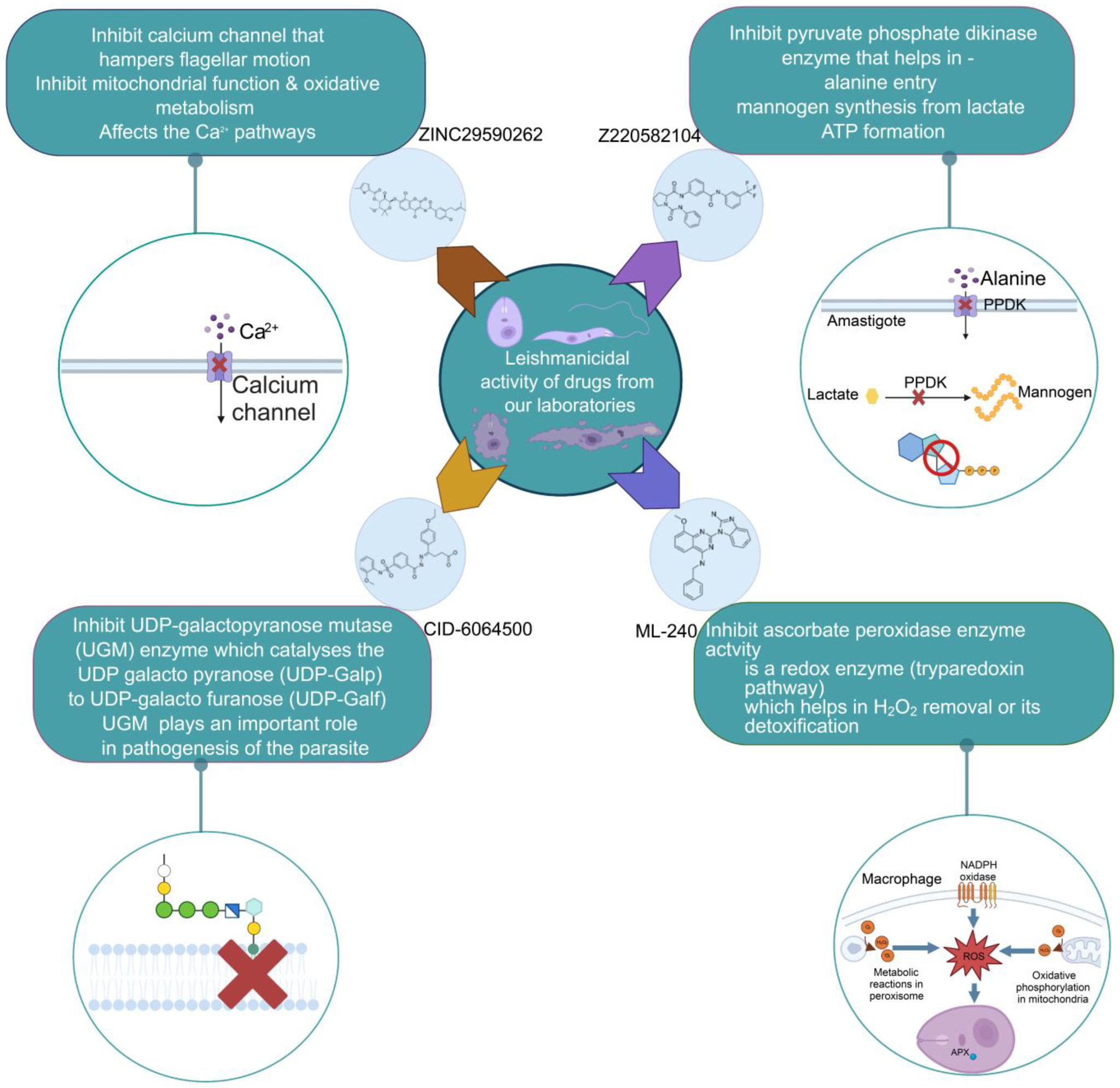
5. Design of Novel Drug Targets: Experience from our Laboratories
5.1. Pyruvate Phosphate Dikinase Inhibitor against Leishmania donovani
5.2. UDP-Galactopyranose Mutase of Leishmania Is a Drug Target
5.3. Targeting Ascorbate Peroxidase of Leishmania
5.4. Screening of Novel Inhibitors against Calcium ion Channels of Leishmania
5.5. Molecular and Cellular Aspects of Novel Drug Design
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | Ascorbate peroxidase |
| CL | Cutaneous leishmaniasis |
| DB | Database |
| DHFR | Dihydrofolate reductase |
| DHFR-TS | Bifunctional dihydrofolate reductase-thymidylate synthase |
| FDA | Food and Drug Administration |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| H2O2 | Hydrogen peroxide |
| HIV | human immunodeficiency virus |
| HMGR | HMG-CoA reductase |
| mRNA | Messenger RNA |
| PDB | Protein Data Bank |
| PKDL | Post Kala-azar dermal leishmaniasis |
| PLGA | poly lactic acid (PLA) and poly glycolic acid (PGA) |
| PRP1 | Proline-rich protein 1 |
| PTR1 | Pteridine reductase 1 |
| ROS | Reactive oxygen species |
| Top1 | DNA topoisomerase I |
| TR | Trypanothione reductase |
| UDP | Uridine diphosphate |
| UGM | UDP-galactopyranose mutase |
| VL | Visceral leishmaniasis |
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Torpiano, P.; Pace, D. Leishmaniasis: Diagnostic Issues in Europe. Expert. Rev. Anti. Infect. Ther. 2015, 13, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 5 November 2022).
- Steverding, D. The History of Leishmaniasis. Parasites Vectors 2017, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Melby, P.C.; Travi, B.L.; Yaneth Osorio, E. Leishmania. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 769–779. ISBN 978-0-12-811736-1. [Google Scholar]
- Gluenz, E.; Ginger, M.L.; McKean, P.G. Flagellum Assembly and Function during the Leishmania Life Cycle. Curr. Opin. Microbiol. 2010, 13, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, T.V.; Mallia Azzopardi, C. Leishmaniasis. Postgrad. Med. J. 2007, 83, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Songumpai, N.; Promrangsee, C.; Noopetch, P.; Siriyasatien, P.; Preativatanyou, K. First Evidence of Co-Circulation of Emerging Leishmania Martiniquensis, Leishmania Orientalis, and Crithidia Sp. in Culicoides Biting Midges (Diptera: Ceratopogonidae), the Putative Vectors for Autochthonous Transmission in Southern Thailand. Trop. Med. Infect. Dis. 2022, 7, 379. [Google Scholar] [CrossRef]
- Srivarasat, S.; Brownell, N.; Siriyasatien, P.; Noppakun, N.; Asawanonda, P.; Rattanakorn, K.; Preativatanyou, K.; Kumtornrut, C. Case Report: Autochthonous Disseminated Cutaneous, Mucocutaneous, and Visceral Leishmaniasis Caused by Leishmania Martiniquensis in a Patient with HIV/AIDS from Northern Thailand and Literature Review. Am. J. Trop. Med. Hyg. 2022, 107, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Arana, B.I. Appraisal of Leishmaniasis Chemotherapy, Current Status and Pipeline StrategiesChapter 1 Leishmaniasis, Impact and Therapeutic Needs; Royal Society of Chemistry: London, UK, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Khan, F.A.K.; Kulkarni, A.A.; Arote, R.; Patil, R.H. Antileishmanial Drug Discovery: Comprehensive Review of the Last 10 Years. RSC Adv. 2015, 5, 32376–32415. [Google Scholar] [CrossRef]
- Lira, R.; Sundar, S.; Makharia, A.; Kenney, R.; Gam, A.; Saraiva, E.; Sacks, D. Evidence That the High Incidence of Treatment Failures in Indian Kala-Azar Is Due to the Emergence of Antimony-Resistant Strains of Leishmania Donovani. J. Infect. Dis. 1999, 180, 564–567. [Google Scholar] [CrossRef]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.; Murray, H.W. Failure of Pentavalent Antimony in Visceral Leishmaniasis in India: Report from the Center of the Indian Epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Adler-Moore, J.; Berenguer, J.; Boelaert, M.; den Boer, M.; Davidson, R.N.; Figueras, C.; Gradoni, L.; Kafetzis, D.A.; Ritmeijer, K.; et al. Liposomal Amphotericin B for the Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 2006, 43, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Meheus, F.; Balasegaram, M.; Olliaro, P.; Sundar, S.; Rijal, S.; Faiz, M.A.; Boelaert, M. Cost-Effectiveness Analysis of Combination Therapies for Visceral Leishmaniasis in the Indian Subcontinent. PLoS Negl. Trop. Dis. 2010, 4, e818. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Neal, R.A.; Pendergast, W.; Chan, J.H. The Activity of Alkyl Phosphorylcholines and Related Derivatives against Leishmania Donovani. Biochem. Pharmacol. 1987, 36, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- Jha, T.K.; Sundar, S.; Thakur, C.P.; Bachmann, P.; Karbwang, J.; Fischer, C.; Voss, A.; Berman, J. Miltefosine, an Oral Agent, for the Treatment of Indian Visceral Leishmaniasis. N. Engl. J. Med. 1999, 341, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Engel, J.; Sindermann, H.; Fischer, C.; Junge, K.; Bryceson, A.; Berman, J. Oral Miltefosine for Indian Visceral Leishmaniasis. N. Engl. J. Med. 2002, 347, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.C.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing Failure of Miltefosine in the Treatment of Kala-Azar in Nepal and the Potential Role of Parasite Drug Resistance, Reinfection, or Noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef]
- Soto, J.; Toledo, J.; Gutierrez, P.; Nicholls, R.S.; Padilla, J.; Engel, J.; Fischer, C.; Voss, A.; Berman, J. Treatment of American Cutaneous Leishmaniasis with Miltefosine, an Oral Agent. Clin. Infect. Dis. 2001, 33, E57–E61. [Google Scholar] [CrossRef]
- Soto, J.; Rea, J.; Balderrama, M.; Toledo, J.; Soto, P.; Valda, L.; Berman, J.D. Efficacy of Miltefosine for Bolivian Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2008, 78, 210–211. [Google Scholar] [CrossRef]
- Pinart, M.; Rueda, J.-R.; Romero, G.A.; Pinzón-Flórez, C.E.; Osorio-Arango, K.; Maia-Elkhoury, A.N.S.; Reveiz, L.; Elias, V.M.; Tweed, J.A. Interventions for American Cutaneous and Mucocutaneous Leishmaniasis. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Jha, T.K.; Olliaro, P.; Thakur, C.P.N.; Kanyok, T.P.; Singhania, B.L.; Singh, I.J.; Singh, N.K.P.; Akhoury, S.; Jha, S. Randomised Controlled Trial of Aminosidine (Paromomycin) v Sodium Stibogluconate for Treating Visceral Leishmaniasis in North Bihar, India. BMJ 1998, 316, 1200–1205. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Sinha, P.K.; Bhattacharya, S.K. Injectable Paromomycin for Visceral Leishmaniasis in India. N. Engl. J. Med. 2007, 356, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Hailu, A.; Musa, A.; Wasunna, M.; Balasegaram, M.; Yifru, S.; Mengistu, G.; Hurissa, Z.; Hailu, W.; Weldegebreal, T.; Tesfaye, S.; et al. Geographical Variation in the Response of Visceral Leishmaniasis to Paromomycin in East Africa: A Multicentre, Open-Label, Randomized Trial. PLoS Negl. Trop. Dis. 2010, 4, e709. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.M.; Younis, B.; Fadlalla, A.; Royce, C.; Balasegaram, M.; Wasunna, M.; Hailu, A.; Edwards, T.; Omollo, R.; Mudawi, M.; et al. Paromomycin for the Treatment of Visceral Leishmaniasis in Sudan: A Randomized, Open-Label, Dose-Finding Study. PLoS Negl. Trop. Dis. 2010, 4, e855. [Google Scholar] [CrossRef]
- Fernández, O.L.; Diaz-Toro, Y.; Ovalle, C.; Valderrama, L.; Muvdi, S.; Rodríguez, I.; Gomez, M.A.; Saravia, N.G. Miltefosine and Antimonial Drug Susceptibility of Leishmania Viannia Species and Populations in Regions of High Transmission in Colombia. PLoS Negl. Trop. Dis. 2014, 8, e2871. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Al-Harrasi, A.; Al-Rawahi, A.; Green, I.R.; Gibbons, S. Fruitful Decade for Antileishmanial Compounds from 2002 to Late 2011. Chem. Rev. 2014, 114, 10369–10428. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J.; Meena, L.P. Leishmaniasis: Treatment, Drug Resistance and Emerging Therapies. Expert. Opin. Orphan Drugs 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Zahedifard, F.; Rafati, S. Prospects for Antimicrobial Peptide-Based Immunotherapy Approaches in Leishmania Control. Expert. Rev. Anti. Infect. Ther. 2018, 16, 461–469. [Google Scholar] [CrossRef]
- Rafferty, J.; Nagaraj, H.; McCloskey, A.P.; Huwaitat, R.; Porter, S.; Albadr, A.; Laverty, G. Peptide Therapeutics and the Pharmaceutical Industry: Barriers Encountered Translating from the Laboratory to Patients. Curr. Med. Chem. 2016, 23, 4231–4259. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the Use of Therapeutic Peptides for Cancer Treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical Application of AMPs. Adv. Exp. Med. Biol. 2019, 1117, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Mendes, B.; Lancellotti, M.; Marangoni, S.; Vale, N.; Passos, Ó.; Ramos, M.J.; Fernandes, P.A.; Gomes, P.; Da Silva, S.L. A Novel Synthetic Peptide Inspired on Lys49 Phospholipase A2 from Crotalus Oreganus Abyssus Snake Venom Active against Multidrug-Resistant Clinical Isolates. Eur. J. Med. Chem. 2018, 149, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.L.; Denny, P.W. Antimicrobial Peptides for Leishmaniasis. Curr. Opin. Investig. Drugs. 2010, 11, 868–875. [Google Scholar]
- Torrent, M.; Pulido, D.; Rivas, L.; Andreu, D. Antimicrobial Peptide Action on Parasites. Curr. Drug. Targets 2012, 13, 1138–1147. [Google Scholar] [CrossRef]
- Marr, A.K.; Cen, S.; Hancock, R.E.W.; McMaster, W.R. Identification of Synthetic and Natural Host Defense Peptides with Leishmanicidal Activity. Antimicrob. Agents Chemother. 2016, 60, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ortega, J.R.; Rivas, L. Characterization of the Leishmanicidal Activity of Antimicrobial Peptides. Methods Mol. Biol. 2010, 618, 393–420. [Google Scholar] [CrossRef]
- González, U.; Pinart, M.; Reveiz, L.; Alvar, J. Interventions for Old World Cutaneous Leishmaniasis. Cochrane Database Syst. Rev. 2008, CD005067. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A. Chemotherapeutics of Visceral Leishmaniasis: Present and Future Developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef]
- Passero, L.F.D.; Brunelli, E.D.S.; Sauini, T.; Amorim Pavani, T.F.; Jesus, J.A.; Rodrigues, E. The Potential of Traditional Knowledge to Develop Effective Medicines for the Treatment of Leishmaniasis. Front. Pharmacol. 2021, 12, 690432. [Google Scholar] [CrossRef]
- Monzote, L. Current Treatment of Leishmaniasis: A Review. Open. Antimicrob. Agents J. 2009, 1, 9–19. [Google Scholar]
- Freitas-Junior, L.H.; Chatelain, E.; Kim, H.A.; Siqueira-Neto, J.L. Visceral Leishmaniasis Treatment: What Do We Have, What Do We Need and How to Deliver It? Int. J. Parasitol. Drugs Drug. Resist. 2012, 2, 11–19. [Google Scholar] [CrossRef] [PubMed]
- No, J.H. Visceral Leishmaniasis: Revisiting Current Treatments and Approaches for Future Discoveries. Acta Trop. 2016, 155, 113–123. [Google Scholar] [CrossRef]
- Singh, K.; Garg, G.; Ali, V. Current Therapeutics, Their Problems and Thiol Metabolism as Potential Drug Targets in Leishmaniasis. Curr. Drug. Metab. 2016, 17, 897–919. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Gil, G. Drug Discovery for Leishmaniasis; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Muthoni Ouattara, G.; Pécoul, B.; Rijal, S.; Rode, J.; et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Clin. Microbiol. Rev. 2018, 31, e00048-18. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Neto, V.V.; Cunha-Junior, E.F.; Dos Santos Faioes, V.; Pereira, T.M.; Silva, R.L.; Leon, L.L.; Torres-Santos, E.C. Leishmaniasis Treatment: Update of Possibilities for Drug Repurposing. Front. Biosci. Landmark 2018, 23, 967–996. [Google Scholar] [CrossRef]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and Promising Novel Drug Candidates against Visceral Leishmaniasis. Pure Appl. Chem. 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of Leishmaniasis: Present Challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Sangenito, L.S.; da Silva Santos, V.; d’Avila-Levy, C.M.; Branquinha, M.H.; Souza Dos Santos, A.L.; de Oliveira, S.S.C. Leishmaniasis and Chagas Disease-Neglected Tropical Diseases: Treatment Updates. Curr. Top. Med. Chem. 2019, 19, 174–177. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Diro, E. Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect. Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef] [PubMed]
- J, B.; M, B.M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 622286. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where Are We and Where Are We Heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J.; Rai, V.K.; Agrawal, N.; Singh, S.P.; Chauhan, V.; Murray, H.W. Amphotericin B Treatment for Indian Visceral Leishmaniasis: Response to 15 Daily versus Alternate-Day Infusions. Clin. Infect. Dis. 2007, 45, 556–561. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.L.; Brustoloni, Y.M.; Fernandes, T.D.; Dorval, M.E.C.; da Cunha, R.V.; Bóia, M.N. Severe Adverse Reactions to Meglumine Antimoniate in the Treatment of Visceral Leishmaniasis: A Report of 13 Cases in the Southwestern Region of Brazil. Trop. Doct. 2009, 39, 180–182. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A.; Tiwari, A.; Shukla, S.; Chakravarty, J.; Rai, M. Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis. ISRN Parasitol. 2014, 2014, 548010. [Google Scholar] [CrossRef]
- de Menezes, J.P.B.; Guedes, C.E.S.; de Oliveira Almeida Petersen, A.L.; Fraga, D.B.M.; Veras, P.S.T. Advances in Development of New Treatment for Leishmaniasis. BioMed Res. Int. 2015, 2015, e815023. [Google Scholar] [CrossRef]
- Andersen, E.M.; Cruz-Saldarriaga, M.; Llanos-Cuentas, A.; Luz-Cjuno, M.; Echevarria, J.; Miranda-Verastegui, C.; Colina, O.; Berman, J.D. Comparison of Meglumine Antimoniate and Pentamidine for Peruvian Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2005, 72, 133–137. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Sinha, P.K.; Sundar, S.; Thakur, C.P.; Jha, T.K.; Pandey, K.; Das, V.R.; Kumar, N.; Lal, C.; Verma, N.; et al. Phase 4 Trial of Miltefosine for the Treatment of Indian Visceral Leishmaniasis. J. Infect. Dis. 2007, 196, 591–598. [Google Scholar] [CrossRef]
- Antinori, S.; Schifanella, L.; Corbellino, M. Leishmaniasis: New Insights from an Old and Neglected Disease. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 109–118. [Google Scholar] [CrossRef]
- Le Pape, P. Development of New Antileishmanial Drugs--Current Knowledge and Future Prospects. J. Enzyme Inhib. Med. Chem. 2008, 23, 708–718. [Google Scholar] [CrossRef]
- Torrado, J.J.; Espada, R.; Ballesteros, M.P.; Torrado-Santiago, S. Amphotericin B Formulations and Drug Targeting. J. Pharm. Sci. 2008, 97, 2405–2425. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.R.; Ferreira, W.A.; Campos, M.A.; Ramos, G.S.; Kato, K.C.; Almeida, G.G.; Corrêa, J.D.; Melo, M.N.; Demicheli, C.; Frézard, F. Amphiphilic Antimony(V) Complexes for Oral Treatment of Visceral Leishmaniasis. Antimicrob. Agents Chemother. 2013, 57, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Acharya, C.; Pal, U.; Chowdhury, S.R.; Sarkar, K.; Maiti, N.C.; Jaisankar, P.; Majumder, H.K. A Novel Spirooxindole Derivative Inhibits the Growth of Leishmania Donovani Parasites Both In Vitro and In Vivo by Targeting Type IB Topoisomerase. Antimicrob. Agents Chemother. 2016, 60, 6281–6293. [Google Scholar] [CrossRef] [PubMed]
- Leañez, J.; Nuñez, J.; García-Marchan, Y.; Sojo, F.; Arvelo, F.; Rodriguez, D.; Buscema, I.; Alvarez-Aular, A.; Bello Forero, J.S.; Kouznetsov, V.V.; et al. Anti-Leishmanial Effect of Spiro Dihydroquinoline-Oxindoles on Volume Regulation Decrease and Sterol Biosynthesis of Leishmania Braziliensis. Exp. Parasitol. 2019, 198, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, N.; Soumya, N.; Singh, S. Antileishmanial Effect of Mevastatin Is Due to Interference with Sterol Metabolism. Parasitol. Res. 2015, 114, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Babu, N.K.; Singh, S.; Babu, N.K. 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGR) Enzyme of the Sterol Biosynthetic Pathway: A Potential Target against Visceral Leishmaniasis; IntechOpen: London, UK, 2018; ISBN 978-1-78984-102-2. [Google Scholar]
- Tabrez, S.; Rahman, F.; Ali, R.; Akand, S.K.; Alaidarous, M.A.; Alshehri, B.M.; Banawas, S.; Dukhyil, A.A.B.; Rub, A. Targeting Sterol Alpha-14 Demethylase of Leishmania Donovani to Fight against Leishmaniasis. J. Cell Biochem. 2021, 122, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, N.; Neelagiri, S.; Kumar, V.; Singh, S. Glycyrrhizic Acid Attenuates Growth of Leishmania Donovani by Depleting Ergosterol Levels. Exp. Parasitol. 2017, 176, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, S.; Lawton, P. In Vitro Effects of Purine and Pyrimidine Analogues on Leishmania Donovani and Leishmania Infantum Promastigotes and Intracellular Amastigotes. Acta Parasitol. 2017, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Soysa, R.; Wilson, Z.N.; Elferich, J.; Forquer, I.; Shinde, U.; Riscoe, M.K.; Yates, P.A.; Ullman, B. Substrate Inhibition of Uracil Phosphoribosyltransferase by Uracil Can Account for the Uracil Growth Sensitivity of Leishmania Donovani Pyrimidine Auxotrophs. J. Biol. Chem. 2013, 288, 29954–29964. [Google Scholar] [CrossRef]
- Yousef, B.; Elwaseela, T.; Ali, T.; Mohammed, F.; Mohammed, W.; Alobaid, M.; Ibrahim Dirar, A. Anti-Malarial Drugs as Potential Inhibitors of Leishmania Glycolytic Enzymes: Development of New Anti-Leishmanial Agents. Pharmacol. Clin. Pharm. Res. 2020, 5, 77–88. [Google Scholar] [CrossRef]
- Verma, A.; Ghosh, S.; Salotra, P.; Singh, R. Artemisinin-Resistant Leishmania Parasite Modulates Host Cell Defense Mechanism and Exhibits Altered Expression of Unfolded Protein Response Genes. Parasitol. Res. 2019, 118, 2705–2713. [Google Scholar] [CrossRef]
- Hendrickx, S.; Caljon, G.; Maes, L. Need for Sustainable Approaches in Antileishmanial Drug Discovery. Parasitol. Res. 2019, 118, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Arciszewska, K.; Maliszewski, D.; Drozdowska, D. Trimethoprim and Other Nonclassical Antifolates an Excellent Template for Searching Modifications of Dihydrofolate Reductase Enzyme Inhibitors. J. Antibiot. 2020, 73, 5–27. [Google Scholar] [CrossRef]
- Sharma, V.K.; Bharatam, P.V. Identification of Selective Inhibitors of LdDHFR Enzyme Using Pharmacoinformatic Methods. J. Comput. Biol. 2021, 28, 43–59. [Google Scholar] [CrossRef] [PubMed]
- das Neves, G.M.; Kagami, L.P.; Gonçalves, I.L.; Eifler-Lima, V.L. Targeting Pteridine Reductase 1 and Dihydrofolate Reductase: The Old Is a New Trend for Leishmaniasis Drug Discovery. Future Med. Chem. 2019, 11, 2107–2130. [Google Scholar] [CrossRef]
- Kapil, S.; Singh, P.K.; Kashyap, A.; Silakari, O. Structure Based Designing of Benzimidazole/Benzoxazole Derivatives as Anti-Leishmanial Agents. SAR QSAR Environ. Res. 2019, 30, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, P.; Parmar, N.; Chandrakar, P.; Sharma, T.; Kathuria, M.; Agnihotri, P.K.; Siddiqi, M.I.; Mitra, K.; Kar, S. Ammonium Trichloro [1,2-Ethanediolato-O,O′]-Tellurate Cures Experimental Visceral Leishmaniasis by Redox Modulation of Leishmania Donovani Trypanothione Reductase and Inhibiting Host Integrin Linked PI3K/Akt Pathway. Cell. Mol. Life Sci. 2018, 75, 563–588. [Google Scholar] [CrossRef]
- Pramanik, P.K.; Chakraborti, S.; Bagchi, A.; Chakraborti, T. Bioassay-Based Corchorus Capsularis L. Leaf-Derived β-Sitosterol Exerts Antileishmanial Effects against Leishmania Donovani by Targeting Trypanothione Reductase. Sci. Rep. 2020, 10, 20440. [Google Scholar] [CrossRef]
- Singh, S.; Kumari, E.; Bhardwaj, R.; Kumar, R.; Dubey, V.K. Molecular Events Leading to Death of Leishmania Donovani under Spermidine Starvation after Hypericin Treatment. Chem. Biol. Drug. Des. 2017, 90, 962–971. [Google Scholar] [CrossRef]
- Singh, S.; Sarma, S.; Katiyar, S.P.; Das, M.; Bhardwaj, R.; Sundar, D.; Dubey, V.K. Probing the Molecular Mechanism of Hypericin-Induced Parasite Death Provides Insight into the Role of Spermidine beyond Redox Metabolism in Leishmania Donovani. Antimicrob. Agents Chemother. 2015, 59, 15–24. [Google Scholar] [CrossRef]
- Alvar, J.; Croft, S.; Olliaro, P. Chemotherapy in the Treatment and Control of Leishmaniasis. Adv. Parasitol. 2006, 61, 223–274. [Google Scholar] [CrossRef]
- Baneth, G.; Shaw, S.E. Chemotherapy of Canine Leishmaniosis. Vet. Parasitol. 2002, 106, 315–324. [Google Scholar] [CrossRef]
- Haldar, A.K.; Sen, P.; Roy, S. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions. Mol. Biol. Int. 2011, 2011, 571242. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.L.; Rainey, P.M. Antileishmanial Activity of Sodium Stibogluconate Fractions. Antimicrob. Agents Chemother. 1993, 37, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Shaked-Mishan, P.; Ulrich, N.; Ephros, M.; Zilberstein, D. Novel Intracellular SbV Reducing Activity Correlates with Antimony Susceptibility in Leishmania Donovani. J. Biol. Chem. 2001, 276, 3971–3976. [Google Scholar] [CrossRef]
- Wyllie, S.; Cunningham, M.L.; Fairlamb, A.H. Dual Action of Antimonial Drugs on Thiol Redox Metabolism in the Human Pathogen Leishmania Donovani. J. Biol. Chem. 2004, 279, 39925–39932. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Monte-Neto, R.; Reis, P.G. Antimony Transport Mechanisms in Resistant Leishmania Parasites. Biophys. Rev. 2014, 6, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Brochu, C.; Wang, J.; Roy, G.; Messier, N.; Wang, X.-Y.; Saravia, N.G.; Ouellette, M. Antimony Uptake Systems in the Protozoan Parasite Leishmania and Accumulation Differences in Antimony-Resistant Parasites. Antimicrob. Agents Chemother. 2003, 47, 3073–3079. [Google Scholar] [CrossRef]
- Marquis, N.; Gourbal, B.; Rosen, B.P.; Mukhopadhyay, R.; Ouellette, M. Modulation in Aquaglyceroporin AQP1 Gene Transcript Levels in Drug-Resistant Leishmania. Mol. Microbiol. 2005, 57, 1690–1699. [Google Scholar] [CrossRef]
- Mandal, S.; Maharjan, M.; Singh, S.; Chatterjee, M.; Madhubala, R. Assessing Aquaglyceroporin Gene Status and Expression Profile in Antimony-Susceptible and -Resistant Clinical Isolates of Leishmania Donovani from India. J. Antimicrob. Chemother. 2010, 65, 496–507. [Google Scholar] [CrossRef]
- Légaré, D.; Richard, D.; Mukhopadhyay, R.; Stierhof, Y.D.; Rosen, B.P.; Haimeur, A.; Papadopoulou, B.; Ouellette, M. The Leishmania ATP-Binding Cassette Protein PGPA Is an Intracellular Metal-Thiol Transporter ATPase. J. Biol. Chem. 2001, 276, 26301–26307. [Google Scholar] [CrossRef] [PubMed]
- Manzano, J.I.; García-Hernández, R.; Castanys, S.; Gamarro, F. A New ABC Half-Transporter in Leishmania Major Is Involved in Resistance to Antimony. Antimicrob. Agents Chemother. 2013, 57, 3719–3730. [Google Scholar] [CrossRef]
- Rai, S.; Bhaskar; Goel, S.K.; Dwivedi, U.N.; Sundar, S.; Goyal, N. Role of Efflux Pumps and Intracellular Thiols in Natural Antimony Resistant Isolates of Leishmania Donovani. PLoS ONE 2013, 8, e74862. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, G.; Miller, R.S.; Oster, C.; Jackson, J.; Aronson, N. A Randomized, Double-Blind Study of the Efficacy of a 10- or 20-Day Course of Sodium Stibogluconate for Treatment of Cutaneous Leishmaniasis in United States Military Personnel. Clin. Infect. Dis. 2002, 35, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic Review of the Adverse Effects of Cutaneous Leishmaniasis Treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.S.; Armstrong, M.S.; Watson, J.; Lockwood, D.N. Monitoring Toxicity Associated with Parenteral Sodium Stibogluconate in the Day-Case Management of Returned Travellers with New World Cutaneous Leishmaniasis [Corrected]. PLoS Negl. Trop. Dis. 2012, 6, e1688. [Google Scholar] [CrossRef]
- Brajtburg, J.; Powderly, W.G.; Kobayashi, G.S.; Medoff, G. Amphotericin B: Current Understanding of Mechanisms of Action. Antimicrob. Agents Chemother. 1990, 34, 183–188. [Google Scholar] [CrossRef]
- Ramos, H.; Valdivieso, E.; Gamargo, M.; Dagger, F.; Cohen, B.E. Amphotericin B Kills Unicellular Leishmanias by Forming Aqueous Pores Permeable to Small Cations and Anions. J. Membr. Biol. 1996, 152, 65–75. [Google Scholar] [CrossRef]
- Solomon, M.; Pavlotsky, F.; Leshem, E.; Ephros, M.; Trau, H.; Schwartz, E. Liposomal Amphotericin B Treatment of Cutaneous Leishmaniasis Due to Leishmania Tropica. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 973–977. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Shirzadi, M.R. Lipsosomal Amphotericin B: A Review of Its Properties, Function, and Use for Treatment of Cutaneous Leishmaniasis. Res. Rep. Trop. Med. 2019, 10, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.R.L.; Rosa, M.E.A.; Guimarães, L.H.; Prates, F.V.O.; Queiroz, A.; Schriefer, A.; Carvalho, E.M. Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B. Clin. Infect. Dis. 2015, 61, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.A.S.; Costa, D.L.; Costa, C.H.N.; de Almeida, R.P.; de Melo, E.V.; de Carvalho, S.F.G.; Rabello, A.; de Carvalho, A.L.; de Queiroz Sousa, A.; Leite, R.D.; et al. Efficacy and Safety of Available Treatments for Visceral Leishmaniasis in Brazil: A Multicenter, Randomized, Open Label Trial. PLoS Negl. Trop. Dis. 2017, 11, e0005706. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, D.V.C.; Martins, V.T.; Lage, D.P.; Dias, D.S.; Ribeiro, P.A.F.; Carvalho, A.M.R.S.; Dias, A.L.T.; Miyazaki, C.K.; Menezes-Souza, D.; Roatt, B.M.; et al. Comparing the Therapeutic Efficacy of Different Amphotericin B-Carrying Delivery Systems against Visceral Leishmaniasis. Exp. Parasitol. 2018, 186, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Gupta, P.K.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Targeted Chemotherapy of Visceral Leishmaniasis by Lactoferrin-Appended Amphotericin B-Loaded Nanoreservoir: In Vitro and in Vivo Studies. Nanomedicine 2015, 10, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A Review of Its Pharmacology and Therapeutic Efficacy in the Treatment of Leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Blanc, S.; Gaudin, K.; Chaminade, P.; Loiseau, P.M. Miltefosine Affects Lipid Metabolism in Leishmania Donovani Promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1425–1430. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A.; Rai, M.; Prajapati, V.K.; Singh, A.K.; Ostyn, B.; Boelaert, M.; Dujardin, J.-C.; Chakravarty, J. Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India after a Decade of Use. Clin. Infect. Dis. 2012, 55, 543–550. [Google Scholar] [CrossRef]
- Godinho, J.L.P.; Simas-Rodrigues, C.; Silva, R.; Ürmenyi, T.P.; de Souza, W.; Rodrigues, J.C.F. Efficacy of Miltefosine Treatment in Leishmania Amazonensis-Infected BALB/c Mice. Int. J. Antimicrob. Agents 2012, 39, 326–331. [Google Scholar] [CrossRef]
- Coelho, A.C.; Oliveira, J.C.; Espada, C.R.; Reimão, J.Q.; Trinconi, C.T.; Uliana, S.R.B. A Luciferase-Expressing Leishmania Braziliensis Line That Leads to Sustained Skin Lesions in BALB/c Mice and Allows Monitoring of Miltefosine Treatment Outcome. PLoS Negl. Trop. Dis. 2016, 10, e0004660. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.C.; Trinconi, C.T.; Costa, C.H.N.; Uliana, S.R.B. In Vitro and In Vivo Miltefosine Susceptibility of a Leishmania Amazonensis Isolate from a Patient with Diffuse Cutaneous Leishmaniasis: Follow-Up. PLoS Negl. Trop. Dis. 2016, 10, e0004720. [Google Scholar] [CrossRef] [PubMed]
- de Morais-Teixeira, E.; Damasceno, Q.S.; Galuppo, M.K.; Romanha, A.J.; Rabello, A. The in Vitro Leishmanicidal Activity of Hexadecylphosphocholine (Miltefosine) against Four Medically Relevant Leishmania Species of Brazil. Mem. Inst. Oswaldo Cruz. 2011, 106, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Victoria, F.J.; Castanys, S.; Gamarro, F. Leishmania Donovani Resistance to Miltefosine Involves a Defective Inward Translocation of the Drug. Antimicrob. Agents Chemother. 2003, 47, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Deep, D.K.; Singh, R.; Bhandari, V.; Verma, A.; Sharma, V.; Wajid, S.; Sundar, S.; Ramesh, V.; Dujardin, J.C.; Salotra, P. Increased Miltefosine Tolerance in Clinical Isolates of Leishmania Donovani Is Associated with Reduced Drug Accumulation, Increased Infectivity and Resistance to Oxidative Stress. PLoS Negl. Trop. Dis. 2017, 11, e0005641. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Monti-Rocha, R.; Costa, D.L.; Molina Sesana, A.; Pansini, L.N.N.; Segatto, M.; Mottram, J.C.; Costa, C.H.N.; Carvalho, S.F.G.; Dietze, R. Natural Resistance of Leishmania Infantum to Miltefosine Contributes to the Low Efficacy in the Treatment of Visceral Leishmaniasis in Brazil. Am. J. Trop. Med. Hyg. 2019, 101, 789–794. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, J.; Gupta, A.K.; Singh, A.; Shankar, P.; Singh, S. Laboratory Confirmed Miltefosine Resistant Cases of Visceral Leishmaniasis from India. Parasit. Vectors 2017, 10, 49. [Google Scholar] [CrossRef]
- Chawla, B.; Jhingran, A.; Panigrahi, A.; Stuart, K.D.; Madhubala, R. Paromomycin Affects Translation and Vesicle-Mediated Trafficking as Revealed by Proteomics of Paromomycin -Susceptible -Resistant Leishmania Donovani. PLoS ONE 2011, 6, e26660. [Google Scholar] [CrossRef]
- Sinha, P.K.; Jha, T.K.; Thakur, C.P.; Nath, D.; Mukherjee, S.; Aditya, A.K.; Sundar, S. Phase 4 Pharmacovigilance Trial of Paromomycin Injection for the Treatment of Visceral Leishmaniasis in India. J. Trop. Med. 2011, 2011, 645203. [Google Scholar] [CrossRef]
- Sundar, S. Drug Resistance in Indian Visceral Leishmaniasis. Trop. Med. Int. Health 2001, 6, 849–854. [Google Scholar] [CrossRef]
- Harhay, M.O.; Olliaro, P.L.; Costa, D.L.; Costa, C.H.N. Urban Parasitology: Visceral Leishmaniasis in Brazil. Trends Parasitol. 2011, 27, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Gradoni, L.; Alvar, J. HIV and the Transmission of Leishmania. Ann. Trop. Med. Parasitol. 2003, 97, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Guerin, P.J.; Gerstl, S.; Haaskjold, A.A.; Rottingen, J.-A.; Sundar, S. Treatment Options for Visceral Leishmaniasis: A Systematic Review of Clinical Studies Done in India, 1980–2004. Lancet Infect. Dis. 2005, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Gourbal, B.; Sonuc, N.; Bhattacharjee, H.; Legare, D.; Sundar, S.; Ouellette, M.; Rosen, B.P.; Mukhopadhyay, R. Drug Uptake and Modulation of Drug Resistance in Leishmania by an Aquaglyceroporin. J. Biol. Chem. 2004, 279, 31010–31017. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Dey, S.; Xu, N.; Gage, D.; Lightbody, J.; Ouellette, M.; Rosen, B.P. Trypanothione Overproduction and Resistance to Antimonials and Arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA 1996, 93, 10383–10387. [Google Scholar] [CrossRef]
- Grondin, K.; Haimeur, A.; Mukhopadhyay, R.; Rosen, B.P.; Ouellette, M. Co-Amplification of the Gamma-Glutamylcysteine Synthetase Gene Gsh1 and of the ABC Transporter Gene PgpA in Arsenite-Resistant Leishmania Tarentolae. EMBO J. 1997, 16, 3057–3065. [Google Scholar] [CrossRef]
- Légaré, D.; Papadopoulou, B.; Roy, G.; Mukhopadhyay, R.; Haimeur, A.; Dey, S.; Grondin, K.; Brochu, C.; Rosen, B.P.; Ouellette, M. Efflux Systems and Increased Trypanothione Levels in Arsenite-ResistantLeishmania. Exp. Parasitol. 1997, 87, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Guimond, C.; Trudel, N.; Brochu, C.; Marquis, N.; Fadili, A.E.; Peytavi, R.; Briand, G.; Richard, D.; Messier, N.; Papadopoulou, B.; et al. Modulation of Gene Expression in Leishmania Drug Resistant Mutants as Determined by Targeted DNA Microarrays. Nucleic Acids Res. 2003, 31, 5886–5896. [Google Scholar] [CrossRef] [PubMed]
- do Monte-Neto, R.L.; Coelho, A.C.; Raymond, F.; Légaré, D.; Corbeil, J.; Melo, M.N.; Frézard, F.; Ouellette, M. Gene Expression Profiling and Molecular Characterization of Antimony Resistance in Leishmania Amazonensis. PLoS Negl. Trop. Dis. 2011, 5, e1167. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Purkait, B.; Singh, R.; Wasnik, K.; Das, S.; Kumar, A.; Paine, M.; Dikhit, M.; Singh, D.; Sardar, A.H.; Ghosh, A.K.; et al. Up-Regulation of Silent Information Regulator 2 (Sir2) Is Associated with Amphotericin B Resistance in Clinical Isolates of Leishmania Donovani. J. Antimicrob. Chemother. 2015, 70, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Mbongo, N.; Loiseau, P.M.; Billion, M.A.; Robert-Gero, M. Mechanism of Amphotericin B Resistance in Leishmania Donovani Promastigotes. Antimicrob. Agents Chemother. 1998, 42, 352–357. [Google Scholar] [CrossRef]
- Rai, K.; Cuypers, B.; Bhattarai, N.R.; Uranw, S.; Berg, M.; Ostyn, B.; Dujardin, J.-C.; Rijal, S.; Vanaerschot, M. Relapse after Treatment with Miltefosine for Visceral Leishmaniasis Is Associated with Increased Infectivity of the Infecting Leishmania Donovani Strain. mBio 2013, 4, e00611-13. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.C.; Rijal, S.; Ostyn, B.; de Vries, P.J.; Singh, R.; Bhattarai, N.; Uranw, S.; Dujardin, J.-C.; Boelaert, M.; Beijnen, J.H.; et al. Failure of Miltefosine in Visceral Leishmaniasis Is Associated With Low Drug Exposure. J. Infect. Dis. 2014, 210, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ortega, J.R.; Rivas, L. Miltefosine (Hexadecylphosphocholine) Inhibits Cytochrome c Oxidase in Leishmania Donovani Promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1327–1332. [Google Scholar] [CrossRef]
- Castanys-Muñoz, E.; Pérez-Victoria, J.M.; Gamarro, F.; Castanys, S. Characterization of an ABCG-Like Transporter from the Protozoan Parasite Leishmania with a Role in Drug Resistance and Transbilayer Lipid Movement. Antimicrob. Agents Chemother. 2008, 52, 3573–3579. [Google Scholar] [CrossRef]
- Coelho, A.C.; Trinconi, C.T.; Costa, C.H.N.; Uliana, S.R.B. In Vitro and In Vivo Miltefosine Susceptibility of a Leishmania Amazonensis Isolate from a Patient with Diffuse Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2014, 8, e2999. [Google Scholar] [CrossRef]
- Sánchez-Cañete, M.P.; Carvalho, L.; Pérez-Victoria, F.J.; Gamarro, F.; Castanys, S. Low Plasma Membrane Expression of the Miltefosine Transport Complex Renders Leishmania Braziliensis Refractory to the Drug. Antimicrob. Agents Chemother. 2009, 53, 1305–1313. [Google Scholar] [CrossRef]
- Obonaga, R.; Fernández, O.L.; Valderrama, L.; Rubiano, L.C.; del Mar Castro, M.; Barrera, M.C.; Gomez, M.A.; Gore Saravia, N. Treatment Failure and Miltefosine Susceptibility in Dermal Leishmaniasis Caused by Leishmania Subgenus Viannia Species. Antimicrob. Agents Chemother. 2014, 58, 144–152. [Google Scholar] [CrossRef]
- Coelho, A.C.; Trinconi, C.T.; Senra, L.; Yokoyama-Yasunaka, J.K.U.; Uliana, S.R.B. Leishmania Is Not Prone to Develop Resistance to Tamoxifen. Int. J. Parasitol. Drugs Drug. Resist. 2015, 5, 77–83. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Sharma, V.; Singh, R.; Salotra, P. Comparative Transcript Expression Analysis of Miltefosine-Sensitive and Miltefosine-Resistant Leishmania Donovani. Parasitol. Res. 2014, 113, 1171–1184. [Google Scholar] [CrossRef]
- Mondelaers, A.; Sanchez-Cañete, M.P.; Hendrickx, S.; Eberhardt, E.; Garcia-Hernandez, R.; Lachaud, L.; Cotton, J.; Sanders, M.; Cuypers, B.; Imamura, H.; et al. Genomic and Molecular Characterization of Miltefosine Resistance in Leishmania Infantum Strains with Either Natural or Acquired Resistance through Experimental Selection of Intracellular Amastigotes. PLoS ONE 2016, 11, e0154101. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.D.; Lonchamp, J.; Downing, T.; Imamura, H.; Freeman, T.M.; Cotton, J.A.; Sanders, M.; Blackburn, G.; Dujardin, J.C.; Rijal, S.; et al. In Vitro Selection of Miltefosine Resistance in Promastigotes of Leishmania Donovani from Nepal: Genomic and Metabolomic Characterization. Mol. Microbiol. 2016, 99, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Basselin, M.; Denise, H.; Coombs, G.H.; Barrett, M.P. Resistance to Pentamidine in Leishmania Mexicana Involves Exclusion of the Drug from the Mitochondrion. Antimicrob. Agents Chemother. 2002, 46, 3731–3738. [Google Scholar] [CrossRef]
- Ac, C.; Sm, B.; Pc, C. Functional Genetic Identification of PRP1, an ABC Transporter Superfamily Member Conferring Pentamidine Resistance in Leishmania Major. Mol. Biochem. Parasitol. 2003, 130, 83–90. [Google Scholar] [CrossRef]
- Gazanion, É.; Fernández-Prada, C.; Papadopoulou, B.; Leprohon, P.; Ouellette, M. Cos-Seq for High-Throughput Identification of Drug Target and Resistance Mechanisms in the Protozoan Parasite Leishmania. Proc. Natl. Acad. Sci. USA 2016, 113, E3012–E3021. [Google Scholar] [CrossRef]
- Prajapati, V.K.; Mehrotra, S.; Gautam, S.; Rai, M.; Sundar, S. In Vitro Antileishmanial Drug Susceptibility of Clinical Isolates from Patients with Indian Visceral Leishmaniasis--Status of Newly Introduced Drugs. Am. J. Trop. Med. Hyg. 2012, 87, 655–657. [Google Scholar] [CrossRef]
- Adaui, V.; Lye, L.-F.; Akopyants, N.S.; Zimic, M.; Llanos-Cuentas, A.; Garcia, L.; Maes, I.; De Doncker, S.; Dobson, D.E.; Arevalo, J.; et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania Braziliensis in Peru and Bolivia. J. Infect. Dis. 2016, 213, 112–121. [Google Scholar] [CrossRef]
- Bourreau, E.; Ginouves, M.; Prévot, G.; Hartley, M.-A.; Gangneux, J.-P.; Robert-Gangneux, F.; Dufour, J.; Sainte-Marie, D.; Bertolotti, A.; Pratlong, F.; et al. Presence of Leishmania RNA Virus 1 in Leishmania Guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J. Infect. Dis. 2016, 213, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Rai, M.; Chakravarty, J.; Agarwal, D.; Agrawal, N.; Vaillant, M.; Olliaro, P.; Murray, H.W. New Treatment Approach in Indian Visceral Leishmaniasis: Single-Dose Liposomal Amphotericin B Followed by Short-Course Oral Miltefosine. Clin. Infect. Dis. 2008, 47, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Sinha, P.K.; Rai, M.; Verma, D.K.; Nawin, K.; Alam, S.; Chakravarty, J.; Vaillant, M.; Verma, N.; Pandey, K.; et al. Comparison of Short-Course Multidrug Treatment with Standard Therapy for Visceral Leishmaniasis in India: An Open-Label, Non-Inferiority, Randomised Controlled Trial. Lancet 2011, 377, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Aparicio, P.; Aseffa, A.; Den Boer, M.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Ter Horst, R.; López-Vélez, R.; Moreno, J. The Relationship between Leishmaniasis and AIDS: The Second 10 Years. Clin. Microbiol. Rev. 2008, 21, 334–359, table of contents. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Diro, E.; Lopez-Velez, R.; Boelaert, M.; Lynen, L.; Zijlstra, E.; Dujardin, J.-C.; Hailu, A. HIV-1 Protease Inhibitors for Treatment of Visceral Leishmaniasis in HIV-Co-Infected Individuals. Lancet Infect. Dis. 2013, 13, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Das, P.; Isaakidis, P.; Sunyoto, T.; Sagili, K.D.; Lima, M.A.; Mitra, G.; Kumar, D.; Pandey, K.; Van geertruyden, J.-P.; et al. Combination Treatment for Visceral Leishmaniasis Patients Coinfected with Human Immunodeficiency Virus in India. Clin. Infect. Dis. 2015, 61, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Mahajan, R.; Sinha, P.K.; van Griensven, J.; Pandey, K.; Lima, M.A.; Sanz, M.G.; Sunyoto, T.; Kumar, S.; Mitra, G.; et al. Visceral Leishmaniasis and HIV Co-Infection in Bihar, India: Long-Term Effectiveness and Treatment Outcomes with Liposomal Amphotericin B (AmBisome). PLoS Negl. Trop. Dis. 2014, 8, e3053. [Google Scholar] [CrossRef] [PubMed]
- Folmer, R.H.A. Integrating Biophysics with HTS-Driven Drug Discovery Projects. Drug. Discovery Today 2016, 21, 491–498. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Lam, K.S. Combinatorial Chemistry in Drug Discovery. Curr. Opin. Chem. Biol. 2017, 38, 117–126. [Google Scholar] [CrossRef]
- Lavecchia, A.; Cerchia, C. In Silico Methods to Address Polypharmacology: Current Status, Applications and Future Perspectives. Drug. Discov. Today 2016, 21, 288–298. [Google Scholar] [CrossRef]
- Cheng, T.; Li, Q.; Zhou, Z.; Wang, Y.; Bryant, S.H. Structure-Based Virtual Screening for Drug Discovery: A Problem-Centric Review. AAPS J. 2012, 14, 133–141. [Google Scholar] [CrossRef]
- Ou-Yang, S.-S.; Lu, J.-Y.; Kong, X.-Q.; Liang, Z.-J.; Luo, C.; Jiang, H. Computational Drug Discovery. Acta Pharmacol. Sin. 2012, 33, 1131–1140. [Google Scholar] [CrossRef]
- Aslett, M.; Aurrecoechea, C.; Berriman, M.; Brestelli, J.; Brunk, B.P.; Carrington, M.; Depledge, D.P.; Fischer, S.; Gajria, B.; Gao, X.; et al. TriTrypDB: A Functional Genomic Resource for the Trypanosomatidae. Nucleic Acids Res. 2010, 38, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E.C.; MacRae, J.I.; Naderer, T.; Ng, M.; McConville, M.J.; Likić, V.A. LeishCyc: A Guide to Building a Metabolic Pathway Database and Visualization of Metabolomic Data. Methods Mol. Biol. 2012, 881, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Real, F.; Vidal, R.O.; Carazzolle, M.F.; Mondego, J.M.C.; Costa, G.G.L.; Herai, R.H.; Würtele, M.; de Carvalho, L.M.; Carmona e Ferreira, R.; Mortara, R.A.; et al. The Genome Sequence of Leishmania (Leishmania) Amazonensis: Functional Annotation and Extended Analysis of Gene Models. DNA Res. 2013, 20, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Logan-Klumpler, F.J.; De Silva, N.; Boehme, U.; Rogers, M.B.; Velarde, G.; McQuillan, J.A.; Carver, T.; Aslett, M.; Olsen, C.; Subramanian, S.; et al. GeneDB--an Annotation Database for Pathogens. Nucleic Acids Res. 2012, 40, D98–D108. [Google Scholar] [CrossRef] [PubMed]
- Aurrecoechea, C.; Barreto, A.; Basenko, E.Y.; Brestelli, J.; Brunk, B.P.; Cade, S.; Crouch, K.; Doherty, R.; Falke, D.; Fischer, S.; et al. EuPathDB: The Eukaryotic Pathogen Genomics Database Resource. Nucleic Acids Res. 2017, 45, D581–D591. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Mandlik, V.; Singh, S. LmSmdB: An Integrated Database for Metabolic and Gene Regulatory Network in Leishmania Major and Schistosoma Mansoni. Genom. Data 2016, 7, 115–118. [Google Scholar] [CrossRef]
- Dikhit, M.R.; Moharana, K.C.; Sahoo, B.R.; Sahoo, G.C.; Das, P. LeishMicrosatDB: Open Source Database of Repeat Sequences Detected in Six Fully Sequenced Leishmania Genomes. Database 2014, 2014, bau078. [Google Scholar] [CrossRef]
- Gazestani, V.H.; Yip, C.W.; Nikpour, N.; Berghuis, N.; Salavati, R. TrypsNetDB: An Integrated Framework for the Functional Characterization of Trypanosomatid Proteins. PLoS Negl. Trop. Dis. 2017, 11, e0005368. [Google Scholar] [CrossRef]
- Torres, F.; Arias-Carrasco, R.; Caris-Maldonado, J.C.; Barral, A.; Maracaja-Coutinho, V.; De Queiroz, A.T.L. LeishDB: A Database of Coding Gene Annotation and Non-Coding RNAs in Leishmania Braziliensis. Database 2017, 2017, bax047. [Google Scholar] [CrossRef]
- Waugh, B.; Ghosh, A.; Bhattacharyya, D.; Ghoshal, N.; Banerjee, R. In Silico Work Flow for Scaffold Hopping in Leishmania. BMC Res. Notes 2014, 7, 802. [Google Scholar] [CrossRef]
- Chavali, A.K.; Whittemore, J.D.; Eddy, J.A.; Williams, K.T.; Papin, J.A. Systems Analysis of Metabolism in the Pathogenic Trypanosomatid Leishmania Major. Mol. Syst. Biol. 2008, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Thomas, M.; Patterson, S.; Crouch, S.; De Rycker, M.; Lowe, R.; Gresham, S.; Urbaniak, M.D.; Otto, T.D.; Stojanovski, L.; et al. Cyclin-Dependent Kinase 12 Is a Drug Target for Visceral Leishmaniasis. Nature 2018, 560, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.K.C.; Teixeira, J.S.; Moreira, D.R.M.; da Silva, T.F.; de Lacerda Barreiro, E.J.; de Freitas, H.F.; da Rocha Pita, S.S.; Teles, A.L.B.; Guimarães, E.T.; Soares, M.B.P. In Vitro, In Vivo and In Silico Effectiveness of LASSBio-1386, an N-Acyl Hydrazone Derivative Phosphodiesterase-4 Inhibitor, Against Leishmania Amazonensis. Front. Pharmacol. 2020, 11, 590544. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Hira, S.K.; Upadhyaya, A.; Gupta, U.; Singh, R.; Paladhi, A.; Khan, F.I.; Rub, A.; Manna, P.P. In Silico Studies and Evaluation of Antiparasitic Role of a Novel Pyruvate Phosphate Dikinase Inhibitor in Leishmania Donovani Infected Macrophages. Int. J. Antimicrob. Agents 2019, 53, 508–514. [Google Scholar] [CrossRef]
- Kashif, M.; Paladhi, A.; Singh, R.; Bhattacharyya, S.; Hira, S.K.; Manna, P.P. Leishmanicidal Activity of an In Silico-Screened Novel Inhibitor against Ascorbate Peroxidase of Leishmania Donovani. Antimicrob. Agents Chemother. 2020, 64, e01766-19. [Google Scholar] [CrossRef]
- Pandey, R.K.; Kumbhar, B.V.; Sundar, S.; Kunwar, A.; Prajapati, V.K. Structure-Based Virtual Screening, Molecular Docking, ADMET and Molecular Simulations to Develop Benzoxaborole Analogs as Potential Inhibitor against Leishmania Donovani Trypanothione Reductase. J. Recept. Signal. Transduct. Res. 2017, 37, 60–70. [Google Scholar] [CrossRef]
- Ochoa, R.; Watowich, S.J.; Flórez, A.; Mesa, C.V.; Robledo, S.M.; Muskus, C. Drug Search for Leishmaniasis: A Virtual Screening Approach by Grid Computing. J. Comput. Aided Mol. Des. 2016, 30, 541–552. [Google Scholar] [CrossRef]
- van Montfort, R.L.M.; Workman, P. Structure-Based Drug Design: Aiming for a Perfect Fit. Essays Biochem. 2017, 61, 431–437. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Dikhit, M.R.; Sahoo, G.C.; Ali, V.; Das, P. Recent Advancement and Treatment of Leishmaniasis Based on Pharmacoinformatics Approach: Current and Future Outlook. Gene Rep. 2017, 9, 86–97. [Google Scholar] [CrossRef]
- Ong, H.B.; Sienkiewicz, N.; Wyllie, S.; Fairlamb, A.H. Dissecting the Metabolic Roles of Pteridine Reductase 1 in Trypanosoma Brucei and Leishmania Major*. J. Biol. Chem. 2011, 286, 10429–10438. [Google Scholar] [CrossRef]
- Casgrain, P.-A.; Martel, C.; McMaster, W.R.; Mottram, J.C.; Olivier, M.; Descoteaux, A. Cysteine Peptidase B Regulates Leishmania Mexicana Virulence through the Modulation of GP63 Expression. PLoS Pathogens 2016, 12, e1005658. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Ferro, S.; Buemi, M.R.; Monforte, A.-M.; Gitto, R.; Schirmeister, T.; Maes, L.; Rescifina, A.; Micale, N. Discovery of Benzimidazole-Based Leishmania Mexicana Cysteine Protease CPB2.8ΔCTE Inhibitors as Potential Therapeutics for Leishmaniasis. Chem. Biol. Drug. Des. 2018, 92, 1585–1596. [Google Scholar] [CrossRef]
- Marreiros, B.C.; Sena, F.V.; Sousa, F.M.; Oliveira, A.S.F.; Soares, C.M.; Batista, A.P.; Pereira, M.M. Structural and Functional Insights into the Catalytic Mechanism of the Type II NADH:Quinone Oxidoreductase Family. Sci. Rep. 2017, 7, 42303. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Perdih, A.; Senćanski, M.; Glišić, S.; Duarte, M.; Tomás, A.M.; Sena, F.V.; Sousa, F.M.; Pereira, M.M.; Solmajer, T. In Silico Discovery of a Substituted 6-Methoxy-Quinalidine with Leishmanicidal Activity in Leishmania Infantum. Molecules 2018, 23, 772. [Google Scholar] [CrossRef]
- Cordeiro, A.T.; Feliciano, P.R.; Pinheiro, M.P.; Nonato, M.C. Crystal Structure of Dihydroorotate Dehydrogenase from Leishmania Major. Biochimie 2012, 94, 1739–1748. [Google Scholar] [CrossRef]
- Mamidala, R.; Majumdar, P.; Jha, K.K.; Bathula, C.; Agarwal, R.; Chary, M.T.; Majumder, H.K.; Munshi, P.; Sen, S. Identification of Leishmania Donovani Topoisomerase 1 Inhibitors via Intuitive Scaffold Hopping and Bioisosteric Modification of Known Top 1 Inhibitors. Sci. Rep. 2016, 6, 26603. [Google Scholar] [CrossRef]
- Pommier, Y.; Sun, Y.; Huang, S.-Y.N.; Nitiss, J.L. Roles of Eukaryotic Topoisomerases in Transcription, Replication and Genomic Stability. Nat. Rev. Mol. Cell. Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- Fiorillo, A.; Colotti, G.; Boffi, A.; Baiocco, P.; Ilari, A. The Crystal Structures of the Tryparedoxin-Tryparedoxin Peroxidase Couple Unveil the Structural Determinants of Leishmania Detoxification Pathway. PLoS Negl. Trop. Dis. 2012, 6, e1781. [Google Scholar] [CrossRef]
- Chen, C.Y.-C. A Novel Integrated Framework and Improved Methodology of Computer-Aided Drug Design. Curr. Top. Med. Chem. 2013, 13, 965–988. [Google Scholar] [CrossRef] [PubMed]
- Yousefinejad, S.; Hemmateenejad, B. Chemometrics Tools in QSAR/QSPR Studies: A Historical Perspective. Chemom. Intell. Lab. Syst. 2015, 149, 177–204. [Google Scholar] [CrossRef]
- Kashif, M.; Tabrez, S.; Husein, A.; Arish, M.; Kalaiarasan, P.; Manna, P.P.; Subbarao, N.; Akhter, Y.; Rub, A. Identification of Novel Inhibitors against UDP-Galactopyranose Mutase to Combat Leishmaniasis. J. Cell. Biochem. 2018, 119, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Manna, P.; Akhter, Y.; Alaidarous, M.; Rub, A. The Screening of Novel Inhibitors against Leishmania Donovani Calcium Ion Channel to Fight Leishmaniasis. Infect. Disord. Drug. Targets (Former. Curr. Drug. Targets Infect. Disord.) 2016, 16, 120–129. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, D.; Hamilton, N. Gluconeogenesis in Leishmania Mexicana: Contribution of Glycerol Kinase, Phosphoenolpyruvate Carboxykinase, and Pyruvate Phosphate Dikinase. J. Biol. Chem. 2014, 289, 32989–33000. [Google Scholar] [CrossRef] [PubMed]
- Palayam, M.; Lakshminarayanan, K.; Radhakrishnan, M.; Krishnaswamy, G. Preliminary Analysis to Target Pyruvate Phosphate Dikinase from Wolbachia Endosymbiont of Brugia Malayi for Designing Anti-Filarial Agents. Interdiscip. Sci. Comput. Life Sci. 2012, 4, 74–82. [Google Scholar] [CrossRef]
- Amaro, R.E.; Baron, R.; McCammon, J.A. An Improved Relaxed Complex Scheme for Receptor Flexibility in Computer-Aided Drug Design. J. Comput. Aided Mol. Des. 2008, 22, 693–705. [Google Scholar] [CrossRef]
- Wu, C.; Dunaway-Mariano, D.; Mariano, P.S. Design, Synthesis, and Evaluation of Inhibitors of Pyruvate Phosphate Dikinase. Available online: https://pubs.acs.org/doi/pdf/10.1021/jo3018473 (accessed on 10 November 2022).
- Beverley, S.M.; Owens, K.L.; Showalter, M.; Griffith, C.L.; Doering, T.L.; Jones, V.C.; McNeil, M.R. Eukaryotic UDP-Galactopyranose Mutase (GLF Gene) in Microbial and Metazoal Pathogens. Eukaryotic Cell. 2005, 4, 1147–1154. [Google Scholar] [CrossRef]
- Oppenheimer, M.; Valenciano, A.L.; Sobrado, P. Isolation and Characterization of Functional Leishmania Major Virulence Factor UDP-Galactopyranose Mutase. Biochem. Biophys. Res. Commun. 2011, 407, 552–556. [Google Scholar] [CrossRef]
- Kumar, A.; Das, S.; Purkait, B.; Sardar, A.H.; Ghosh, A.K.; Dikhit, M.R.; Abhishek, K.; Das, P. Ascorbate Peroxidase, a Key Molecule Regulating Amphotericin B Resistance in Clinical Isolates of Leishmania Donovani. Antimicrob. Agents Chemother. 2014, 58, 6172–6184. [Google Scholar] [CrossRef]
- Dolai, S.; Yadav, R.K.; Pal, S.; Adak, S. Overexpression of Mitochondrial Leishmania Major Ascorbate Peroxidase Enhances Tolerance to Oxidative Stress-Induced Programmed Cell Death and Protein Damage. Eukaryotic Cell. 2009, 8, 1721–1731. [Google Scholar] [CrossRef]
- Pal, S.; Dolai, S.; Yadav, R.K.; Adak, S. Ascorbate Peroxidase from Leishmania Major Controls the Virulence of Infective Stage of Promastigotes by Regulating Oxidative Stress. PLoS ONE 2010, 5, e11271. [Google Scholar] [CrossRef] [PubMed]
- Sardar, A.H.; Kumar, S.; Kumar, A.; Purkait, B.; Das, S.; Sen, A.; Kumar, M.; Sinha, K.K.; Singh, D.; Equbal, A.; et al. Proteome Changes Associated with Leishmania Donovani Promastigote Adaptation to Oxidative and Nitrosative Stresses. J. Proteom. 2013, 81, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-F.; Bulfer, S.L.; Weihl, C.C.; Li, K.; Lis, L.G.; Walters, M.A.; Schoenen, F.J.; Lin, H.J.; Deshaies, R.J.; Arkin, M.R. Specific Inhibition of P97/VCP ATPase and Kinetic Analysis Demonstrate Interaction between D1 and D2 ATPase Domains. J. Mol. Biol. 2014, 426, 2886–2899. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-F.; Li, K.; Frankowski, K.J.; Schoenen, F.J.; Deshaies, R.J. Structure–Activity Relationship Study Reveals ML240 and ML241 as Potent and Selective Inhibitors of P97 ATPase. ChemMedChem 2013, 8, 297–312. [Google Scholar] [CrossRef]
- Chou, T.-F.; Brown, S.J.; Minond, D.; Nordin, B.E.; Li, K.; Jones, A.C.; Chase, P.; Porubsky, P.R.; Stoltz, B.M.; Schoenen, F.J.; et al. Reversible Inhibitor of P97, DBeQ, Impairs Both Ubiquitin-Dependent and Autophagic Protein Clearance Pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4834–4839. [Google Scholar] [CrossRef]
- Guedes Aguiar, B.; Padmanabhan, P.K.; Dumas, C.; Papadopoulou, B. Valosin-Containing Protein VCP/P97 Is Essential for the Intracellular Development of Leishmania and Its Survival under Heat Stress. Cell. Microbiol. 2018, 20, e12867. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Naskar, K.; Sarkar, D.; Ghosh, D.K. Role of Ca2+ Ion on Leishmania-Macrophage Attachment. Mol. Cell. Biochem. 1991, 102, 13–18. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and Cell Death Mechanisms: A Perspective from the Cell Death Community. Cell. Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Batool, M.; Ahmad, B.; Choi, S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019, 20, 2783. [Google Scholar] [CrossRef]
- Raj, S.; Sasidharan, S.; Balaji, S.N.; Saudagar, P. An Overview of Biochemically Characterized Drug Targets in Metabolic Pathways of Leishmania Parasite. Parasitol. Res. 2020, 119, 2025–2037. [Google Scholar] [CrossRef]
- Dykhuizen, E.C.; May, J.F.; Tongpenyai, A.; Kiessling, L.L. Inhibitors of UDP-Galactopyranose Mutase Thwart Mycobacterial Growth. J. Am. Chem. Soc. 2008, 130, 6706–6707. [Google Scholar] [CrossRef] [PubMed]
- Kizjakina, K.; Tanner, J.J.; Sobrado, P. Targeting UDP-Galactopyranose Mutases from Eukaryotic Human Pathogens. Curr. Pharm. Des. 2013, 19, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.L.; Turco, S.J. Galactofuranose Metabolism: A Potential Target for Antimicrobial Chemotherapy. CMLS Cell. Mol. Life Sci. 2003, 60, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Damveld, R.A.; Franken, A.; Arentshorst, M.; Punt, P.J.; Klis, F.M.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. A Novel Screening Method for Cell Wall Mutants in Aspergillus Niger Identifies UDP-Galactopyranose Mutase as an Important Protein in Fungal Cell Wall Biosynthesis. Genetics 2008, 178, 873–881. [Google Scholar] [CrossRef]
- Schmalhorst, P.S.; Krappmann, S.; Vervecken, W.; Rohde, M.; Müller, M.; Braus, G.H.; Contreras, R.; Braun, A.; Bakker, H.; Routier, F.H. Contribution of Galactofuranose to the Virulence of the Opportunistic Pathogen Aspergillus Fumigatus. Eukaryotic Cell. 2008, 7, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Kleczka, B.; Lamerz, A.-C.; van Zandbergen, G.; Wenzel, A.; Gerardy-Schahn, R.; Wiese, M.; Routier, F.H. Targeted Gene Deletion of Leishmania Major UDP-Galactopyranose Mutase Leads to Attenuated Virulence. J. Biol. Chem. 2007, 282, 10498–10505. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Valicherla, G.R.; Shahab, M.; Gupta, J.; Gayen, J.R.; Misra-Bhattacharya, S. UDP-Galactopyranose Mutase, a Potential Drug Target against Human Pathogenic Nematode Brugia Malayi. Pathog. Dis. 2016, 74, ftw072. [Google Scholar] [CrossRef]
- de Souza Moreira, D.; Xavier, M.V.; Murta, S.M.F. Ascorbate Peroxidase Overexpression Protects Leishmania Braziliensis against Trivalent Antimony Effects. Mem. Inst. Oswaldo Cruz 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Singh, K.; Ali, V.; Pratap Singh, K.; Gupta, P.; Suman, S.S.; Ghosh, A.K.; Bimal, S.; Pandey, K.; Das, P. Deciphering the Interplay between Cysteine Synthase and Thiol Cascade Proteins in Modulating Amphotericin B Resistance and Survival of Leishmania Donovani under Oxidative Stress. Redox Biol. 2017, 12, 350–366. [Google Scholar] [CrossRef]
- Das, S.; Aich, A.; Shaha, C. The Complex World of Cellular Defense in the Leishmania Parasite. Proc. Indian. Natl. Sci. Acad. 2015, 81, 629–641. [Google Scholar] [CrossRef]
- Adak, S.; Datta, A.K. Leishmania Major Encodes an Unusual Peroxidase That Is a Close Homologue of Plant Ascorbate Peroxidase: A Novel Role of the Transmembrane Domain. Biochem. J. 2005, 390, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Dolai, S.; Yadav, R.K.; Pal, S.; Adak, S. Leishmania Major Ascorbate Peroxidase Overexpression Protects Cells against Reactive Oxygen Species-Mediated Cardiolipin Oxidation. Free. Radic. Biol. Med. 2008, 45, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Seguin, S.J.; Morelli, F.F.; Vinet, J.; Amore, D.; De Biasi, S.; Poletti, A.; Rubinsztein, D.C.; Carra, S. Inhibition of Autophagy, Lysosome and VCP Function Impairs Stress Granule Assembly. Cell. Death Differ. 2014, 21, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, R.; Kumar, A.; Rana, S.; Panthi, B.; Ansari, M.Y.; Das, S.; Dikhit, M.R.; Sahoo, G.C.; Das, P. In Vitro Evaluation of Antileishmanial Activity of Computationally Screened Compounds against Ascorbate Peroxidase To Combat Amphotericin B Drug Resistance. Antimicrob. Agents Chemother. 2017, 61, e02429-16. [Google Scholar] [CrossRef]
- Saavedra-Lira, E.; Pérez-Montfort, R. Energy Production in Entamoeba Histolytica: New Perspectives in Rational Drug Design. Arch. Med. Res. 1996, 27, 257–264. [Google Scholar]
- de Carvalho Gallo, J.C.; de Mattos Oliveira, L.; Araújo, J.S.C.; Santana, I.B.; dos Santos Junior, M.C. Virtual Screening to Identify Leishmania Braziliensis N-Myristoyltransferase Inhibitors: Pharmacophore Models, Docking, and Molecular Dynamics. J. Mol. Model. 2018, 24, 260. [Google Scholar] [CrossRef]
- Gundampati, R.K.; Sahu, S.; Shukla, A.; Pandey, R.K.; Patel, M.; Banik, R.M.; Jagannadham, M.V. Tryparedoxin Peroxidase of Leishmania Braziliensis: Homology Modeling and Inhibitory Effects of Flavonoids for Anti-Leishmanial Activity. Bioinformation 2014, 10, 353–357. [Google Scholar] [CrossRef]
- Venkatesan, S.K.; Saudagar, P.; Shukla, A.K.; Dubey, V.K. Screening Natural Products Database for Identification of Potential Antileishmanial Chemotherapeutic Agents. Interdiscip. Sci. 2011, 3, 217–231. [Google Scholar] [CrossRef]
- Maamri, S.; Benarous, K.; Yousfi, M. Identification of 3-Methoxycarpachromene and Masticadienonic Acid as New Target Inhibitors against Trypanothione Reductase from Leishmania Infantum Using Molecular Docking and ADMET Prediction. Molecules 2021, 26, 3335. [Google Scholar] [CrossRef]
- Inacio, J.D.F.; Fonseca, M.S.; Limaverde-Sousa, G.; Tomas, A.M.; Castro, H.; Almeida-Amaral, E.E. Epigallocathechin-O-3-Gallate Inhibits Trypanothione Reductase of Leishmania Infantum, Causing Alterations in Redox Balance and Leading to Parasite Death. Front. Cell. Infect. Microbiol. 2021, 11, 640561. [Google Scholar] [CrossRef]
- Amiri-Dashatan, N.; Rezaei-Tavirani, M.; Ranjbar, M.M.; Koushki, M.; Mousavi Nasab, S.D.; Ahmadi, N. Discovery of Novel Pyruvate Kinase Inhibitors Against Leishmania Major Among FDA Approved Drugs Through System Biology and Molecular Docking Approach. Turk. J. Pharm. Sci. 2021, 18, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]

| Pathway | Drug Target | Drug Candidate | Mode of Action | Refs. |
|---|---|---|---|---|
| Sterol Biosynthesis Pathway | Squalene epoxidase | Spiro[indole-3,3′-pyrrolizidine]-2-one | DNA topoisomerase IB inhibitor. | [67,68] |
| HMGR enzyme | Mevastatin | Hampers HMGR activity. | [69,70] | |
| Sterol alpha-14 demethylase | Avodart | Induces ROS and causes apoptosis in the parasite. | [71] | |
| HMGR enzyme | Glycyrrhizic acid | Inhibits HMGR enzyme. | [72] | |
| Purine Salvage Pathway | mRNA translation | 5-fluorouracil 4-thiouracil | Binds to RNA and blocks cell growth. | [73,74] |
| Glycolytic Pathway | GAPDH | Artesunate | Inhibits the parasites’ glycolytic enzymes GPDH. | [75,76] |
| Quinine | [75] | |||
| Mefloquine | [75] | |||
| Folate Biosynthesis Pathway | DHFR | Methotrexate (MTX, 1) | Inhibits DHFR. | [77] |
| Cycloguanil | [77] | |||
| Trimethoprim (TMP, 2) | [77,78] | |||
| ZINC57774418 (Z18) | Inhibits DHFR activity. | [79] | ||
| ZINC69844431 (Z31) | [79] | |||
| ZINC71746025 (Z25) | [79] | |||
| D11596 (DB96) | [79] | |||
| 3,4-dihydropyrimidine-2-one | [80] | |||
| 5-(3,5-dimethoxybenzyl) pyrimidine-2,4-diamine | [80] | |||
| DHFR and PTR1 | 2-(4-((2,4-dichlorobenzyl)oxy)phenyl)-1H-benzo[d]imidazole | DHFR-TS/PTR1 inhibitors. | [81] | |
| 2-(4-((2,4-dichlorobenzyl)oxy)phenyl)-1H-benzo[d]imidazole-1H-benzo[d]oxazole | [81] | |||
| Trypanothione Pathway | TR | Trichloro [1,2-ethanediolato-O,O’]-tellurate (AS101) | Induces ROS-mediated apoptosis by binding to TR cysteine residues. | [82] |
| β-sitosterol CCL | Inhibit TR activity. | [83] | ||
| Hypusine Pathway | Spermidine synthase | Hypericin | ROS and spermidine reduction. | [84,85] |
| S. No | Resources | Descriptions | Weblink | Ref. |
|---|---|---|---|---|
| 1. | TriTrypDB | For Leishmania and Trypanosoma, an integrated genomic and functional genomic resource is available. | http://tritrypdb.org (accessed on 8 December 2022) | [166] |
| 2. | LeishCyc | L. major biochemical pathway database. | http://biocyc.org/LEISH/organism-summary?object¼LEISH (accessed on 7 December 2022) | [167] |
| 3. | L. amazonensis genome DB | The genome of L. amazonensis has been sequenced and annotated. | http://bioinfo08.ibi.unicamp.br/leishmania (accessed on 6 December 2022) | [168] |
| 4. | GeneDB (Kinetoplastid Protozoa section) | Annotations and sequences of 5 Leishmania species were curated. | http://www.genedb.or (accessed on 8 December 2022) | [169] |
| 5. | EuPathDB | For eukaryotic pathogens, there is a pathogen genomics resource. | http://eupathdb.org (accessed on 9 December 2022) | [170] |
| 6. | LmSmdB | Regulatory pathways and biological networks of L. major. | http://www.nccs.res.in/LmSmdb (accessed on 9 December 2022) | [171] |
| 7. | LeishMicrosatDB | Repeat sequences from six Leishmania species are included in a database. | http://biomedinformri.com/leishmicrosat (accessed on 11 December 2022) | [172] |
| 8. | TrypsNetDB | Protein interactions and annotations for trypanosomatid parasites that have been experimentally verified as well as predicted. | http://trypsNetDB.org (accessed on 5 December 2022) | [173] |
| 9. | LeishDB | Noncoding RNAs and coding gene reannotation in L. braziliensis. | http://www.leishdb.com (accessed on 7 December 2022) | [174] |
| 10. | List of putative anti-leishmanials | Lead compounds and drug targets with predicted antileishmanial activity. | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4247209/(accessed on 6 December 2022) | [175] |
| 11. | L. major metabolic network | Genome-scale metabolic network of Leishmania major (iAC560). | https://www.ebi.ac.uk/biomodels/MODEL1507180059 (accessed on 10 December 2022) | [176] |
| Drug | Structure | Pathway | Target Protein | Mode of Action | Ref. |
|---|---|---|---|---|---|
| Z220582104 |  | Glucose synthesis and alanine influx | Pyruvate phosphate dikinase (PPDK) | Inhibits the pyruvate phosphate dikinase enzyme that helps in
| [179] |
| CID 6064500 | 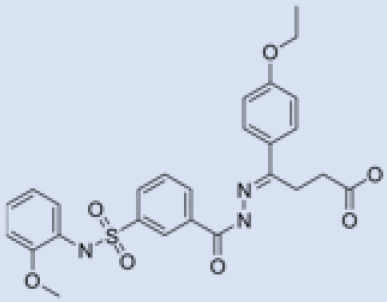 | Β-Galf synthesis Role in pathogenesis | UDP-galactopyranose mutase (UGM) | Inhibits the UDP-galactopyranose mutase (UGM) enzyme
| [197] |
| ZINC96021026 | 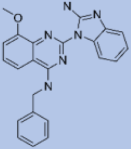 | Tryparedoxin | Ascorbate peroxidase (APX) | Inhibits the ascorbate peroxidase enzyme activity
| [180] |
| ZINC29590262 |  | Ca2+ related pathways | Calcium channel | Inhibits calcium channel that hampers flagellar motion
| [198] |
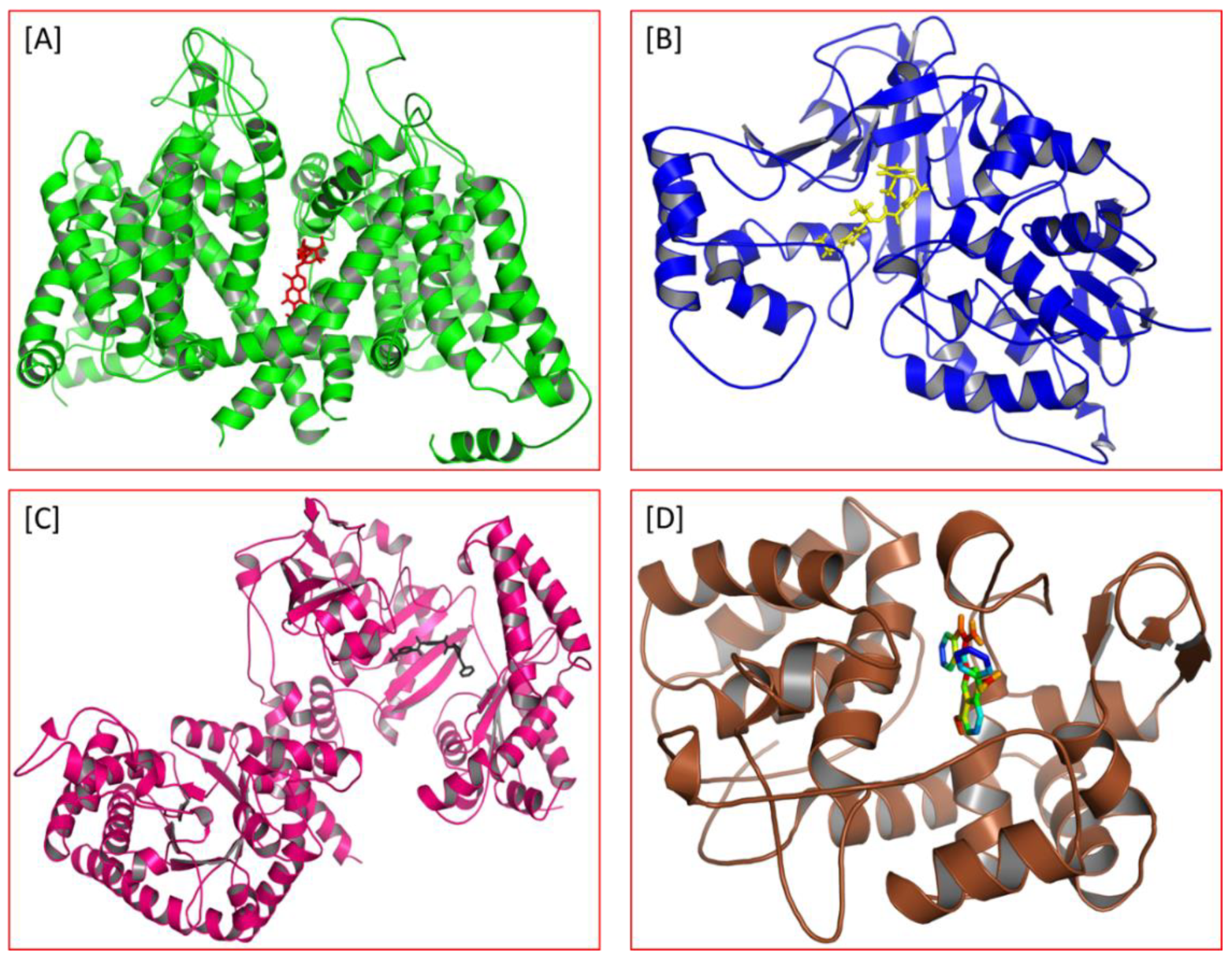
| S. No | Leishmania Spp. | Target Proteins | Structure of the Protein | Compound | Ref. |
|---|---|---|---|---|---|
| 1. | Leishmania major | N-myristoyl transferase (PDBID: 5A27) | 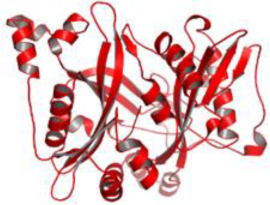 | ZINC35426134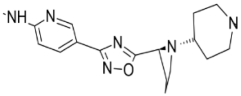 | [232] |
| 2. | Leishmania major | Tryparedoxin peroxidase (PDB ID: 3TUE) | 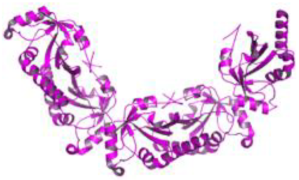 | Taxifolin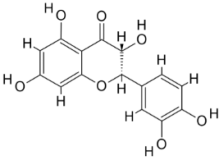 | [233] |
Quercetin | |||||
| 3. | Leishmania infantum | Trypanothione reductase (PDB ID: 2JK6) | 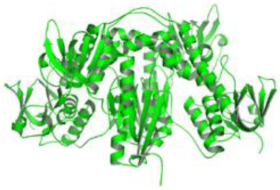 | Beta-Amyrin Acetate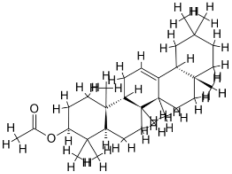 | [234] |
Ginkgetin | |||||
Fucostanol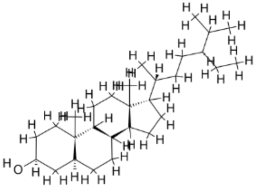 | |||||
Lunarine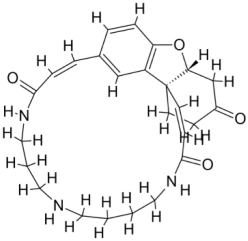 | |||||
| 4. | Leishmania infantum | Trypanothione reductase (PDB ID: 5EBK) | 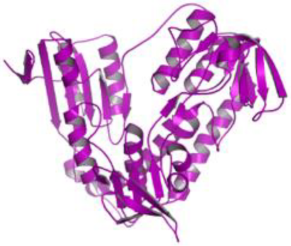 | Masticadienonic acid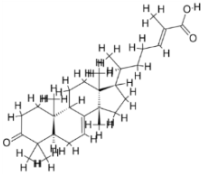 | [235] |
| 5. | Leishmania infantum | Trypanothione reductase (PDB ID: 2JK6) |  | Epigallocatechin Gallate  (EGCG) | [236] |
| 6. | Leishmania mexicana | Pyruvate kinase (PDB ID: 3PP7) |  | Irinotecan | [237] |
 | |||||
| Coniveptan | |||||
 | |||||
| Valstar | |||||
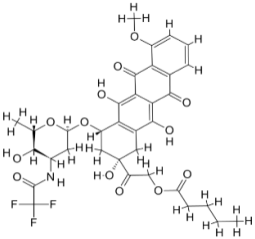 | |||||
| Nilotinib | |||||
 | |||||
| Netupitant | |||||
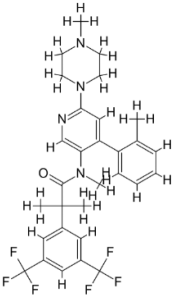 | |||||
| Lomitapide | |||||
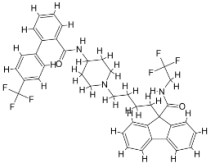 | |||||
| Trametinib | |||||
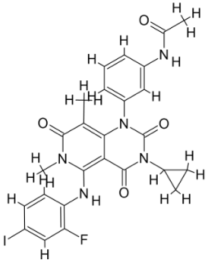 | |||||
| Naldemedine | |||||
 | |||||
| Vumon | |||||
 | |||||
| Eltrombopag | |||||
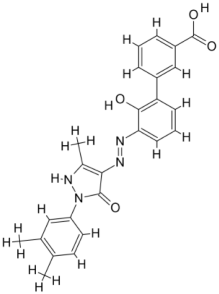 | |||||
| 7. | Leishmania mexicana | Glucose-6-phosphate isomerase (PDB ID: 1T10) | 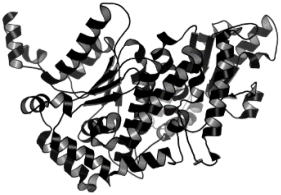 | Artesunate | [75] |
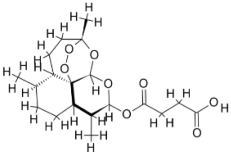 | |||||
| Quinine | |||||
 | |||||
| Leishmania mexicana | Triosephosphate isomerase (PDB ID: 2Y63) |  | Mefloquine | ||
| Leishmania mexicana | Glycerol-3-phosphate dehydrogenase (PDB ID: 1M67) | 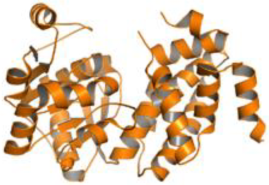 | |||
| Leishmania mexicana | Glyceraldehyde-3-phosphate dehydrogenase (PDB ID: 1I33) | 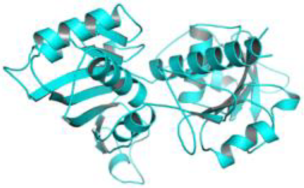 |  | ||
| Leishmania mexicana | Pyruvate kinase (PDB ID: 3PP7) | 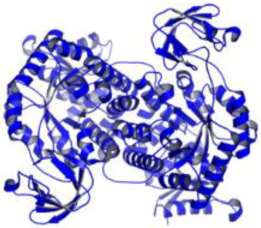 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Kashif, M.; Srivastava, P.; Manna, P.P. Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host. Pathogens 2023, 12, 706. https://doi.org/10.3390/pathogens12050706
Singh R, Kashif M, Srivastava P, Manna PP. Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host. Pathogens. 2023; 12(5):706. https://doi.org/10.3390/pathogens12050706
Chicago/Turabian StyleSingh, Ranjeet, Mohammad Kashif, Prateek Srivastava, and Partha Pratim Manna. 2023. "Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host" Pathogens 12, no. 5: 706. https://doi.org/10.3390/pathogens12050706
APA StyleSingh, R., Kashif, M., Srivastava, P., & Manna, P. P. (2023). Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host. Pathogens, 12(5), 706. https://doi.org/10.3390/pathogens12050706







