The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H. COVID-19 and Antimicrobial Resistance: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance-WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016; p. 20. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 18 August 2021).
- WHO. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 16 April 2023).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016–2020; ECDC: Stockholm, Sweden, 2022.
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Constantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R. Key considerations on the potential impacts of the COVID-19 pandemic on antimicrobial resistance research and surveillance. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1122–1129. [Google Scholar] [CrossRef]
- CLS Clinical and Laboratory Standards Institute. Available online: https://clsi.org/standards/products/microbiology/ (accessed on 24 April 2023).
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ (accessed on 24 April 2023).
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Sridhar, D. The pandemic legacy of antimicrobial resistance in the USA. Lancet Microb. 2022, 3, e726–e727. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. World Organisation for Animal Health. WHO Global Database for the Tripartite Antimicrobial Resistance (AMR) Country Self-assessment Survey (TrACSS). Available online: https://amrcountryprogress.org/#/response-overview (accessed on 16 April 2023).
- Center for Disease Control and Prevention. COVID-19: US Impact on Antimicrobial Resistance, Special Report 2022; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022.
- Liew, Y.; Lee, W.H.L.; Tan, L.; Kwa, A.L.H.; Thien, S.Y.; Cherng, B.P.Z.; Chung, S.J. Antimicrobial stewardship programme: A vital resource for hospitals during the global outbreak of coronavirus disease 2019 (COVID-19). Int. J. Antimicrob. Agents 2020, 56, 106145. [Google Scholar] [CrossRef]
- Molla, M.M.A.; Yeasmin, M.; Islam, M.K.; Sharif, M.M.; Amin, M.R.; Nafisa, T.; Ghosh, A.K.; Parveen, M.; Arif, M.M.H.; Alam, J.A.J.; et al. Antibiotic prescribing patterns at COVID-19 dedicated wards in Bangladesh: Findings from a single center study. Infect. Prev. Pract. 2021, 3, 100134. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Castaldi, S.; Luconi, E.; Marano, G.; Auxilia, F.; Maraschini, A.; Bono, P.; Ungaro, R.; Bandera, A.; Boracchi, P.; Biganzoli, E. Hospital acquired infections in COVID-19 patients in sub intensive care unit. Acta Biomed. 2020, 91, e2020017. [Google Scholar] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Sharifipour, E.; Shams, S.; Esmkhani, M.; Khodadadi, J.; Fotouhi-Ardakani, R.; Koohpaei, A.; Doosti, Z.; EJ Golzari, S. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 2020, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Vilbrun, S.C.; Mathurin, L.; Pape, J.W.; Fitzgerald, D.; Walsh, K.F. Case report: Multidrug-resistant tuberculosis and COVID-19 coinfection in Port-au-Prince, Haiti. Am. J. Trop. Med. Hyg. 2020, 103, 1986–1988. [Google Scholar] [CrossRef]
- Yousaf, Z.; Khan, A.A.; Chaudhary, H.A.; Mushtaq, K.; Parengal, J.; Aboukamar, M.; Khan, M.U.; Mohamed, M.F.H. Cavitary pulmonary tuberculosis with COVID-19 coinfection. IDCases 2020, 22, e00973. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Ventilator-associated pneumonia (VAP) caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19: Two problems, one solution? Med. Hypotheses 2020, 144, 110139. [Google Scholar] [CrossRef]
- Yock-Corrales, A.; Lenzi, J.; Ulloa-Gutiérrez, R.; Gómez-Vargas, J.; Yassef, A.-M.O.; Rios Aida, J.A.; del Aguila, O.; Arteaga-Menchaca, E.; Campos, F.; Uribe, F.; et al. Antibiotic prescriptions in children with COVID-19 and multisystem inflammatory syndrome: A multinational experience in 990 cases from Latin America. Acta Paediatr. 2021, 110, 1902–1910. [Google Scholar] [CrossRef]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Flandre, P.L.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Polemis, M.; Tryfinopoulou, K.; Giakkoupi, P.; Vatopoulos, A.; WHONET-Greece Study Group. Eight-year trends in the relative isolation frequency and antimicrobial susceptibility among bloodstream isolates from Greek hospitals: Data from the Greek electronic system for the surveillance of antimicrobial resistance—WHONET Greece, 2010 to 2017. Eurosurveillance 2020, 25, 1900516. [Google Scholar] [CrossRef]
- Mędrzycka-Dąbrowska, W.; Lange, S.; Zorena, K.; Dąbrowski, S.; Ozga, D.; Tomaszek, L. Carbapenem-resistant Klebsiella pneumoniae infections in ICU COVID-19 patients—A scoping review. J. Clin. Med. 2021, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Teich, V.D.; Klajner, S.; Santiago de Almeida, F.A.; Batista Dantas, A.C.; Laselva, C.R.; Torritesi, M.G.; Canero, T.R.; Berwanger, O.; Rizzo, L.V.; Reis, E.P. Epidemiologic and clinical features of patients with COVID-19 in Brazil. Einstein 2020, 18, eAO6022. [Google Scholar] [CrossRef]
- Gonzalez-Zorn, B. Antibiotic use in the COVID-19 crisis in Spain. Clin. Microbiol. Infect. 2021, 27, 646–647. [Google Scholar] [CrossRef]
- Barrasa, H.; Rello, J.; Tejada, S.; Martin, A.; Balziskueta, G.; Vinuesa, C.; Fernandez-Miret, B.; Villagra, A.; Vallejo, A.; San Sebastian, A.; et al. SARS-CoV-2 in Spanish intensive care units: Early experience with 15-day survival in Vitoria. Anaesth. Crit. Care Pain Med. 2020, 39, 553–561. [Google Scholar] [CrossRef]
- Saunderson, R.B.; Gouliouris, T.; Nickerson, E.K.; Cartwright, E.J.; Kidney, A.; Aliyu, S.H.; Brown, N.M.; Limmathurotsakul, D.; Peacock, S.J.; Török, M.E. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteraemia in adults. Clin. Microbiol. Infect. 2015, 21, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Forsblom, E.; Ruotsalainen, E.; Ollgren, J.; Jarvinen, A. Telephone Consultation Cannot Replace Bedside Infectious Disease Consultation in the Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2013, 56, 527–535. [Google Scholar] [CrossRef] [PubMed]



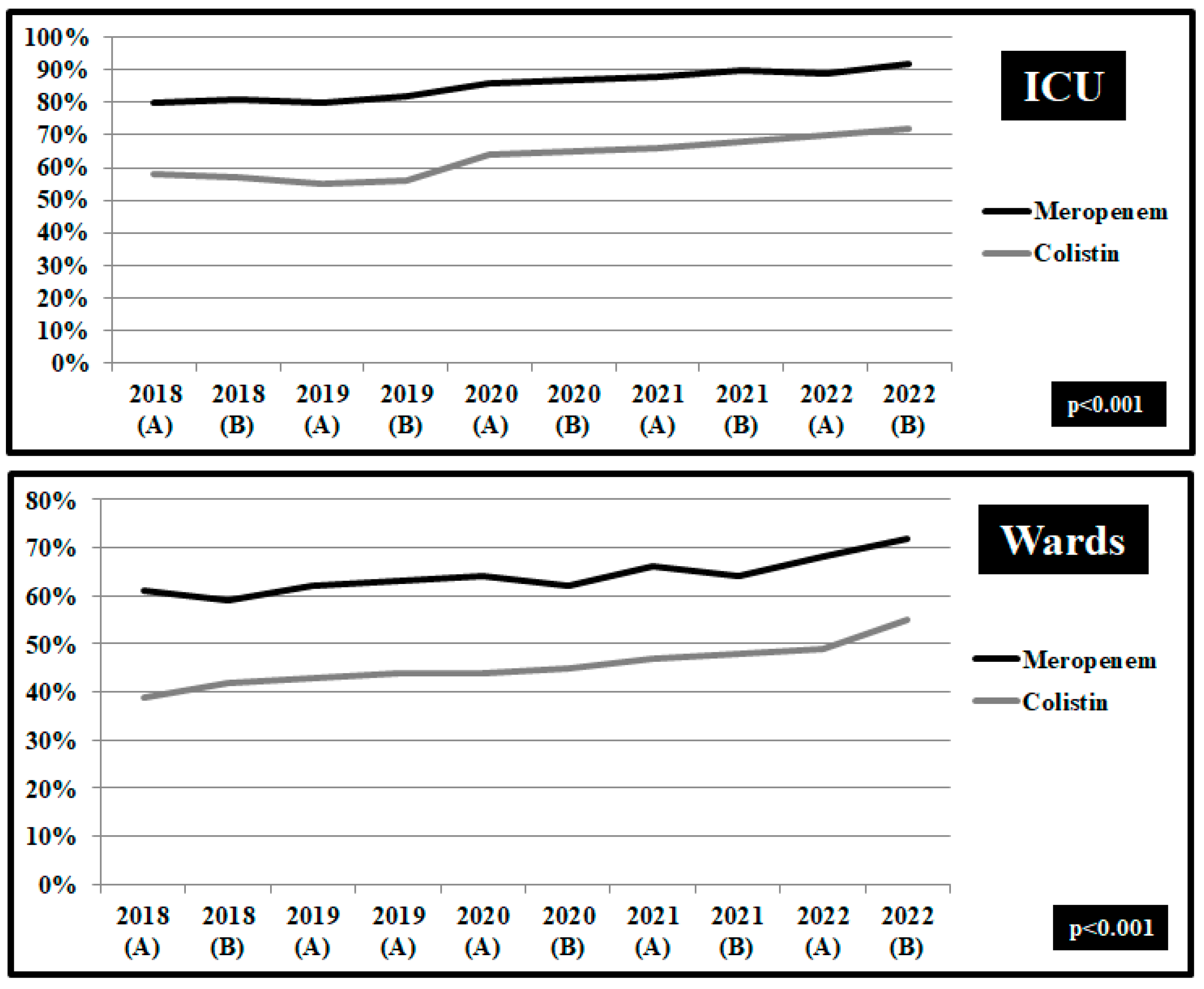


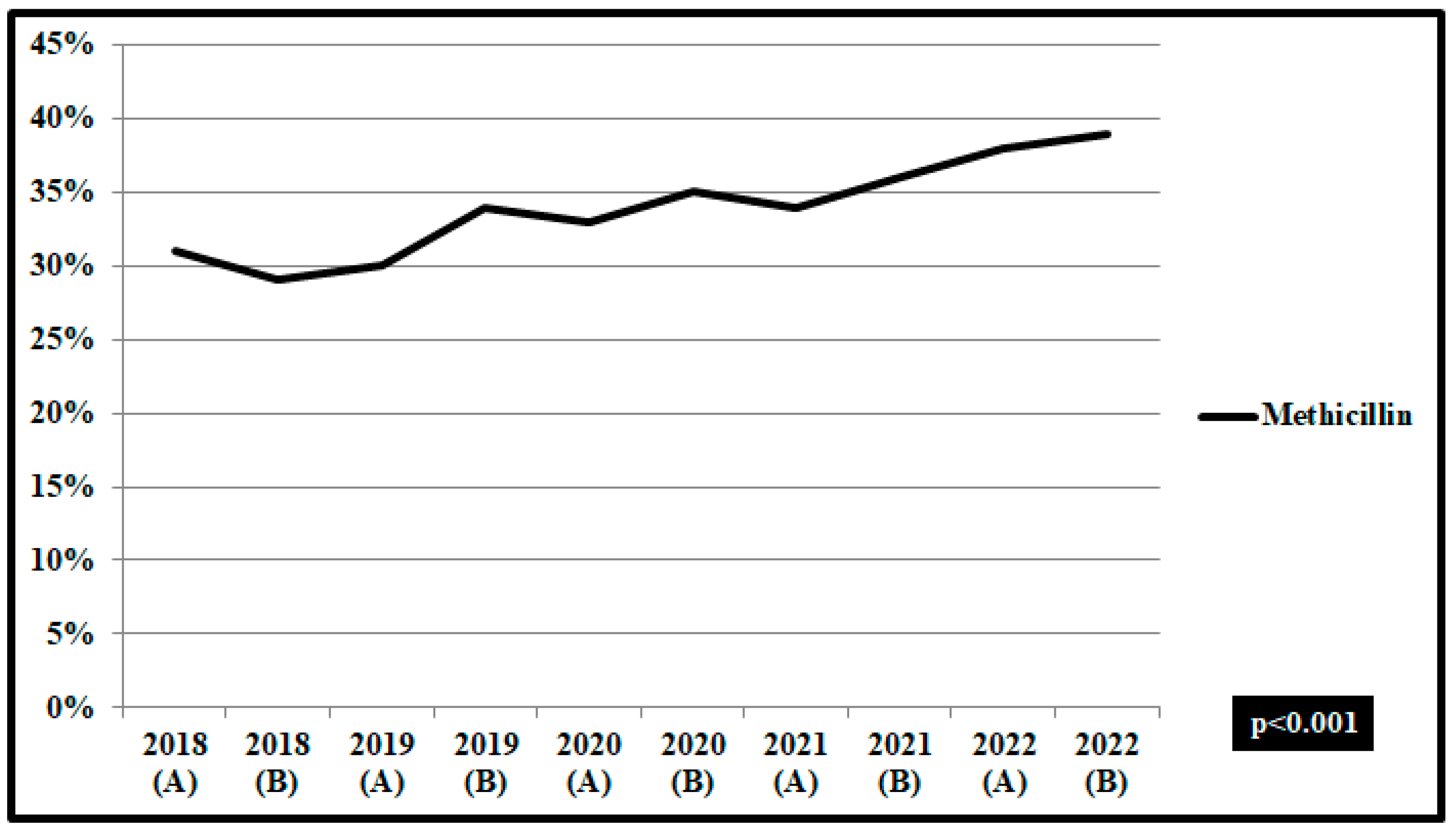
| Risk of Death, OR (95% CI) | Risk of Prolonged Hospital Stay, OR (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2022 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Acinetobacter baumannii Carvapenem resistant | 3.6 (2.1–4.5) n = 145 | 3.5 (2.4–5.1) n = 136 | 3.9 (3.1–6.1) n = 125 | 4.1 (3.4–6.1) n = 162 | 4.2 (3.5–5.2) n = 174 | 7.6 (5.3–9.5) n = 145 | 7.8 (6.2–9.2) n = 136 | 7.9 (7.2–9.4) n = 125 | 7.8 (6.9–9.2) n = 162 | 8.2 (7.6–10.1) n = 174 |
| Acinetobacter baumannii Non Carvapenem resistant | 2.1 (1.4–2.8) n = 44 | 2.2 (1.5–2.7) n = 38 | 2.4 (1.8–3.1) n = 33 | 2.3 (1.7–2.9) n = 20 | 2.4 (1.6–2.9) n = 26 | 4.3 (3.9–5.4) n = 44 | 4.5 (3.8–5.9) n = 38 | 4.3 (3.7–5.4) n = 33 | 4.4 (3.9–5.8) n = 20 | 4.5 (3.7–5.3) n = 26 |
| Klebsiella pneumoniae Carvapenem resistant | 3.1 (2.4–5.1) n = 98 | 3.3 (2.2–5.1) n = 109 | 3.6 (2.8–5.7) n = 106 | 3.5 (2.9–4.3) n = 110 | 3.8 (2.6–4.1) n = 126 | 6.5 (5.4–7.9) n = 98 | 6.7 (5.6–8.4) n = 109 | 6.7 (6.2–8.6) n = 106 | 6.9 (5.9–7.8) n = 110 | 7.1 (6.5–8.9) n = 126 |
| Klebsiella pneumoniae Non Carvapenem resistant | 2.2 (1.4–2.8) n = 46 | 1.9 (1.3–2.7) n = 38 | 2.1 (1.6–2.9) n = 40 | 2.2 (1.5–3.1) n = 36 | 2.3 (1.6–2.8) n = 30 | 3.1 (2.7–4.2) n = 46 | 3.3 (2.7–4.5) n = 38 | 3.4 (2.8–4.7) n = 40 | 3.5 (2.7–4.9) n = 36 | 3.5 (2.8–4.8) n = 30 |
| Pseudomonas aeruginosa MDR, Multidrug resistant | 3.1 (2.4–4.7) n = 87 | 3.4 (2.4–4.9) n = 121 | 3.8 (2.4–5.9) n = 101 | 3.7 (2.9– 2.8) n = 131 | 3.9 (3.1–4.3) n = 129 | 5.6 (4.6–7.3) n = 87 | 5.8 (4.5–7.9) n = 121 | 5.9 (4.5–7.2) n = 101 | 6.4 (5.9–7.3) n = 131 | 6.5 (4.9–8.2) n = 129 |
| Pseudomonas aeruginosa Non MDR, Non Multidrug resistant | 1.9 (1.3–3.7) n = 31 | 2.0 (1.4–3.8) n = 30 | 2.1 (1.5–3.7) n = 35 | 2.1 (1.6–3.9) n = 43 | 2.2 (1.7–3.8) n = 40 | 3.2 (2.8–4.3) n = 31 | 3.3 (2.7–4.9) n = 30 | 2.9 (2.6–4.5) n = 35 | 3.0 (2.4–4.2) n = 43 | 3.1 (2.6–4.9) n = 40 |
| Enterococcus faecium VRE, Vancomycin resistant | 1.4 (0.9–1.8) n = 8 | 1.6 (1.1–3.6) n = 12 | 1.6 (1.2–3.4) n = 15 | 1.9 (1.1– 2.2) n = 17 | 2.1 (1.7–2.9) n = 18 | 3.9 (3.1–5.3) n = 8 | 3.8 (3.1–5.7) n = 12 | 4.2 (3.6–6.3) n = 15 | 4.6 (4.5–7.6) n = 17 | 4.8 (4.1–7.3) n = 18 |
| Enterococcus faecium Non VRE, Non Vancomycin resistant | 1.1 (0.7–1.7) n = 27 | 1.2 (0.7–2.1) n = 29 | 1.3 (0.8–2.4) n = 32 | 1.4 (0.9–2.6) n = 30 | 1.4 (0.9–2.5) n = 36 | 2.5 (1.9–3.8) n = 27 | 2.6 (2.1–3.9) n = 29 | 2.5 (1.8–3.7) n = 32 | 2.7 (2.0–3.9) n = 30 | 2.6 (1.9–3.7) n = 36 |
| Staphylococcus aureus MRSA, Methicillin resistant | 3.0 (2.1–4.9) n = 48 | 3.0 (2.2–4.7) n = 41 | 3.1 (2.3– 4.5) n = 52 | 3.2 (2.4–4.1) n = 59 | 3.4 (2.8–4.9) n = 55 | 2.3 (2.1–4.6) n = 48 | 2.3 (2.1–4.3) n = 41 | 2.4 (2.1–4.1) n = 52 | 2.4 (1.8–4.3) n = 59 | 2.6 (2.2–5.1) n = 55 |
| Staphylococcus aureus Non MRSA, Non Methicillin resistant | 1.7 (0.8–2.6) n = 95 | 1.6 (0.9–2.6) n = 90 | 1.6 (1.0–2.6) n = 90 | 1.8 (1.1–2.9) n = 98 | 1.8 (1.0–2.8) n = 90 | 2.1 (1.4–3.5) n = 95 | 2.2 (1.4–3.7) n = 90 | 2.3 (1.6–3.7) n = 90 | 2.3 (1.5–3.8) n = 98 | 2.4 (1.5–3.9) n = 90 |
| p-value < 0.001 | p-value < 0.001 | |||||||||
| Pre-Pandemic Period 2018–2019 (n = 246) Group A | COVID-19 Pandemic 2020–2022 (n = 154) Group B | |
|---|---|---|
| Gender, male | 166 (67.2%) | 98 (63.6%) |
| Age, years, mean ± SD | 65.6 (50.4–76.4) | 65.8 (50.5–77.4) |
| Duration of bacteraemia symptoms before treatment initiation | ||
| 0–24 h | 158 (64.2%) | 73 (47.4%) |
| 25–72 h | 25 (10.2%) | 34 (22.1%) |
| >72 h | 55 (22.4%) | 36 (23.4%) |
| Unknown | 8 (3.2%) | 11 (7.1%) |
| Telephone consultation | 37 (15%) | 117 (76%) |
| Bedside consultation | 209 (85%) | 37 (24%) |
| Group A, 2018–2019 (n = 246) | Group B, 2020–2022 (n = 154) | p-Value | |
|---|---|---|---|
| Operation within 30 days | 34 (13.8%) | 29 (18.8%) | 0.04 |
| Diabetes mellitus type 2 | 89 (36.2%) | 68 (44.2%) | 0.12 |
| Heart failure | 26 (10.6%) | 19 (12.3%) | 0.02 |
| Coronary disease | 49 (19.9%) | 18 (11.7%) | 0.45 |
| Peripheral Vascular disease | 11 (4.5%) | 12 (7.8%) | 0.12 |
| Cerebrovascular disease | 18 (7.3%) | 17 (11%) | 0.05 |
| Chronic respiratory disease | 9 (3.7%) | 8 (5.2%) | 0.04 |
| Malignancies | 25 (10.2%) | 35 (22.7%) | 0.24 |
| Transplantation | 14 (5.7%) | 11 (7.14%) | 1.02 |
| Immunosuppresion | 38 (15.4%) | 24 (15.6%) | 0.87 |
| Chronic renal disease | 22 (8.9%) | 19 (12.3%) | 0.04 |
| Prosthetic device | 56 (22.8%) | 47 (30.5%) | 0.02 |
| Charlson comorbidity index Score ≥ 3 | 102 (41%) | 67 (43.5%) | 0.02 |
| Group A 2018–2019 (n = 246) | Group B 2020–2022 (n = 154) | p-Value | |
|---|---|---|---|
| Community-acquired infection | 96 (39%) | 56 (36.3%) | 0.001 |
| Hospital-acquired infection | 150 (61%) | 98 (63.6%) | 0.001 |
| Multidrug-resistant bacteria | 83 (33.7%) | 57 (37%) | 0.001 |
| Focus of infection | |||
| Unknown | 16 (6.5%) | 18 (11.7%) | 0.004 |
| Central venous catheter | 46 (18.7%) | 31 (20.1%) | 0.156 |
| Peripheral venous catheter | 34 (13.8%) | 21 (13.6%) | 0.458 |
| Thrombophlebitis | 12 (4.9%) | 27 (17.5%) | 0.024 |
| Implanted vascular device | 21 (8.5%) | 16 (10.4%) | 0.048 |
| Infective endocarditis | 11 (4.5%) | 16 (10.4%) | 0.678 |
| Native valve | 6 (2.4%) | 7 (4.5%) | 0.465 |
| Prosthetic valve | 5 (2%) | 9 (5.8%) | 0.247 |
| Joint infection | 10 (4.1%) | 9 (5.8%) | 0.765 |
| Prosthetic joint infection | 15 (6.1%) | 19 (12.3%) | 0.223 |
| Vertebral osteomyelitis | 13 (5.3%) | 17 (11%) | 0.058 |
| Intra-abdominal infections | 26 (10%) | 18 (11.7%) | 0.047 |
| Osteomyelitis/diabetic foot ulcers | 29 (11.8%) | 20 (13%) | 0.023 |
| Skin and soft-tissue infections | 24 (9.8%) | 19 (12.3%) | 0.027 |
| Respiratory infections | 32 (13%) | 21 (13.6%) | 0.057 |
| Urinary tract infections | 19 (7.7%) | 16 (10.4%) | 0.077 |
| Central nervous system infections | 9 (3.7%) | 7 (4.5%) | 0.065 |
| Complicated infection | 134 (54.5%) | 87 (56.5%) | 0.001 |
| Group A 2018–2019 (n = 246) | Group B 2020–2022 (n = 154) | p-Value | |
|---|---|---|---|
| Septic shock | 8 (3.3%) | 7 (4.5%) | 0.118 |
| Hospitalization in ICU | 11 (4.5%) | 12 (7.8%) | 0.245 |
| Hospital stay, days, mean ± SD | 29 (17–52) | 30 (16–51) | 0.457 |
| Mortality | |||
| Within 28 days | 12 (4.9%) | 16 (10.4%) | 0.001 |
| Within 90 days | 19 (7.7%) | 23 (14.9%) | 0.001 |
| Repeated blood culture | 137 (55.7%) | 56 (36.4%) | 0.001 |
| Negative blood culture within 7 days | 98 (40%) | 48 (31.2%) | 0.001 |
| Recurrent disease | 9 (3.6%) | 6 (3.9%) | 0.458 |
| Duration of antibiotic treatment, days, mean ± SD | 15 (8–19) | 11 (6–12) | 0.04 |
| Repeated clinical estimation | 112 (45.5%) | 36 (23.4%) | 0.001 |
| Combination of antibiotics | 26 (10.6%) | 11 (7.1%) | 0.001 |
| Recorded bloodstream infection (isolated pathogen) in discharge summary | 124 (50.4%) | 44 (28.6%) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens 2023, 12, 780. https://doi.org/10.3390/pathogens12060780
Petrakis V, Panopoulou M, Rafailidis P, Lemonakis N, Lazaridis G, Terzi I, Papazoglou D, Panagopoulos P. The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens. 2023; 12(6):780. https://doi.org/10.3390/pathogens12060780
Chicago/Turabian StylePetrakis, Vasilios, Maria Panopoulou, Petros Rafailidis, Nikolaos Lemonakis, Georgios Lazaridis, Irene Terzi, Dimitrios Papazoglou, and Periklis Panagopoulos. 2023. "The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections" Pathogens 12, no. 6: 780. https://doi.org/10.3390/pathogens12060780
APA StylePetrakis, V., Panopoulou, M., Rafailidis, P., Lemonakis, N., Lazaridis, G., Terzi, I., Papazoglou, D., & Panagopoulos, P. (2023). The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens, 12(6), 780. https://doi.org/10.3390/pathogens12060780






