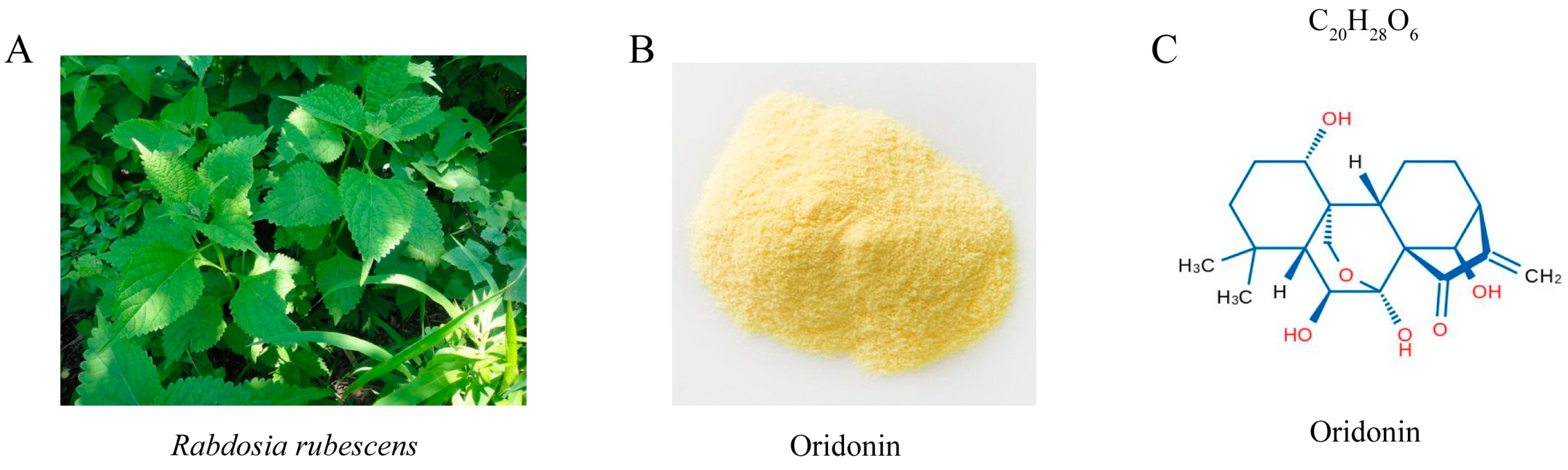
Oridonin Inhibits Mycobacterium marinum Infection-Induced Oxidative Stress In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and CCK8 Assay
2.2. Mm Culture
2.3. Mm Infection and Colony-Forming Units (CFU) Assay
2.4. Microinjection of Mm into Zebrafish
2.5. RNA Isolation and qRT-PCR
2.6. Western Blot Analysis
2.7. Statistics
3. Results
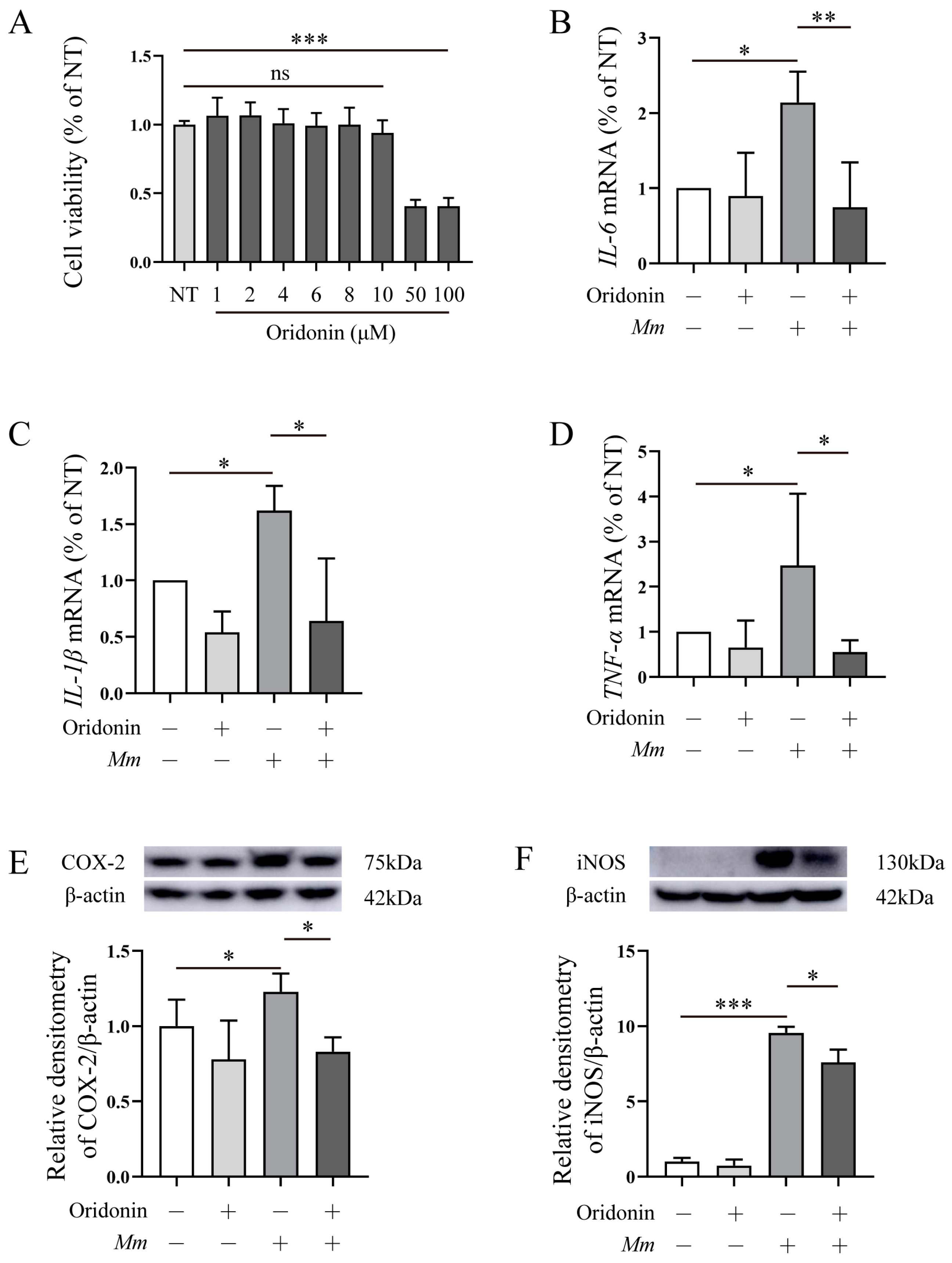
3.1. Ori Hinders Mm Entry into Lung Epithelial Cells
3.2. Ori Inhibits the Pro-Inflammatory Response Induced by Mm Infection in RAW264.7 Cells
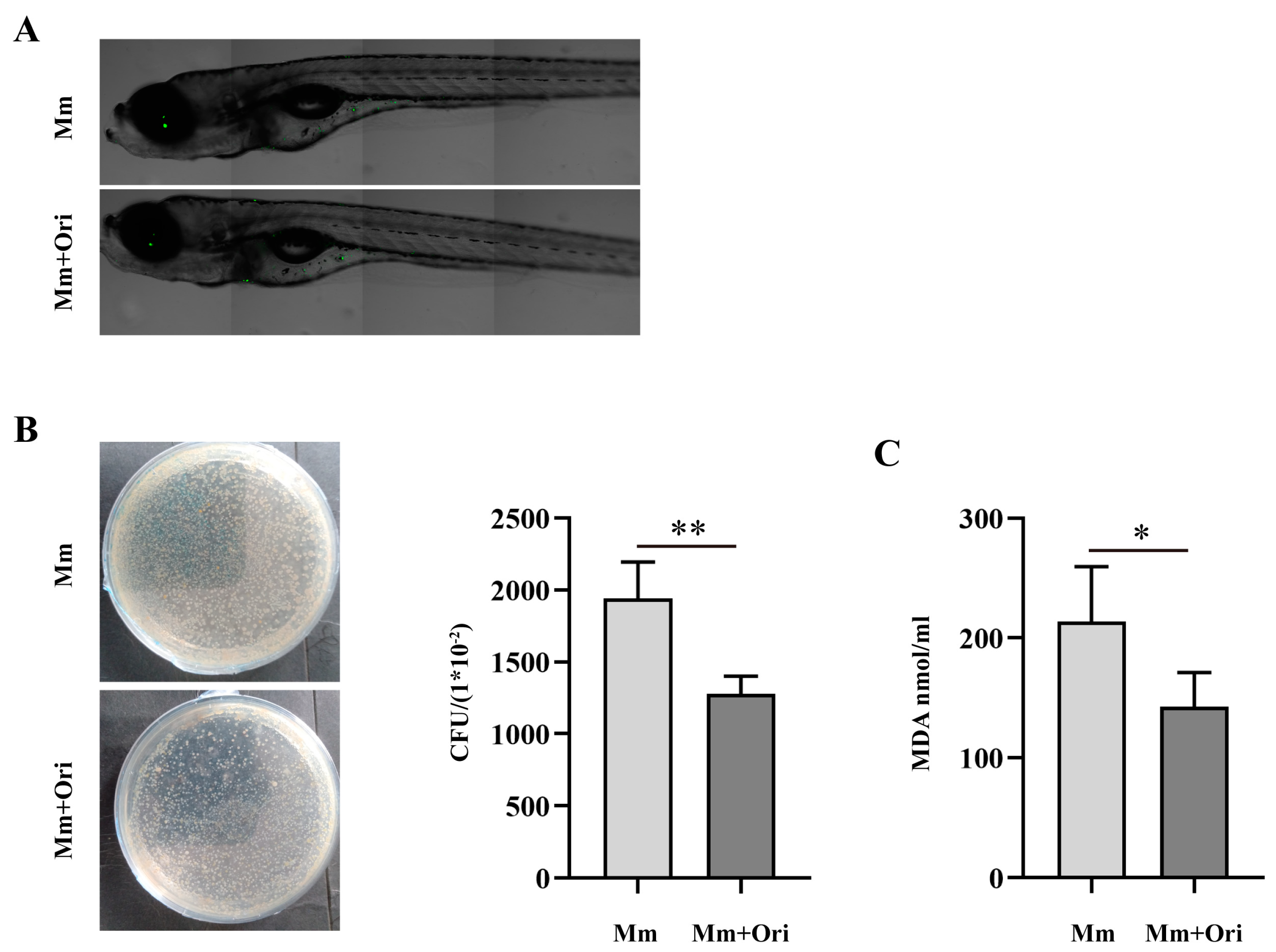
3.3. Ori Inhibits the Proliferation of Mm in Zebrafish and Alleviates Oxidative Stress in Mm-Infected Zebrafish
3.4. The Effects of Ori on NRF2/HO-1/NQO-1, and AKT/GSK-3β/AMPK-α1 Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suliman, S.; Pelzer, P.T.; Shaku, M.; Rozot, V.; Mendelsohn, S.C. Meeting report: Virtual Global Forum on Tuberculosis Vaccines, 20–22 April 2021. Vaccine 2021, 39, 7223–7229. [Google Scholar] [CrossRef]
- Dutta, N.K.; Klinkenberg, L.G.; Vazquez, M.J.; Segura-Carro, D.; Colmenarejo, G.; Ramon, F.; Rodriguez-Miquel, B.; Mata-Cantero, L.; Porras-De Francisco, E.; Chuang, Y.M.; et al. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci. Adv. 2019, 5, eaav2104. [Google Scholar] [CrossRef]
- Nathavitharana, R.R.; Friedland, J.S. A tale of two global emergencies: Tuberculosis control efforts can learn from the Ebola outbreak. Eur. Respir. J. 2015, 46, 293–296. [Google Scholar] [CrossRef]
- World Health Organization. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management; World Health Organization: Geneva, Switzerland, 2018.
- Emery, J.C.; Richards, A.S.; Dale, K.D.; McQuaid, C.F.; White, R.G.; Denholm, J.T.; Houben, R. Self-clearance of Mycobacterium tuberculosis infection: Implications for lifetime risk and population at-risk of tuberculosis disease. Proc. Biol. Sci. 2021, 288, 20201635. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; Edelstein, P.H.; Ramakrishnan, L. Is Mycobacterium tuberculosis infection life long? BMJ 2019, 367, l5770. [Google Scholar] [CrossRef] [PubMed]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C.; Sathananthan, A.; Venketaraman, V. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef]
- Zumla, A.; George, A.; Sharma, V.; Herbert, R.H.; Ilton, B.M.O.; Oxley, A.; Oliver, M. The WHO 2014 global tuberculosis report—Further to go. Lancet. Glob. Health 2015, 3, e10–e12. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, V.; Aithal, G.P. Hepatotoxicity Related to Anti-tuberculosis Drugs: Mechanisms and Management. J. Clin. Exp. Hepatol. 2013, 3, 37–49. [Google Scholar] [CrossRef]
- Singh, P.; Subbian, S. Harnessing the mTOR Pathway for Tuberculosis Treatment. Front. Microbiol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.M.; Kuo, C.Y.; Lin, C.Y.; Hung, M.F.; Shen, J.J.; Hwang, T.L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014, 19, 3327–3344. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Xia, Y.X.; Liang, Z.M.; Tsang, S.W.; Zhang, H.J. Mechanistic Pathways and Molecular Targets of Plant-Derived Anticancer ent-Kaurane Diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef]
- Chen, K.; Ye, J.; Qi, L.; Liao, Y.; Li, R.; Song, S.; Zhou, C.; Feng, R.; Zhai, W. Oridonin inhibits hypoxia-induced epithelial-mesenchymal transition and cell migration by the hypoxia-inducible factor-1alpha/matrix metallopeptidase-9 signal pathway in gallbladder cancer. Anti-Cancer Drugs 2019, 30, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Wang, D.; Zou, L.; Fu, C.; Zhang, J.; Leung, G.P. Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacol. Res. 2019, 146, 104313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Dai, M.; Nai, J.; Zhu, L.; Sheng, H. Solubility and Bioavailability Enhancement of Oridonin: A Review. Molecules 2020, 25, 332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, L.; Zha, D.; Zhang, L.; Gao, P.; Zhang, J.; Wu, X. Oridonin protects against the inflammatory response in diabetic nephropathy by inhibiting the TLR4/p38-MAPK and TLR4/NF-kappaB signaling pathways. Int. Immunopharmacol. 2018, 55, 9–19. [Google Scholar] [CrossRef]
- Xu, J.; Wold, E.A.; Ding, Y.; Shen, Q.; Zhou, J. Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection. Molecules 2018, 23, 474. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.T.; Ma, W.; Xie, X.; Huang, Q. Oridonin: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2021, 12, 645824. [Google Scholar] [CrossRef]
- Takaki, K.; Davis, J.M.; Winglee, K.; Ramakrishnan, L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat. Protoc. 2013, 8, 1114–1124. [Google Scholar] [CrossRef]
- Kobayashi, E.; Suzuki, T.; Yamamoto, M. Roles nrf2 plays in myeloid cells and related disorders. Oxidative Med. Cell. Longev. 2013, 2013, 529219. [Google Scholar] [CrossRef]
- Rizvi, F.; Mathur, A.; Kakkar, P. Morin mitigates acetaminophen-induced liver injury by potentiating Nrf2 regulated survival mechanism through molecular intervention in PHLPP2-Akt-Gsk3beta axis. Apoptosis Int. J. Program. Cell Death 2015, 20, 1296–1306. [Google Scholar] [CrossRef]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet. Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Stinear, T.P.; Seemann, T.; Harrison, P.F.; Jenkin, G.A.; Davies, J.K.; Johnson, P.D.; Abdellah, Z.; Arrowsmith, C.; Chillingworth, T.; Churcher, C.; et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008, 18, 729–741. [Google Scholar] [CrossRef]
- Cosma, C.L.; Klein, K.; Kim, R.; Beery, D.; Ramakrishnan, L. Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect. Immun. 2006, 74, 3125–3133. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zheng, X.C.; Wang, T.; Zhang, T.Y. Effects of oridonin on immune cells, Th1/Th2 balance and the expression of BLys in the spleens of broiler chickens challenged with Salmonella pullorum. Res. Vet. Sci. 2018, 119, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Zheng, X.C.; Wang, T.; Zhang, T.Y. Effects of dietary supplementation with oridonin on the growth performance, relative organ weight, lymphocyte proliferation, and cytokine concentration in broiler chickens. BMC Vet. Res. 2018, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Zheng, X.C.; Wang, T.; Zhang, T.Y. Effect of dietary oridonin supplementation on growth performance, gut health, and immune response of broilers infected with Salmonella pullorum. Ir. Vet. J. 2018, 71, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, T.; Xu, S.; Zhou, T.; Tian, K.; Hu, X.; Cheng, K.; Li, Z.; Hua, H.; Xu, J. Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao. Molecules 2016, 21, 575. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pei, L.; Li, D.; Yao, H.; Cai, H.; Yao, H.; Wu, X.; Xu, J. Synthesis and antimycobacterial evaluation of natural oridonin and its enmein-type derivatives. Fitoterapia 2014, 99, 300–306. [Google Scholar] [CrossRef]
- Tan, R.Z.; Yan, Y.; Yu, Y.; Diao, H.; Zhong, X.; Lin, X.; Liao, Y.Y.; Wang, L. Renoprotective Effect of Oridonin in a Mouse Model of Acute Kidney Injury via Suppression of Macrophage Involved Inflammation. Biol. Pharm. Bull. 2021, 44, 714–723. [Google Scholar] [CrossRef]
- Tian, L.; Sheng, D.; Li, Q.; Guo, C.; Zhu, G. Preliminary safety assessment of oridonin in zebrafish. Pharm. Biol. 2019, 57, 632–640. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Wang, L.; Yan, C.; Liu, H.; Zhang, W.; Zhao, H.; Cheng, C.; Chen, Z.; Xu, T.; et al. Oridonin attenuates hind limb ischemia-reperfusion injury by modulating Nrf2-mediated oxidative stress and NLRP3-mediated inflammation. J. Ethnopharmacol. 2022, 292, 115206. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Yang, Z.; Wen, D.; Li, P.; Xiong, Q.; Wu, C. Oridonin Inhibits Mycobacterium marinum Infection-Induced Oxidative Stress In Vitro and In Vivo. Pathogens 2023, 12, 799. https://doi.org/10.3390/pathogens12060799
Chen G, Yang Z, Wen D, Li P, Xiong Q, Wu C. Oridonin Inhibits Mycobacterium marinum Infection-Induced Oxidative Stress In Vitro and In Vivo. Pathogens. 2023; 12(6):799. https://doi.org/10.3390/pathogens12060799
Chicago/Turabian StyleChen, Guangxin, Ziyue Yang, Da Wen, Ping Li, Qiuhong Xiong, and Changxin Wu. 2023. "Oridonin Inhibits Mycobacterium marinum Infection-Induced Oxidative Stress In Vitro and In Vivo" Pathogens 12, no. 6: 799. https://doi.org/10.3390/pathogens12060799
APA StyleChen, G., Yang, Z., Wen, D., Li, P., Xiong, Q., & Wu, C. (2023). Oridonin Inhibits Mycobacterium marinum Infection-Induced Oxidative Stress In Vitro and In Vivo. Pathogens, 12(6), 799. https://doi.org/10.3390/pathogens12060799






